Abstract
Objective
to assess the safety and efficacy of influenza vaccination in patients with systemic lupus erythematosus (SLE), and to evaluate the influence of immunosuppressive drugs on the immune response.
Methods
SLE patients (n = 56) and healthy controls (n = 18) were studied. All patients had quiescent disease (SLE disease activity index ⩽5). Four patient groups were defined on the basis of their drug use: (1) no drug treatment; (2) hydroxychloroquine treatment; (3) azathioprine treatment; (4) prednisone treatment. Participants received trivalent influenza subunit vaccine during October/November 2003. Disease activity scores and side effects were recorded. Antibody titres against influenza virus were measured before and 30 days after vaccination using the haemagglutination inhibition assay.
Results
Influenza vaccination did not result in changes in disease activity and was well tolerated. SLE patients had fewer seroconversions or fourfold titre rises for A/H1N1 (p<0.001) and A/H3N2 (p<0.001) than healthy controls, while for B/Hong Kong the difference was of borderline significance (p = 0.051). With regard to immunosuppressive treatment, fewer SLE patients using azathioprine developed fourfold titre rises against A/H3N2 (p = 0.041), and fewer achieved titres of ⩾40 against A/H3N2 (p = 0.030) compared with the other patient groups.
Conclusions
Influenza vaccination in SLE patients with quiescent disease is safe but is less effective than in controls. Use of azathioprine was associated with a trend to decreased vaccination efficacy.
Keywords: SLE, influenza vaccination, safety, efficacy
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterised by relapsing and remitting disease activity. Immunosuppressive drugs are often needed to control disease activity rendering patients more susceptible to infection. Immunocompromised patients have an increased risk of morbidity and mortality following influenza infection.1 Thus influenza vaccination should be considered in SLE patients. It is, however, still questionable whether vaccination might induce disease activity in patients with established autoimmune disease. Limited numbers of studies have been undertaken to establish whether influenza vaccination is safe in SLE patients.2,3,4,5,6,7,8,9 These studies have their limitations, as most dealt with small numbers of patients3,4,6,8,9 and included patients irrespective of their level of disease activity.4,5,7,8,9
Furthermore, it is unclear whether vaccination is effective in SLE patients, as they are assumed to have decreased primary and secondary immune responses.10 In addition, the use of immunosuppressive drugs may further decrease the immune response following vaccination. In some studies it has been shown that SLE patients have a reduced antibody response after vaccination compared with healthy adults.4,7,11 In contrast, other studies suggested a normal vaccination efficacy.2,3,5,6 In transplantation patients the use of drugs such as corticosteroids, azathioprine, and ciclosporine decreases the antibody response after vaccination12,13,14,15,16; however, the influence of immunosuppressive drugs on efficacy of influenza vaccination in SLE patients has not been thoroughly examined.
In this study we assessed the safety and efficacy of influenza vaccination and the effect of drugs on vaccination efficacy in an (immunosuppressed) cohort of SLE patients.
Methods
Patients
Patients were eligible for the study when they fulfilled at least four of the American College of Rheumatology criteria for SLE17 and had quiescent disease, defined as a SLE disease activity index (SLEDAI) of ⩽5.18 Based on predefined drug treatment criteria, patients were divided in four groups. Group A consisted of patients who were not using immunosuppressive drugs. Patients in group B used hydroxychloroquine ⩾400 mg/day. Patients in group C used azathioprine ⩾50 mg/day. In both group B and group C, a stable dose of prednisone of less than 10 mg/day was allowed. Finally, group D consisted of patients who used a stable dose of prednisone of ⩾10 mg/day. “Stable” was defined as a constant dose, unaltered for a period of at least two months before vaccination. Patients were excluded when no informed consent was given; in cases of pregnancy; and if immunosuppressive drugs other than hydroxychloroquine, azathioprine, or prednisone were used. In all, five patients using methotrexate and 12 patients treated with a variety of other immunosuppressive drugs (cyclophosphamide, ciclosporine, and mycophenolate mofetil) were excluded. Age and sex matched healthy volunteers were used as controls.
Vaccines
Influvac®, a trivalent influenza vaccine (2003/2004), was supplied by Solvay Pharmaceuticals (Weesp, Netherlands). The vaccine contained surface antigens (haemagglutinin and neuramidase) of viruses bred on chicken eggs, of the following strains: A/Moscow/10/99‐like (A/H3N2) (A/Panama/2007/99 RESVIR‐17 reass.), A/New Caledonia/20/99‐like (A/H1N1) (A/New Caledonia/20/99 IVR‐116 reass.), B/Hong Kong/330/2001‐like (B/Shangdong/797). There were 15 μg of haemagglutinin per virus preparation.
Procedures
Patients and controls were vaccinated with Influvac®, a subunit vaccine, in October and November 2003. The patients were vaccinated at a regular outpatient visit. SLEDAI was recorded for measuring disease activity. After 30 (3) days (mean (range)), patients and controls were seen again. At this visit the SLEDAI scores were once more recorded in the patients. In addition, patients were asked to fill in a visual analogue score (VAS) on a scale of 0 to 10 (patient VAS, disease activity as experienced by the patient) during both visits. In all participants, information on previous influenza vaccination was obtained and adverse effects following vaccination were recorded. Adverse effects were classified into local (itching, pain, erythema, and induration at the site of vaccination), systemic (fever, tiredness, sweating, myalgia, chills, headache, arthralgia, diarrhoea, common cold‐like complaints), and other adverse effects.
At the time of vaccination and at the follow up visit 10 ml blood were drawn. After sampling, serum was stored at −20°C until the end of the study.
Haemagglutination inhibition test
For quantitative detection of influenza antibodies the haemagglutination inhibition (HAI) test was used. HAI tests were carried out with guinea pig erythrocytes following standard procedures19 with slight modifications as described elsewhere.20 Sera were tested against all three vaccine strains. The antibody response was evaluated in three ways: by assessment of a ⩾fourfold titre rise, by means of a titre rise to ⩾40, and by geometric mean titres (GMTs). Fourfold titre rises and seroconversions are widely in use as indices for efficacy of vaccination. Seroconversions were defined as those samples that tested negative (below 1:10) before vaccination, rising to at least 40 after vaccination. Titres ⩾40 can be considered as protective in healthy adults,21 and a median titre of 28 protects 50% of healthy adult vaccinees.22
Statistical analysis
Data were analysed using SPSS 11 (SPSS Inc). The Mann–Whitney U test, Wilcoxon signed rank test, Fisher's exact test, and the Kruskal–Wallis test were used where appropriate. A probability (p) value of <0.05 was considered significant.
Results
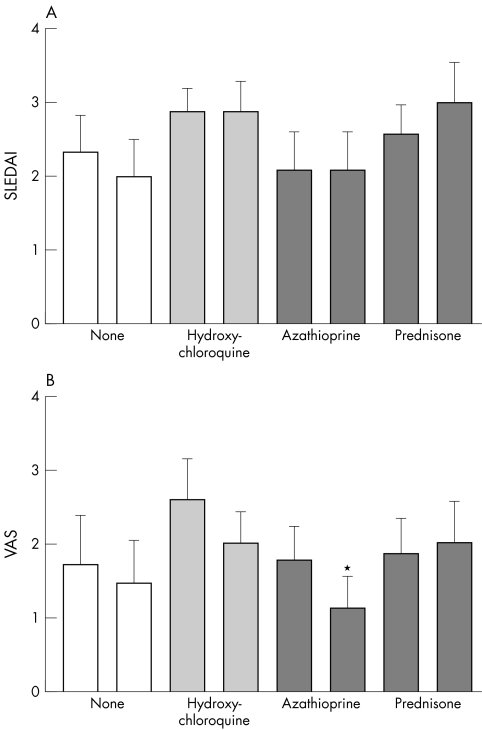
Fifty six SLE patients and 18 healthy controls were studied. Forty three (77%) of the SLE patients had received influenza vaccination in the past compared with four (22%) of the healthy controls (p<0.001). Thus more patients (34 of 56) than controls (1 of 17) had received influenza vaccination the year before (2002/2003; p<0.001), which consisted of the same viral antigens. Data on the four study groups are shown in table 1. Median doses for the drugs which defined the groups were 400 mg/day for hydroxychloroquine in group B, 100 mg/day for azathioprine in group C, and 10 mg/day for prednisone in group D. Baseline characteristics were equally distributed among the groups. Patient groups did not differ before vaccination for duration of SLE, patient VAS, or SLEDAI (fig 1, p = 0.644). Within patient groups, the numbers of patients who had received influenza vaccination in the past were comparable (p = 0.231); however, more patients in the azathioprine group had received vaccination in the previous influenza season (2002/2003) than patients in the other groups (p = 0.026).
Table 1 Baseline characteristics of patients with systemic lupus erythematosus and controls.
| Variable | No drugs | Hydroxychloroquine | Azathioprine | Prednisone | Healthy controls | p Value |
|---|---|---|---|---|---|---|
| Number | 12 | 17 | 13 | 14 | 18 | |
| Age (years) | 45 (29 to 78) | 42 (26 to 66) | 47 (28 to 64) | 46.5 (18 to 71) | 40.5 (21 to 57) | 0.518 |
| (median (range)) | ||||||
| Sex (male/female) | 4/8 | 1/16 | 1/12 | 0/14 | 4/14 | 0.068 |
| Duration of disease (years) | 8 (2 to 43) | 9 (3 to 45) | 10 (4 to 29) | 5 (1 to 36) | 0.730 | |
| (median (range)) | ||||||
| Influenza vaccination in | 8/4 | 1/6 | 12/1 | 2/2 | 4/14† | <0.001 |
| the past (yes/no) | ||||||
| Influenza vaccination last | 6/6 | 7/10 | 12/1* | 9/5 | 1/17† | <0.001 |
| season (yes/no) |
The Kruskal‐Wallis test was used to compare all groups for age and duration of disease, Fisher's exact test was used to compare all groups for sex and for influenza vaccination in the past/last season.
*p = 0.026, patients on azathioprine v other patient groups.
†p<0.001, SLE patients v healthy controls.
Figure 1 Influence of vaccination on disease activity in the different patient groups. Disease activity was measured by the SLE disease activity index (SLEDAI) (A) and patient visual analogue score (VAS) (B), depicting patients' perception of disease activity. Columns are means, error bars = SEM. The left bars in each pair represent results before, the right bars 30 days after vaccination. *p<0.05 (Wilcoxon signed rank test).
Safety of vaccination
SLEDAI scores after vaccination did not differ significantly from scores before vaccination in any of the patient groups. However, in the azathioprine group, patient VAS scores were significantly lower after vaccination. In the other patient groups no significant changes in patient VAS scores were observed (fig 1).
In relation to side effects, three SLE patients reported local adverse reactions and 19 reported systemic adverse reactions. One healthy control reported a local adverse reaction and one a systemic adverse reaction. The difference in systemic adverse reactions between SLE patients and controls was significant (p = 0.02). In particular, tiredness, sweating, and myalgia were reported. All adverse reactions were mild.
Efficacy of vaccination
The GMT values in SLE patients and controls are shown in table 2. As expected, as more SLE patients were vaccinated with the same vaccine the previous season, GMTs before vaccination were significantly higher in SLE patients than in controls (p<0.001 for A/H1N1, p = 0.036 for A/H3N2, and p<0.001 for B/Hong Kong). In both patients and controls GMT increased after vaccination and did not differ significantly between both groups. However, SLE patients had fewer seroconversions or fourfold titre rises against A/H1N1 (p<0.001) and A/H3N2 (p = 0.001) than controls; for B/Hong Kong this difference was marginally significant (p = 0.051; table 3). Seventy five per cent of SLE patients achieved a titre of ⩾40 after vaccination for both influenza A strains together, compared with 100% of healthy controls (p = 0.030). No significant differences were found in the percentage of patients who achieved a post‐vaccination titre ⩾40 for separate influenza strains compared with the controls, although a trend towards a lower percentage in the patients could be seen.
Table 2 Efficacy of influenza vaccination: geometric mean titres to influenza.
| GMT to A/H1N1 | GMT to A/H1N1 | GMT to A/H3N2 | GMT to A/H3N2 | GMT to B/Hong Kong before | GMT to B/Hong Kong after | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before vaccination | after vaccination | before vaccination | after vaccination | vaccination | vaccination | |||||||
| SLE (n = 56) | 32.4 | 142 | 50.0 | 183 | 16.2 | 64.0 | ||||||
| Healthy controls (n = 17) | 6.93** | 130 | 21.7* | 272 | 5.65** | 49.0 |
The Mann–Whitney U test was used for all variables.
*p<0.05.
**p<0.001.
GMT, geometric mean titre; SLE, systemic lupus erythematosus.
Table 3 Efficacy of influenza vaccination: seroconversions or fourfold titre rises.
| SLE (n = 56) | Healthy controls (n = 17) | p Value | |
|---|---|---|---|
| A/H1N1 | 24 (43%) | 16 (94%) | <0.001 |
| A/H3N2 | 22 (39%) | 15 (88%) | 0.001 |
| B/Hong Kong | 23 (41%) | 12 (71%) | 0.051 |
Fisher's exact test was used for all variables.
SLE, systemic lupus erythematosus.
Because more SLE patients than controls had an antibody titre ⩾40 against influenza A/H1N1 and B/Hong Kong before vaccination (table 4), we assumed that this could reduce the number of patients achieving seroconversion or a fourfold increase in titre. To exclude effects of an influenza vaccination the previous season, we examined those participants who did not receive an influenza vaccination in 2002 separately. SLE patients showed significant fewer seroconversions or fourfold titre rises to A/H1N1 and A/H3N2 (table 5).
Table 4 Efficacy of influenza vaccination: titres ⩾40 to influenza.
| SLE patients (n = 56) | Healthy controls (n = 17) | p Value | |
|---|---|---|---|
| A/H1N1 ⩾40 before vaccination | 27 (48%) | 1 (6%) | 0.001 |
| A/H1N1 ⩾40 after vaccination | 47 (84%) | 17 (100%) | 0.105 |
| A/H3N2 ⩾40 before vaccination | 35 (63%) | 7 (41%) | 0.163 |
| A/H3N2 ⩾40 after vaccination | 48 (86%) | 17 (100%) | 0.185 |
| B/Hong Kong ⩾40 before vaccination | 14 (25%) | 0 (0%) | 0.030 |
| B/Hong Kong ⩾40 after vaccination | 39 (70%) | 12 (71%) | 1.000 |
Fisher's exact test was used for all variables.
SLE, systemic lupus erythematosus.
Table 5 Response of participants with no influenza vaccination in the previous year (2002).
| SLE patients (n = 22) | Healthy Controls (n = 16) | p Value | |
|---|---|---|---|
| A/H1N1 titre ⩾40 after vaccination | 18 (82%) | 16 (100%) | 0.124 |
| A/H1N1 seroconversion or fourfold titre rise | 14 (64%) | 16 (100%) | 0.012 |
| A/H3N2 titre ⩾40 after vaccination | 19 (86%) | 16 (100%) | 0.249 |
| A/H3N2 seroconversion or fourfold titre rise | 10 (45%) | 15 (94%) | 0.002 |
| B/Hong Kong titre ⩾40 after vaccination | 15 (68%) | 12 (75%) | 0.729 |
| B/Hong Kong seroconversion or fourfold titre rise | 13 (59%) | 12 (75%) | 0.490 |
Fisher's exact test was used for all variables.
SLE, systemic lupus erythematosus.
Effect of drug treatment on vaccination efficacy
To evaluate the influence of immunosuppressive drug treatment on vaccination efficacy we compared the percentage of seroconversions or fourfold titre rises and protective titres after vaccination in patients without drugs with those in patients using immunosuppressive agents. For this purpose we combined patient groups B to D in which immunosuppressive drug treatment was used. This analysis showed no difference between patients without drugs compared with patients using immunosuppressive agents in the percentage of seroconversions or fourfold titre rises (p = 0.325 for A/H1N1, p = 0.184 for A/H3N2) or in achievement of titres ⩾40 (p = 0.666 for A/H1N1, p = 0.180 for A/H3N2). Next, we conducted a subanalysis in which all patient groups were compared with each other (tables 6 and 7). For A/H1N1 and B/Hong Kong no difference was found in the percentage of seroconversions or fourfold titre rises (p = 0.619 for A/H1N1, p = 0.316 for B/Hong Kong) or in the achievement of titres ⩾40 (p = 0.396 for A/H1N1, p = 0.226 for B/Hong Kong). However, for A/H3N2, SLE patients receiving azathioprine had fewer fourfold titre rises than the other patient groups (p = 0.041). Furthermore, a smaller proportion of the azathioprine group achieved titres of ⩾40 against A/H3N2 (p = 0.030) compared with the other patient groups.
Table 6 Influence of immunosuppressive drug treatment on vaccination efficacy: seroconversions or fourfold titre rises.
| No drug treatment (n = 12) | Hydroxychloroquine (n = 17) | Azathioprine (n = 13) | Prednisone (n = 14) | p Value | ||
|---|---|---|---|---|---|---|
| A/H1N1 | 7 (58%) | 7 (41%) | 4 (31%) | 6 (43%) | 0.619 | |
| A/H3N2 | 7 (58%) | 8 (47%) | 1 (8%) | 6 (43%) | 0.041 | |
| B/Hong Kong | 7 (58%) | 8 (47%) | 3 (23%) | 5 (36%) | 0.316 |
Fisher's exact test was used for all variables.
Table 7 Influence of immunosuppressive drug treatment on vaccination efficacy: titres ⩾40 to influenza.
| No drug treatment (n = 12) | Hydroxychloroquine (n = 17) | Azathioprine (n = 13) | Prednisone (n = 14) | p Value | |
|---|---|---|---|---|---|
| A/H1N1 ⩾40 before vaccination | 6 (50%) | 8 (47%) | 7 (54%) | 6 (43%) | 0.982 |
| A/H1N1 ⩾40 after vaccination | 11 (92%) | 14 (82%) | 9 (69%) | 13 (93%) | 0.396 |
| A/H3N2 ⩾40 before vaccination | 8 (67%) | 11 (65%) | 7 (54%) | 9 (64%) | 0.929 |
| A/H3N2 ⩾40 after vaccination | 12 (100%) | 16 (94%) | 8 (62%) | 12 (86%) | 0.030 |
| B/Hong Kong ⩾40 before vaccination | 5 (42%) | 2 (12%) | 4 (31%) | 3 (21%) | 0.295 |
| B/Hong Kong ⩾40 after vaccination | 11 (92%) | 12 (71%) | 8 (62%) | 8 (57%) | 0.226 |
Fisher's exact test was used for all variables.
Discussion
Our study shows that influenza vaccination is safe in SLE patients with quiescent disease but has decreased efficacy, in particular in those taking azathioprine. It can be argued that disease activity might increase over a longer period than the follow up used in this study. However, the immune response to influenza is generated during the first weeks following vaccination. If vaccination were to enhance established autoimmunity, this would be expected to occur particularly during this early period. We therefore carried out our second assessment of disease activity four weeks after the vaccination.
We found no increase in SLE disease activity or in patient perception of disease activity, as measured by patient VAS, four weeks after the vaccination. This corresponds with previous studies,2,3,4,5,6,7,8,9 in which clinical and laboratory assessed lupus disease activity did not increase after vaccination. In one study, increased disease activity was reported, though it was infrequent and usually mild.2 Another study reported one patient (of 11) with significantly more disease activity following vaccination.6 Although SLE patients had more systemic side effects of influenza vaccination, these were all mild. Symptoms such as tiredness, sweating, and myalgia—which were considered as side effects—are common in SLE patients, although these are not criteria for disease activity in SLEDAI. Whereas SLEDAI scores did not change, the symptoms mentioned above occurred in some patients following vaccination. This suggests at the least a temporal relation. However, the higher frequency of side effects might have been a result of a reporting bias in patients. It is known that many SLE patients with quiescent disease experience a decreased sense of wellbeing,23,24,25 which contributes to such a bias. We conclude that influenza vaccination in SLE patients appears to be safe.
Studies concerning the efficacy of influenza vaccination thus far are conflicting, as some indicate normal efficacy in SLE patients2,3,5,6 whereas others conclude that efficacy is reduced.4,7,11 An overview is given in table 8. In general, these studies contained fewer patients than our study, efficacy was partially analysed, and effects of previous vaccinations were not mentioned. In addition, the effects of differences in drug use were often not sufficiently taken into account. Furthermore, previous influenza vaccinations were not recorded. In summary, conflicting data can be explained by methodological differences.
Table 8 Studies dealing with efficacy of influenza vaccination in SLE patients.
| Study | Year | SLE patients | Controls; study design | Parameters | Humoral response of | Influence of drug treatment | |
|---|---|---|---|---|---|---|---|
| SLE patients | |||||||
| Brodman et al5 | 1978 | 46 | 58 healthy controls | GMTs | Similar/decreased | No significant effect of prednisone, azathioprine, or hydroxychloroquine | |
| 23 patients on prednisone, mean | Titres ⩾40 | ||||||
| 20 mg/d | |||||||
| 3 patients on azathioprine, 50 mg/d | |||||||
| 28 patients on hydroxychloroquine | |||||||
| Louie et al6 | 1978 | 11 | 8 healthy controls | 4‐fold rises | Similar | – | |
| GMTs | |||||||
| Ristow et al4 | 1978 | 29 | 29 healthy controls, matched for prevaccination antibody titre | 4‐fold rises | Decreased/similar | No significant effect | |
| GMTs | (trend towards lower immunogenicity) | ||||||
| Williams et al7 | 1978 | 19 | 36 healthy controls | 4‐fold rises | Decreased | Trend towards lower immunogenicity when using prednisone | |
| Influenza vaccination in 19 patients and 18 controls, placebo vaccination in 21 patients and 18 controls, double blind; controls were matched for prevaccination antibody titre | GMTs | ||||||
| Titres ⩾40 | |||||||
| Herron et al2 | 1979 | 20 | 32 healthy controls, open label study | 4‐fold rises | Similar | Trend towards lower immunogenicity when using prednisone | |
| GMTs | |||||||
| Kanakoudi‐Tsakalidou et al3 | 2001 | 11 | Both patients and healthy controls (5) were children | 4‐fold rises | Similar | No significant effect | |
| GMTs | |||||||
| Titres ⩾40 | |||||||
| Abu‐Shakra et al11 | 2002 | 24 | None, immunogenicity of vaccination was compared with expected immunogenicity | 4‐fold rises | Decreased | Trend towards lower immunogenicity in case of azathioprine or ⩾10 mg prednisone/day | |
| Titres ⩾40 |
GMT, geometric mean titre; SLE, systemic lupus erythematosus.
We therefore evaluated the efficacy of influenza vaccination in SLE patients in several ways. With respect to the percentage of patients who achieved seroconversion or a fourfold titre rise we found that influenza vaccination was less effective for A/H1N1 and A/H3N2 in SLE patients. Accordingly, fewer SLE patients achieved a protective titre after vaccination against both the influenza A strains together when compared with healthy controls, despite the fact that more patients than controls had received a vaccination with the same viral antigens the year before. We suggest that the GMT in SLE patients after vaccination did not differ from the controls because GMT before vaccination was higher in the patients—which can easily be accounted for by their higher rate of previous vaccination. The conclusion that SLE patients appear to have a decreased immune response compared with healthy controls is supported by the subanalysis of those patients and controls who did not have influenza vaccination the previous season. In these subgroups there was also a significantly decreased humoral response to A/H1N1 and A/H3N2 in the SLE patients compared with the controls.
To evaluate whether our group of healthy controls was representative we compared the GMTs of this group with those of a healthy control group vaccinated in the course of a routine survey of the 2002/2003 Influvac vaccine (data kindly provided by Solvay Pharmaceuticals, Weesp, Netherlands). The 2002/2003 vaccine was identical to the 2003/2004 vaccine used in our study. A group of 17 healthy persons, age and sex matched, was compared with our group of controls. In the Solvay survey, the GMT of A/H1N1 increased from 7.5 to 221.4, of A/H3N2 from 16.0 to 247.2, and of B/Hong Kong from 8.2 to 90.0. The change in GMTs did not differ statistically from the results obtained in the controls in the present study (Mann–Whitney U test). Why patients showed a decreased humoral responses to both influenza A strains, but not to the B/Hong Kong strain is open to discussion. As the healthy controls in our study appeared to have a decreased response to the influenza B strain, a possible explanation is that the immunogenicity of the influenza B strain was lower than that of the influenza A strains included. This might have caused a smaller difference in response between patients and controls, in which case the power of our study could have been too low to detect such a difference.
It is reported that the H3N2 subtype of influenza A causes more severe illness than A/H1N1 or influenza B,26 and in most seasons the prevalence of influenza A infections is higher than influenza B infections.27 So sufficient protection against influenza A (especially A/H3N2) is clinically more relevant than sufficient protection against influenza B.
Why SLE patients have a decreased response to influenza vaccination is not entirely clear. Ioannou et al showed that vaccinations in SLE patients generally tend to give rise to lowered immune responses.28 Another study showed that pneumococcal vaccination in SLE patients in general is immunogenic, but that a subset of patients may remain unprotected by the currently available vaccine.29 It is conceivable that SLE patients have an intrinsic immunological defect that results in decreased responsiveness to vaccination. The assumption of an intrinsic immune defect is supported by studies reporting decreased cellular immune responses to influenza in SLE patients.30,31
In addition, the use of immunosuppressive drugs may influence the efficacy of vaccination. To assess this effect, we included patients using hydroxychloroquine, azathioprine, or prednisone, and analysed data from these groups of patients separately, as there are considerable differences in pharmacological effects between these drugs. Patients using other immunosuppressive agents were excluded to prevent the formation of small heterogeneous subgroups. SLE patients receiving azathioprine showed a trend towards a decreased immune response against influenza A/H3N2 compared with the other patient groups. This is in concordance with the study of Abu‐Shakra et al, in which a trend towards a decreased immune response to influenza vaccination was observed in SLE patients who received azathioprine.11 In renal transplant patients the use of azathioprine was reported to lower the antibody response to influenza vaccination compared with healthy controls,32 but this could not be confirmed by others.16 Although the number of patients included in this study is quite substantial, the subgroups (according to treatment) are quite small. Data on the effects of immunosuppressive drugs on the efficacy of the vaccination should therefore be interpreted with caution.
Twenty five per cent of SLE patients achieved titres of <40 against both the influenza A strains together and are not expected to be protected from influenza A infection.22 Moreover, one might expect that SLE patients experience less protection from influenza vaccination because cellular immunity also seems to be impaired after vaccination.30,31
To improve the antibody response of immunosuppressed patients several studies have been conducted in which a second vaccination was given. In general, in immunocompromised patients an increased antibody response could not be achieved after a booster injection,33,34 although Soesman et al did find an increased response in liver transplant patients.15 Recent studies have shown that virosomal vaccines generate better cellular immune responses, and they enhance the humoral immune response following vaccination as well.35,36,37,38 Regarding the hampered humoral and cellular immune response to influenza vaccination in SLE patients, these new vaccines are of particular interest as one might expect them to improve the efficacy of vaccination in SLE patients.
Acknowledgements
We thank Solvay Pharmaceuticals for kindly supplying the vaccines and additional efficacy data from healthy controls.
Abbreviations
GMT - geometric mean titre
SLE - systemic lupus erythematosus
SLEDAI - SLE disease activity index
VAS - visual analogue score
References
- 1.Hayden F G. Prevention and treatment of influenza in immunocompromised patients. Am J Med 199710255–60. [DOI] [PubMed] [Google Scholar]
- 2.Herron A, Dettleff G, Hixon B, Brandwin L, Ortbals D, Hornick R.et al Influenza vaccination in patients with rheumatic diseases. Safety and efficacy. JAMA 197924253–56. [PubMed] [Google Scholar]
- 3.Kanakoudi‐Tsakalidou F, Trachana M, Pratsidou‐Gertsi P, Tsitsami E, Kyriazopoulou‐Dalaina V. Influenza vaccination in children with chronic rheumatic diseases and long‐term immunosuppressive therapy. Clin Exp Rheumatol 200119589–594. [PubMed] [Google Scholar]
- 4.Ristow S C, Douglas R G, Condemi J J. Influenza vaccination of patients with systemic lupus erythematosus. Ann Intern Med 197888786–789. [DOI] [PubMed] [Google Scholar]
- 5.Brodman R, Gilfillan R, Glass D, Schur P H. Influenzal vaccine response in systemic lupus erythematosus. Ann Intern Med 197888735–740. [DOI] [PubMed] [Google Scholar]
- 6.Louie J S, Nies K M, Shoji K T, Fraback R C, Abrass C, Border W.et al Clinical and antibody responses after influenza immunization in systemic lupus erythematosus. Ann Intern Med 197888790–792. [DOI] [PubMed] [Google Scholar]
- 7.Williams G W, Steinberg A D, Reinertsen J L, Klassen L W, Decker J L, Dolin R. Influenza immunization in systemic lupus erythematosus. A double‐blind trial. Ann Intern Med 197888729–734. [DOI] [PubMed] [Google Scholar]
- 8.Abu‐Shakra M, Zalmanson S, Neumann L, Flusser D, Sukenik S, Buskila D. Influenza virus vaccination of patients with systemic lupus erythematosus: effects on disease activity. J Rheumatol 2000271681–1685. [PubMed] [Google Scholar]
- 9.Abu‐Shakra M, Press J, Sukenik S, Buskila D. Influenza virus vaccination of patients with SLE: effects on generation of autoantibodies. Clin Rheumatol 200221369–372. [DOI] [PubMed] [Google Scholar]
- 10.Kallenberg C G, Limburg P C, Van Slochteren C, Van der Woude F J, The T H. B cell activity in systemic lupus erythematosus: depressed in vivo humoral immune response to a primary antigen (haemocyanin) and increased in vitro spontaneous immunoglobulin synthesis. Clin Exp Immunol 198353371–383. [PMC free article] [PubMed] [Google Scholar]
- 11.Abu‐Shakra M, Press J, Varsano N, Levy V, Mendelson E, Sukenik S.et al Specific antibody response after influenza immunization in systemic lupus erythematosus. J Rheumatol 2002292555–2557. [PubMed] [Google Scholar]
- 12.Dengler T J, Strnad N, Buhring I, Zimmermann R, Girgsdies O, Kubler W E.et al Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation 1998661340–1347. [DOI] [PubMed] [Google Scholar]
- 13.Duchini A, Hendry R M, Nyberg L M, Viernes M E, Pockros P J. Immune response to influenza vaccine in adult liver transplant recipients. Liver Transpl 20017311–313. [DOI] [PubMed] [Google Scholar]
- 14.Mazzone P J, Mossad S B, Mawhorter S D, Mehta A C, Schilz R J, Maurer J R. The humoral immune response to influenza vaccination in lung transplant patients. Eur Respir J 200118971–976. [DOI] [PubMed] [Google Scholar]
- 15.Soesman N M, Rimmelzwaan G F, Nieuwkoop N J, Beyer W E, Tilanus H W, Kemmeren M H.et al Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol 20006185–93. [PubMed] [Google Scholar]
- 16.Versluis D J, Beyer W E, Masurel N, Wenting G J, Weimar W. Impairment of the immune response to influenza vaccination in renal transplant recipients by cyclosporine, but not azathioprine. Transplantation 198642376–379. [DOI] [PubMed] [Google Scholar]
- 17.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 18.Bombardier C, Gladman D D, Urowitz M B, Caron D, Chang C H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 199235630–640. [DOI] [PubMed] [Google Scholar]
- 19.Harmon M W. Influenza viruses. In: Lennette EH, editor. Laboratory diagnosis of viral infections, 2nd edition. New York: Marcel Dekker, 2004515–534.
- 20.de Jong J C, Ronde‐Verloop F M, Veenendaal‐van Herk T M, Weijers T F, Bijlsma K, Osterhaus A D. Antigenic heterogeneity within influenza A (H3N2) virus strains. Bull WHO 19886647–55. [PMC free article] [PubMed] [Google Scholar]
- 21.Gross P A, Hermogenes A W, Sacks H S, Lau J, Levandowski R A. The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature. Ann Intern Med 1995123518–527. [DOI] [PubMed] [Google Scholar]
- 22.de Jong J C, Palache A M, Beyer W E, Rimmelzwaan G F, Boon A C, Osterhaus A D. Haemagglutination‐inhibiting antibody to influenza virus. Dev Biol (Basel) 200311563–73. [PubMed] [Google Scholar]
- 23.Bruce I N, Mak V C, Hallett D C, Gladman D D, Urowitz M B. Factors associated with fatigue in patients with systemic lupus erythematosus. Ann Rheum Dis 199958379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tench C M, McCurdie I, White P D, D'Cruz D P. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology (Oxford) 2000391249–1254. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Gladman D D, Urowitz M B. Fatigue in lupus is not correlated with disease activity. J Rheumatol 199825892–895. [PubMed] [Google Scholar]
- 26.Nicholson K G, Wood J M, Zambon M. Influenza. Lancet 20033621733–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y P, Gregory V, Bennett M, Hay A. Recent changes among human influenza viruses. Virus Res 200410347–52. [DOI] [PubMed] [Google Scholar]
- 28.Ioannou Y, Isenberg D A. Immunisation of patients with systemic lupus erythematosus: the current state of play. Lupus 19998497–501. [DOI] [PubMed] [Google Scholar]
- 29.Elkayam O, Paran D, Caspi D, Litinsky I, Yaron M, Charboneau D.et al Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin Infect Dis 200234147–153. [DOI] [PubMed] [Google Scholar]
- 30.Bermas B L, Petri M, Goldman D, Mittleman B, Miller M W, Stocks N I.et al T helper cell dysfunction in systemic lupus erythematosus (SLE): relation to disease activity. J Clin Immunol 199414169–177. [DOI] [PubMed] [Google Scholar]
- 31.Turner‐Stokes L, Cambridge G, Corcoran T, Oxford J S, Snaith M L. In vitro response to influenza immunisation by peripheral blood mononuclear cells from patients with systemic lupus erythematosus and other autoimmune diseases. Ann Rheum Dis 198847532–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang K L, Armstrong J A, Ho M. Antibody response after influenza immunization in renal transplant patients receiving cyclosporin A or azathioprine. Infect Immun 198340421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelhard D, Nagler A, Hardan I, Morag A, Aker M, Baciu H.et al Antibody response to a two‐dose regimen of influenza vaccine in allogeneic T cell‐depleted and autologous BMT recipients. Bone Marrow Transplant 1993111–5. [PubMed] [Google Scholar]
- 34.Kroon F P, van Dissel J T, de Jong J C, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV‐seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 19948469–476. [DOI] [PubMed] [Google Scholar]
- 35.Bungener L, Idema J, ter Veer W, Huckriede A, Daemen T, Wilschut J. Virosomes in vaccine development: induction of cytotoxic T lymphocyte activity with virosome‐encapsulated protein antigens. J Liposome Res 200212155–163. [DOI] [PubMed] [Google Scholar]
- 36.Conne P, Gauthey L, Vernet P, Althaus B, Que J U, Finkel B.et al Immunogenicity of trivalent subunit versus virosome‐formulated influenza vaccines in geriatric patients. Vaccine 1997151675–1679. [DOI] [PubMed] [Google Scholar]
- 37.Gluck R, Mischler R, Finkel B, Que J U, Scarpa B, Cryz S J. Immunogenicity of new virosome influenza vaccine in elderly people. Lancet 1994344160–163. [DOI] [PubMed] [Google Scholar]
- 38.Huckriede A, Bungener L, ter Veer W, Holtrop M, Daemen T, Palache A M.et al Influenza virosomes: combining optimal presentation of hemagglutinin with immunopotentiating activity. Vaccine 200321925–931. [DOI] [PubMed] [Google Scholar]