Abstract
The localization of orexin neuropeptides in the lateral hypothalamus has focused interest on their role in ingestion. The orexigenic neurones in the lateral hypothalamus, however, project widely in the brain, and thus the physiological role of orexins is likely to be complex. Here we describe an investigation of the action of orexin A in modulating the arousal state of rats by using a combination of tissue localization and electrophysiological and behavioral techniques. We show that the brain region receiving the densest innervation from orexinergic nerves is the locus coeruleus, a key modulator of attentional state, where application of orexin A increases cell firing of intrinsic noradrenergic neurones. Orexin A increases arousal and locomotor activity and modulates neuroendocrine function. The data suggest that orexin A plays an important role in orchestrating the sleep-wake cycle.
Keywords: immunocytochemistry, hypothalamus, sleep, locomotor activity, grooming
Since the discovery of the orexins (1) investigations of their functions have been guided by evidence for their hypothalamic distribution (1, 2), focusing on feeding, energy homeostasis (1, 3), and neurocrine functions (3). Our studies now show the presence of orexin A immunoreactive fibers and varicosities in extrahypothalamic areas, particularly the locus coeruleus, and demonstrate that the functions of orexin A extend beyond the hypothalamus.
Orexin A and B are derived from a 130-aa precursor, prepro-orexin, which is encoded by a gene localized to human chromosome 17q21 (1). Prepro-orexin, or preprohypocretin (2), was identified in the rat hypothalamus by directional tag PCR subtractive hybridization (2) and has been shown by Northern blot analysis to be abundant in the brain and detectable at low levels in testes but not in a variety of other tissues (1, 2). Hypocretins had been identified as hypothalamic neuropeptides, but their biological role was not described (2). Nucleotide sequence alignment shows that hypocretins 1 and 2 have sequence in common with orexins A and B, respectively, but additional amino acids are present in both hypocretins. In situ hybridization maps confirm dense prepro-orexin mRNA expression in the hypothalamus (1, 2). Immunocytochemical mapping of orexin A has identified a population of medium-sized neurones within the hypothalamus, median eminence (3), and ventral thalamic nuclei of rat brain (1, 3). This distribution has been confirmed in human tissue (4).
Orexin A binds with high affinity to the novel G protein-coupled receptors orexin1 (OX1) (IC50 20 nM) and orexin2 (OX2) (IC50 38 nM). Calcium mobilization assays in transfected HEK293 cells confirm that orexin A is a potent agonist at both OX1 (EC50 30 nM) and OX2 (EC50 34 nM) (1). Emerging evidence suggests the existence of an extensive extrahypothalamic projection of orexin-immunoreactive neurones. Peyron et al. (5), in addition to confirming the presence of immunoreactive cell somata within the hypothalamus, reported immunolabeled fibers throughout extrahypothalamic regions, including septal nuclei, substantia nigra, and raphe nuclei, with a particularly dense innervation of the locus coeruleus. Furthermore, in situ hybridization data confirm the presence of OX1 mRNA in the locus coeruleus, hippocampal formation, dorsal raphe, and other brain areas (6) and demonstrate the presence of OX2 mRNA in the cortex, nucleus accumbens, and paraventricular nuclei of the thalamus and hypothalamus. Taken together these data suggest that orexins A and B are likely to play a broad regulatory role in the central nervous system and could regulate arousal, hormonal control and other functions (3, 5, 6). Nevertheless, the individual contributions of orexin A, orexin B, and prepro-orexin are uncertain, as the antisera used to map the extrahypothalamic fibers did not distinguish among the various neuropeptides (5).
Thus, we have characterized the distribution of orexin A immunoreactivity in rat brain tissue by using an antiserum selective for orexin A over orexin B. Studies were undertaken in vitro to characterize the effects of synthetic orexin A administration on noradrenergic cell firing in locus coeruleus slices. Experiments in vivo were conducted to identify the effects of orexin A on neuroendocrine function, monoamine and amino acid levels in the brain, the sleep-wake cycle, locomotor activity (LMA), and other behavioral models. Doses were selected on the basis of published findings (1). Orexin A immunoreactive fibers and varicosities were detected in extrahypothalamic areas, particularly the locus coeruleus, and electrophysiological studies demonstrated that orexin A modulates locus coeruleus cell firing rates in vitro. Intracerebroventricular (ICV) administration of orexin A increases arousal and LMA and induces an intense grooming response and modulates plasma levels of prolactin, growth hormone, and corticosterone but not thyroid-stimulating hormone. These data, in accord with our neuroanatomical distribution data, confirm that orexin A is involved in modulating a range of functions within the central nervous system.
MATERIALS AND METHODS
Immunocytochemistry. Rabbit polyclonal antibodies were raised against synthetic full-length orexin A peptide. Competition studies with synthetic, soluble, full-length orexin B by using an ELISA assay indicated less than 4% crossreactivity with the antiserum. A mAb was prepared against a 19-aa fragment of orexin A (RLYELLHGAGNHAAGILTL) as follows. Mice were immunized with the synthetic peptide conjugated to keyhole limpet hemocyanin. The mouse sera were analyzed by immunoassay to determine the best responder. The spleen was removed and splenocytes were isolated for fusion with X63 AG8 653 myelomas to produce hybridomas. Hybridoma supernatants were screened in immunoassay with orexin A-ovalbumin conjugate to identify anti-orexin A hybridomas. ELISA competition studies with synthetic, soluble, full-length orexin B peptide indicated <2% crossreactivity with the isolated antibody.
Localization of orexin A was carried out by using standard indirect immuno-fluorescence techniques. Male Sprague–Dawley rats (250–300 g) were deeply anesthetized with Sagital (National Veterinary Supplies, Stoke on Trent, U.K.; 60 mg/kg i.p.) and perfuse-fixed transcardially with 500 ml of 4% paraformaldehyde (Sigma) in 0.1 M sodium phosphate buffer (pH 7.4). Brains were dissected free and stored in the same fixative overnight. Frozen coronal sections (50 μm) were taken from the fore-, mid- and hind-brain regions at intervals of ca. 1 mm and collected in PBS (Sigma, pH 7.4). Sections were incubated with rabbit polyclonal antibodies raised against the 33-aa orexin A peptide, for 5 hr at room temperature (1:200 dilution in PBS containing 0.1% Triton X-100, Sigma). Other sections were incubated, under similar conditions, in a 1:50 dilution (PBS containing 0.1% Triton X-100, Sigma) of the mAb supernatant. A number of control experiments were conducted to check for specificity of the immunoreactivity seen with both types of antibodies. These included: (i) omitting the primary antiserum or monoclonal supernatant; (ii) substituting normal rabbit serum (1:200 dilution) for the primary antibody solution; (iii) preadsorbing the primary antiserum or monoclonal supernatant with excess synthetic orexin A peptide (50 μg/ml), or (iv) preadsorbing the primary antiserum or monoclonal supernatant with excess synthetic orexin B peptide (50 μg/ml). Sections were washed three times in PBS and then incubated with Texas Red-conjugated goat anti-rabbit (or anti-mouse in the case of the mAb) secondary antibody (Vector Laboratories, 30 μg/ml in PBS containing 0.1% Triton X-100). Sections were washed three times in PBS, floated onto gelatin-coated slides, mounted by using Vectashield mounting medium (Vector Laboratories), and examined under a fluorescence microscope (Leica, Deerfield, IL, DMRB, 596-nm excitation, 615-nm emission) fitted with an Ultrapix 400 charge-coupled device camera system (Astrocam, Life Science Resources, Cambridge, U.K.) by using DataCell (DataCell Ltd, Yately, Hampshire, U.K.) image capture facilities and Optimas (DataCell, Ltd) software, or a confocal microscope (Leica TCS SP). No immunoreactivity was observed in any of the control experiments in which primary antiserum or antibody was substituted with buffer, normal rabbit serum, or orexin A peptide-preadsorbed antiserum or supernatant. In contrast, preadsorption of the primary antiserum or supernatant solution with synthetic orexin B peptide had no effect on the immunostaining, indicating that the antiserum and the mAb specifically recognized orexin A over orexin B. Because the orexin A sequence is contained entirely within the prepro-orexin molecule, the antiserum and the mAb probably recognized prepro-orexin as well.
Electrophysiological Studies in Vitro. Male Hooded Lister rats (50–150 g) were used to provide coronal or horizontal brain tissue slices containing the locus coeruleus. Slices were prepared by using a vibrating blade slicer, then fixed in a submersion chamber perfused with artificial cerebrospinal fluid at 36°C. Conventional intracellular and extracellular techniques (7) were used to record from locus coeruleus neurones, which were identified by anatomical position within the slice, regular firing rate (0.5–5 Hz), an action potential duration of approximately 2 ms, and inhibition of firing by 30 μM noradrenaline. Orexin A was applied in the perfusion line.
In some experiments 4% Neurobiotin (Vector Laboratories) was included in the intracellular microelectrode and injected via depolarizing current pulses. At the end of each experiment slices were fixed in 4% paraformaldehyde, permeabilized in 0.4% Triton X-100, and treated with FITC-conjugated extravidin (Sigma) before being processed for immunofluorescent histochemistry using the orexin A antisera as described above.
In Vivo Studies.
All in vivo studies were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986 and conformed to SmithKline Beecham ethical standards.
Electroencephalogram (EEG) Sleep-Wake Analysis. To maintain consistency with the in vitro electrophysiological studies EEG sleep-wake studies were carried out by using naïve male Hooded Lister rats (Charles River Breeding Laboratories; 250–300 g at the time of surgery). Animals were anesthetized with a mixture of Domitor (medetomidine HCl, 0.4 mg/kg s.c.; Pfizer) and Sublimaze (fentanyl, 0.45 mg/kg i.p.; Janssen-Cilag). A cannula was aimed at the left or right lateral ventricle (±1.6 mm from midline, 0.8 mm caudal to bregma, −4.1 mm from skull surface according to Paxinos and Watson, ref. 8) by using standard stereotaxic procedures. Silver chloride ball EEG electrodes were implanted through bore holes in the skull over the left/right frontal cortex and the left/right occipital cortex. Silver disc electromyogram electrodes also were placed in the left/right musculature of the neck. All electrodes were prepared in house. Anesthesia was reversed with Antisedan (atipamezole HCl, 2.5 mg/kg s.c.; Pfizer), and postoperative analgesia was provided by Nubain (nalbuphine HCl, 2 mg/kg s.c.; DuPont). After recovery animals were housed in pairs in a temperature-controlled environment (20 ± 1°C) with access to food and water ad libitum. After a recovery period of 7 days, cannula placement was confirmed by an intense drinking response to angiotensin II (100 ng ICV) (9). At least 7 days elapsed before further testing.
Rats were maintained on a reverse 12-hr light-dark cycle (lights on 18:00 hr) throughout. On test days, rats first were acclimatized to the test chambers for 9 hr and then dosed on a randomized crossover basis with vehicle or orexin A (10 μg/rat ICV) immediately before the start of their normal sleep cycle (i.e., 18:00 hr). Seventy-two hours later each animal received the remaining treatment. Food and water were available ad libitum throughout the study. The dose of orexin A was selected on the basis of literature data (1) and the outcome of grooming and LMA studies (see below). EEG and electromyogram signals were recorded for the duration of the 12-hr sleep cycle and the subsequent 12-hr active period. Percentage time in each sleep stage (arousal, slow wave sleep 1 and 2, paradoxical sleep) was calculated (Sleep Stage Analysis version 3.03, SmithKline Beecham), by using the area under the curve (mean ± SEM) for specified periods post dosing, and the effects of orexin A were compared with vehicle.
Behavioral Studies. Behavioral studies were carried out by using male Sprague–Dawley rats (Charles River Breeding Laboratories; 250–300 g at the start of the experiment) equipped with ICV cannula according to the procedures outlined above. Naïve animals were used throughout. After surgery rats were housed singly, in a temperature-controlled environment (20 ± 1°C) with 12-hr light/dark cycle (lights on 07:00) and ad libitum access to food and water. Rats were acclimatized to the experimental room overnight and testing was conducted between 08:00 and 18:00. Treatment groups were balanced throughout the day. Food and water were not available during the experimental observation periods.
LMA, grooming, and body temperature experiments were conducted in clear Perspex cages (42 × 21 × 20 cm) to which the rats were habituated for 60 min before testing. LMA changes in response to orexin A (1, 3, 10, 30 μg/rat ICV) were registered automatically by using an IR beam system (AM1052 Activity Monitor, Benwick Electronics, Diss, Norfolk, U.K.), which measured beam breaks and transits (movement along the whole length of the cage) for 90 min. The grooming response to orexin A (1, 3, 10, 30 μg/rat ICV) was measured by observing the animals for 60 min, and the duration and number of grooming bouts (each bout separated by at least 5 s) recorded in 10-min time bins. Body temperature changes after orexin A (10, 30 μg/rat ICV) were determined by using an electronic thermometer (Comark, Welwyn Garden City, U.K., model 9001) coupled to a rectal probe (Comark, model BS4937K). Temperature measurements were taken 30 min before treatment, immediately before treatment, and then at 15-, 30-, 45-, and 60-min posttreatment. Temperature changes were calculated from body temperature immediately before injection.
Rats were tested in an X-maze (modified from Handley and Mithani, ref. 10) which consisted of two opposing open arms and two opposing closed arms (45 × 15 cm) made of matte black Perspex. The walls of the closed arms (11 cm high) extended in a sloping fashion 5 cm into the open arms, and the open arms were edged with a small lip (1.5 cm high). Sixty minutes after dosing (10, 30 μg/rat ICV) rats were placed on the central zone of the maze and movement was tracked for 5 min by using an automated videotracking system (Videotrack, CPL Systems, Cambridge, U.K.). The doses were selected on the basis of dose ranging studies carried out using grooming and LMA. Percentage time in open arms ([time in open arms/total time on both arms] × 100), the % number of entries into the open arms ([number of entries into open arms/total number of entries onto both arms] × 100) and the total distance traveled (m) were recorded.
Startle reactivity was measured in startle chambers (clear Perspex 18 × 9 × 14 cm) equipped with a spring-mounted metal grid floor, an overhead light, and a loudspeaker for delivery of the acoustic stimulus (Instrument Design Technologies, SmithKline Beecham). A floor-mounted accelerometer measured startle, and each startle chamber was contained within a sound-attenuated box. Thirty minutes after dosing (1, 3, 10 μg/rat ICV) animals were placed in test chambers, and after a 5-min acclimatization period (background noise level 70 dB), they were exposed to a series of 10 50-ms bursts of white noise (100 dB) at 60-s intervals.
Neurochemistry and Neuroendocrine Markers.
Studies were conducted by using male Sprague–Dawley rats (400–500 g), which previously had been tested in a behavioral study and allowed a 14-day recovery period. Animals were injected with orexin A (0.3, 1, 3, 10, 30 μg/rat ICV), and, 40 min later, were killed by rapid decapitation. The doses were based on the results obtained in the behavioral studies, and the sampling times were selected to coincide with peak grooming and LMA effects. Trunk blood was collected into prechilled EDTA tubes and centrifuged at 3,000 rpm for 15 min to harvest plasma, which then was stored at −70°C for RIA of prolactin and growth hormone (Amersham Pharmacia) or corticosterone (Immunodiagnostic Systems Limited, Newcastle-Upon-Tyne, U.K.). For analysis of dopamine and 5-hydroxytryptamine turnover brains were removed and the following brain regions were dissected out on ice (medial prefrontal cortex, hypothalamus, nucleus accumbens, striatum, hippocampus, and either the occipital cortex or the cerebellum). These were frozen on dry ice and stored at −70°C. Samples were weighed, homogenized in 0.4 M perchloric acid, and centrifuged at 3,000 g, and the supernatant was analyzed for dopamine, 5-hydroxytryptamine, and their metabolites by HPLC with electrochemical detection. For amino acid analysis, the supernatant was derivatized and analyzed by HPLC with fluorescence detection (11).
Preparation of Orexin A.
For all studies, orexin A or vehicle were injected ICV (2.5–5 μl in 60 s), and the injection needle was left in place for an additional 90 s to allow diffusion. Orexin A was obtained from California Peptide Research (Napa, CA) and was dissolved in sterile water. The pH for the orexin A solution was ca. 6.5, and vehicle solutions were adjusted to the same pH with 500 nM NaOH. Individual aliquots were prepared for each experiment and were frozen (−20°C) and thawed immediately before use.
Data Analysis.
LMA (log10 transformation to correct for heterogeneity of variance), body temperature, X-maze, startle reactivity, and EEG sleep-wake studies were analyzed by one-way ANOVA followed by Dunnett’s t test (SAS-RA, SAS Institute, Cary, NC) where appropriate. Grooming data were analyzed by using two-way ANOVA with day and treatment as the independent variables. Neurochemical and plasma hormone data were analyzed by using one-way ANOVA followed by least-significance difference t test (Statistica, Statsoft, Tulsa, OK), with the exception of the prolactin study that was analyzed nonparametrically by using Kruskall-Wallis one-way ANOVA by ranks. In all cases P < 0.05 was taken as the level of significance.
RESULTS
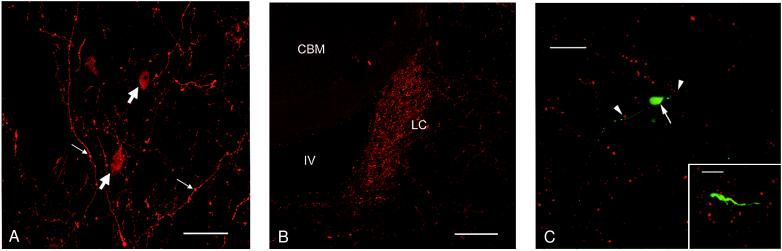
The polyclonal antiserum revealed orexin A-like immunoreactivity in cells distributed within the hypothalamus, with the highest densities of labeled cells in perifornical and lateral hypothalamic nuclei (Fig. 1A). Intensely labeled varicose fibers were scattered throughout the hypothalamus (with particularly dense labeling in the lateral hypothalamic area and the perifornical nucleus), in the dorsal raphe nucleus, and throughout the periaqueductal gray matter (central gray). There were abundant labeled fibers in the septal area and in several subnuclei of the thalamus (paraventricular and centromedial nuclei in particular). Many labeled varicose fibers were found in lower densities throughout other cortical and subcortical regions, suggesting that these hypothalamic cells projected very widely throughout the brain. Brain regions exhibiting sparser orexin A immunoreactive fiber labeling included the olfactory bulb, corpus striatum, hippocampus, and amygdala.
Figure 1.
Immunofluorescence images of orexin A immunoreactive profiles in the rat brain. All of these micrographs were generated by using the polyclonal antiserum against orexin A. (A) Coronal section through rat lateral hypothalamus showing orexin A immunofluorescence labeling in cell bodies (block arrows) and varicose axonal fibers (small arrows). (Bar = 50 μm.) (B) Coronal section through rat locus coeruleus (LC) showing dense plexus of immunolabeled axon fibers and terminals. CBM, cerebellum; IV, fourth ventricle. (Bar = 200 μm.) (C) An orexin A-responsive cell in a locus coeruleus brain slice, filled with Neurobiotin via the recording electrode. The red signal illustrates orexin A immunoreactivity, and the green signal indicates intracellular Neurobiotin. The large arrow indicates a neuronal soma bearing two main dendrites. The dendrites extend throughout the field of dense orexin A-positive varicosities. The arrowheads indicate varicosities that are closely apposed to a dendritic structure. The image is a brightest point projection of 64 confocal optical sections taken at 2-μm intervals. (Bar = 35 μm.) (Inset) Higher-power micrograph of a similarly treated section showing detail of proximity of orexin A-immunoreactive fiber varicosities to a process of an intrinsic locus coeruleus neurone. (Bar = 10 μm.)
The most prominent fiber labeling, consisting of a very dense plexus of immunolabeled varicose fibers, was evident in the locus coeruleus of the rhombencephalon (Fig. 1B). Exactly the same pattern of orexin A-like immunoreactivity was revealed by a further investigation using the mAb raised against a synthetic peptide fragment of the orexin A molecule (data not shown).
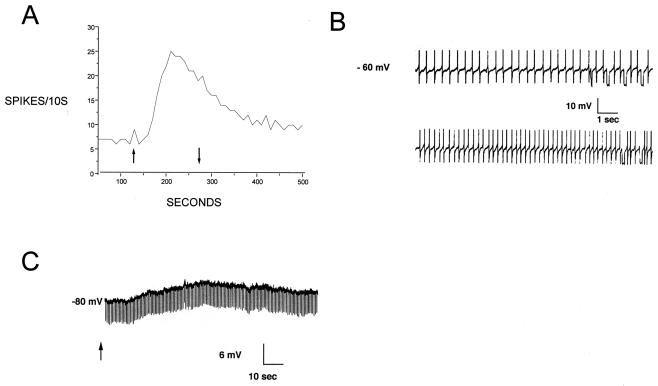
The intense fiber labeling seen in the locus coeruleus suggested strongly that orexin A might play an important functional role in modulating intrinsic neurones of this nucleus. Extracellular recording of the spontaneous action potential activity of locus coeruleus neurones from the rat revealed an increase of firing rate in response to application of orexin A over the range 30 nM to 3 μM. At 1 μM, the firing rate increased by 2.87 ± 0.5 (n = 6)-fold over the baseline rate. The response threshold was between 10 and 30 nM. The maximum increase in firing rate occurred at 1 μM. The response reached peak amplitude within 2 min and only slowly declined during a continuous 3-min perfusion of orexin A. On washout of orexin A, firing rate returned to near baseline levels within 10 min, and the rate of decline was similar to the desensitization rate seen during orexin A administration. Similar amplitude responses could be obtained after an additional 10 min of wash (Fig. 2A).
Figure 2.
Orexin A excitation in the locus coeruleus. (A) Extracellular recording of a single unit. Vertical axis is the number of suprathreshold spikes per 10-s period. Orexin A (1 μM) was applied between the arrows. (B) Intracellular recording of identified locus coeruleus neurone. (Upper) Baseline firing at the resting potential in normal artificial cerebrospinal fluid. (Lower) Increased action potential frequency at the peak of the response to 1 μM orexin A. (C) Intracellular recording of a locus coeruleus cell hyperpolarized to −80 mV by steady current injection. In the absence of action potentials, a clear depolarization is observed as a response to 1 μM orexin A (applied at arrow). Downward deflections in the trace are responses to 200-ms current pulses to monitor cell resistance. Note the absence of a clear change of resistance at the peak of the orexin A response.
Intracellular recordings revealed a depolarization in response to 1 μM orexin A application of 6.50 ± 0.8 mV (n = 5) at or near the resting membrane potential (−59 to − 66 mV). This depolarization was accompanied by an increase in firing of action potentials (Fig. 2B). Hyperpolarizing current injections were applied during the response to monitor membrane resistance but did not reveal a clear conductance change (Fig. 2C). This finding could be explained if the depolarizing response to orexin A is complex and mediated by a simultaneous decrease in a hyperpolarizing conductance and increase in a depolarizing conductance.
When filled with Neurobiotin, neurones that responded to orexin A were shown to have the characteristic morphology of the principal neurones of the locus coeruleus (12). Double labeling of these cells with orexin A immunoreactivity revealed that extensive portions of the distal dendrites of the cells extended into the regions of orexin A immunoreactivity (Fig. 1C). These dendrites were seen to occur in very close apposition to immunolabeled fibers bearing large (up to 2.5 μm in diameter) varicosities. The smallest distance measured between an immunolabeled varicosity and a Neurobiotin-labeled cell process was 3.7 μm.
The in vitro data suggest a potential role for modulation of arousal states by orexin A through the locus coeruleus noradrenergic pathway. So, we next conducted in vivo studies to test this hypothesis, examining the effect of orexin A on the sleep-wake cycle. Orexin A, (10 μg/rat ICV) administered at the onset of the normal sleep period, produced a significant increase (F = 4.49; df = 1,16; P < 0.05) in the proportion (area under the curve) of arousal during the second and third hour after dosing (Table 1). During the same period there was a significant decrease (F = 4.82; df = 1, 16; P < 0.05) in the proportion of paradoxical sleep, no change in light slow wave sleep (SWS 1; F = 0.003; df = 1,16; P > 0.05) and a nonsignificant (F = 1.28; df = 1,16; P > 0.05) decrease in deep slow wave sleep (SWS 2). The vehicle baseline data obtained compared favorably with data from previous experiments. Changes in sleep stage distribution normalized within 4 hr postdose, and no further disruption was apparent during the remainder of the sleep period or the subsequent awake period (i.e., 12–24 hr postdose).
Table 1.
Mean (± SEM) percentage of total time in each sleep stage determined during the second and third hour postdose using area under the curve calculations
| Sleep stage | Control | 10.0 (μg/rat ICV), n = 9 |
|---|---|---|
| Arousal | 13.4 ± 4.9% | 34.5 ± 8.7%* |
| Paradoxical sleep | 5.9 ± 1.8% | 1.9 ± 0.9%† |
| SWS1 | 13.1 ± 1.8% | 13.0 ± 2.9%; NS |
| SWS2 | 67.6 ± 5.9% | 50.7 ± 8.2%; NS |
NS, nonsignificant. SWS, slow wave sleep.
Increased relative to vehicle, P < 0.05.
Decreased relative to vehicle, P < 0.05.
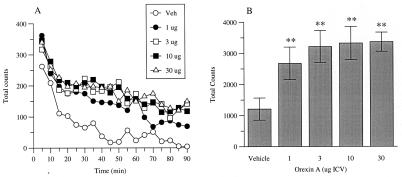
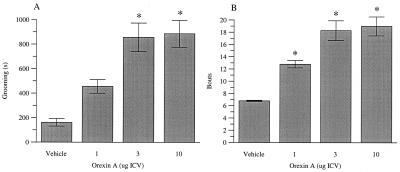
Orexin A caused a significant increase in LMA (F = 7.07; df = 4,32; P < 0.001) at all doses (1, 3, 10 and 30 μg/rat ICV, P < 0.05) and throughout the 90-min observation period (Fig. 3). Orexin A dose-dependently induced an increase in time spent grooming (F = 19.75; df = 3,12; P < 0.001), which reached significance at 3 and 10 μg/rat ICV, and an increase in grooming bouts (F = 13.66: df = 3,12; P < 0.001), which was significant at 1, 3, and 10 μg/rat (Fig. 4). No significant effect on body temperature (10, 30 μg/rat ICV) was observed (F = 1.61; df = 2,15; P = >0.05) (data not shown). In addition, orexin A did not significantly affect any of the behavioral parameters measured in the X-maze (10, 30 μg/rat ICV) (F < 1) or alter startle reactivity (1, 3, 10, 30 μg/rat ICV) (F < 1) (data not shown).
Figure 3.
Effects of orexin A on LMA. Data are shown as (A) mean total beam breaks during 5-min time bins for a 90-min recording period and (B) mean (±SEM) cumulative beam breaks for total period. Significant differences from vehicle-treated (Veh) rats are shown by **P < 0.01 (n = 7–8 per treatment group).
Figure 4.
The effects of orexin A on (A) grooming duration (s) and (B) number of grooming bouts recorded for a 60-min period immediately after peptide administration. Significant differences from vehicle-treated (Veh) rats are shown by *P < 0.05 (n = 6 per treatment group).
ICV administration of orexin A (0.3, 1, 3, 10, 30 μg/rat ICV) caused a dose-dependent decrease in plasma growth hormone levels (F = 4.68., df = 5,30, P < 0.05), which was significant at 1, 3, 10, and 30 μg (P < 0.05). A marked and highly significant (Kruskall-Wallis H = 23.7, P < 0.005) decrease in plasma prolactin levels also was observed. Prolactin levels were below the limit of detection (0.1 ng/ml) in the RIA assay after 1, 3, 10, and 30 μg ICV of orexin A. In contrast, orexin A evoked a dose-dependent increase in plasma corticosterone levels (F = 5.39, df = 5,33, P < 0.05) that reached significance at 10 and 30 μg (P < 0.05). No significant effect on thyroid-stimulating hormone levels was detected (F < 1; see Table 2).
Table 2.
Effects of orexin A on neuroendocrine markers
| Markers | Dose of orexin A (μg/rat ICV)
|
|||||
|---|---|---|---|---|---|---|
| Control | 0.3 | 1.0 | 3.0 | 10.0 | 30.0 | |
| Prolactin, ng/ml | 4.84 ± 1.4 | 1.08 ± 0.86* | ND < 0.1* | ND < 0.1* | ND < 0.1* | ND < 0.1* |
| Growth hormone, ng/ml | 29.8 ± 12.5 | 37.9 ± 8.98 | 9.6 ± 2.9* | 3.8 ± 0.95* | 3.5 ± 1.09* | 1.7 ± 0.29* |
| Corticosterone, ng/ml | 44.0 ± 8.99 | 38.8 ± 5.7 | 74.6 ± 13.02 | 60.3 ± 14.7 | 104.2 ± 24.8* | 140.5 ± 21.1* |
| Thyroid-stimulating hormone, ng/ml | 6.8 ± 1.74 | 7.9 ± 0.95 | 6.6 ± 0.71 | 6.1 ± 1.30 | 7.6 ± 1.32 | NA |
NA, experiment not done; ND, below limit of detection. ∗, P < 0.05 c.f. vehicle.
A significant increase in dopamine turnover (dihydroxyphenyl acetic acid + homovanillic acid/dopamine) in the medial prefrontal cortex was observed after 30 μg/rat ICV of orexin A (F = 4.09, df = 2, 15, P < 0.05); however, no effect was observed at lower doses (0.3, 1, 3, 10 μg/rat ICV) in any other brain area. In addition, orexin A had no significant effect on 5-hydroxytryptamine (5-HT) (5-hydroxyindole acetic acid/5-HT) turnover in any brain region studied. Orexin A had no significant effects on regional amino acid levels, as measured by HPLC, in any brain area (data not shown).
DISCUSSION
The immunocytochemical experiments reported here confirm and extend the previous distribution studies reported by Sakurai et al. (1) and Peyron et al. (5). Our results showed that immunoreactivity to orexin A, using an antibody that did not recognize orexin B, occurred throughout the hypothalamus. In addition, we found intensely labeled fibers in many extrahypothalamic brain regions, with highest densities of fibers and varicosities in the dorsal raphe nucleus, the central gray matter, the trigeminal mesencephalic nucleus, and especially the locus coeruleus. The distribution of fiber labeling is essentially in agreement with that reported by Sakurai et al. (1), who used a polyclonal antiserum raised against a fragment of the orexin A molecule, and Peyron et al. (5), whose antiserum was directed against fragments of the prepro-orexin molecule. In addition, the distribution of immunolabeled fibers and varicosities found in the present study is consistent with the OX1 mRNA distribution reported by in situ hybridization studies (6). The results of the present study suggest that either the study by Sakurai et al. (1) used an antiserum that predominantly recognized orexin A (rather than orexin B) or alternatively that the distribution of orexin B cells and fibers in the rat brain is contained within the same anatomical regions exhibiting orexin A immunoreactivity. Further studies with reagents that recognize only orexin B must be done to resolve this issue.
The presence of a dense network of orexin A immunoreactive processes in the locus coeruleus suggests that this peptide might play a role in regulating neuronal activity in this structure. This proposal is supported by the observation that orexin A markedly increased the basal firing rate of locus coeruleus neurones in vitro. Orexin A-induced enhancement of locus coeruleus neuronal firing highlights a potential involvement of the peptide in maintaining the waking state, because cells in this region fire maximally during arousal but are virtually quiescent during rapid eye movement sleep (equivalent to paradoxical sleep) (12, 13). We have confirmed this hypothesis by sleep studies in conscious animals, which show that orexin A increases the proportion of time spent awake, mainly at the expense of a reduction in paradoxical sleep, when given at the onset of the normal sleep period.
Evidence from both electrophysiological studies in vitro and EEG sleep-wake studies suggest that orexin A acts to increase arousal mechanisms and the observed increases in LMA in response to orexin A are consistent with this hypothesis. Enhanced LMA also may be linked to increased dopamine turnover, although this mechanism appears less likely as changes in dopamine turnover were observed only at the 30 μg/rat ICV dose, whereas LMA changes were evident even after 1 μg/rat ICV.
In addition to its effects on arousal and LMA in conscious rats, orexin A potently inhibits plasma levels of prolactin and growth hormone and stimulates corticosterone levels. Inhibition of prolactin secretion is most sensitive to the effects of orexin A (significant effect at 0.3 μg ICV). Neuroendocrine effects are consistent with the hypothalamic distribution of orexin A immunoreactive cell bodies and fibers, described in this and other studies (2, 3, 5) and suggest that mediation of the responses may occur in the hypothalamus or the pituitary gland. In support of this hypothesis, Risold et al. (14) have demonstrated that orexin- and prolactin-like immunoreactivity are colocalized in the lateral hypothalamus. Furthermore, orexin A has been shown to have excitatory effects on hypothalamic cells in vitro (2), and van den Pol et al. (3) have shown that orexin A modulated the input controlling neuroendocrine neurones in the arcuate nucleus in hypothalamic slices in vitro. Finally, Pu et al. (15) have suggested a hypothalamic site of action of orexin A on luteinizing hormone-releasing hormone. Thus, several lines of evidence suggest that orexin A may modulate neuroendocrine release at the level of the hypothalamus. As levels of growth hormone are normally at their lowest during the awake state and increase at the onset of slow wave sleep (16, 17), our data suggest that orexin A, acting in multiple different brain regions, may play an integrative role in the sleep-wake cycle.
Intense grooming was observed in response to orexin A administration. This is a characteristic behavioral response to some hypothalamic neuropeptides, including corticotropin-releasing factor (CRF) and corticotropin (18, 19). Grooming has been linked to elevated stress levels (19), a suggestion consistent with the observations that orexin A increases plasma corticosterone levels. However, orexin A lacked any effect on behavior in the X-maze or on startle reactivity, both of which are known to be sensitive to other modulators of hypothalamic pituitary adrenal axis activity, including CRF (18, 20), and to anxiolytic and anxiogenic drugs. This profile of neuroendocrine and behavioral effects clearly distinguishes the effects of orexin A from those of CRF.
In summary, we have shown that orexin A increases locus coeruleus neuronal firing in vitro and increases levels of arousal and LMA in rats after ICV administration of the peptide in the dose range shown previously to be orexigenic (1). Orexin A also regulates prolactin, growth hormone, and corticosterone levels, possibly through a direct effect on hypothalamic neurones. Taken together, these observations suggest that orexin A plays an important role in orchestrating the electrophysiological, behavioral, and endocrine aspects of the sleep-wake cycle. Both OX1 and OX2 are present within the locus coeruleus, hypothalamus, and other brain areas likely to be involved in mediating these responses. However, the selectivity of orexin A for OX1 and OX2 is poor, and therefore additional studies will be required to determine the specific roles of each member of the orexin receptor family.
Acknowledgments
We are very grateful for the surgical and technical assistance provided by Karen Davies, Heather Lloyd, Susan Barber, Jackie Colledge, and Alan White.
ABBREVIATIONS
- LMA
locomotor activity
- ICV
intracerebroventricular
- OX1
orexin1 receptor
- OX2
orexin2 receptor
- EEG
electroencephalogram
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemilli R M, Tanaka H, Clay Williams S, Richardson J A, Kozlowski G P, Wilson S, et al. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 2.De Lecea L, Kilduff T S, Peyron C, Gao X-B, Foye P E, Danielson P E, Fukuhara C, Battenberg E L F, Gautvik V T, Bartlett II F S, et al. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Pol A N, Gao X B, Obrietan K, Kilduff T S, Belousov A B. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias C F, Saper C B, Maratos-Flier E, Tritos N A, Lee C, Kelly J, Tatro J B, Hoffman G E, Ollman M M, Barsh G S, et al. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 5.Peyron C, Tighe D K, van den Pol A N, de Lecea L, Heller H C, Sutcliffe J G, Kilduff T S. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivedi P, Yu H, MacNeil D J, Van der Ploeg L H T, Guan X M. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 7.Ishimatsu M, Williams J T. J Neurosci. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Ed. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 9.Simpson J B, Epstein A N, Camardo J S., Jr J Comp Phys Psychol. 1978;92:581–604. doi: 10.1037/h0077503. [DOI] [PubMed] [Google Scholar]
- 10.Handley S L, Mithani S. Naunyn-Schmiederberg’s Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- 11.Shah, A., de Biasi, V., Taylor, S. G., Roberts, C., Hemmati, P., Munton, R., West, A., Routledge, C. & Camilleri, P. (1999) J. Chromatogr. B, in press. [DOI] [PubMed]
- 12.Aston-Jones G, Bloom F E. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge C W, Foote S L. J Neurosci. 1996;16:6999–7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risold P Y, Griffond B, Kilduff T S, Sutcliffe J G, Fellmann D. Neurosci Lett. 1999;259:153–156. doi: 10.1016/s0304-3940(98)00906-9. [DOI] [PubMed] [Google Scholar]
- 15.Pu S, Jain M R, Kalra P S, Kalra S P. Regul Pept. 1998;78:133–136. doi: 10.1016/s0167-0115(98)00128-1. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Kipnis D M, Daughaday W H. J Clin Invest. 1968;47:2079–2090. doi: 10.1172/JCI105893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendelson W B, Jacobs L S, Gillin J C, Wyall R J. Psychoneuroendocrinology. 1979;4:341–349. doi: 10.1016/0306-4530(79)90017-9. [DOI] [PubMed] [Google Scholar]
- 18.Jones D N C, Kortekaas R, Slade P D, Middlemiss D N, Hagan J J. Psychopharmacology. 1998;138:124–132. doi: 10.1007/s002130050654. [DOI] [PubMed] [Google Scholar]
- 19.Dunn A J, Guild A L, Kramarcy N R, Ware M D. Pharmacol Biochem Behav. 1981;15:605–608. doi: 10.1016/0091-3057(81)90217-3. [DOI] [PubMed] [Google Scholar]
- 20.Moreau J L, Kilpatrick G, Jenck F. NeuroReport. 1997;8:1697–1701. doi: 10.1097/00001756-199705060-00027. [DOI] [PubMed] [Google Scholar]