Abstract
Objective
To describe the occurrence of baseline comorbidity in subjects with active rheumatoid arthritis starting treatment with biological agents. Such data are necessary to interpret the reported occurrence of adverse events following treatment.
Methods
Baseline comorbidity was recorded in a large national cohort of patients with rheumatoid arthritis newly starting biological agents. The distribution of the number and types of comorbidities is presented.
Results
In all, 7818 patients treated with biological agents (infliximab 3332, etanercept 3302, adalimumab 1059, anakinra 132) were included in the analysis. Comorbidity was common, with 58% of patients having at least one comorbid condition and 25% having more than one. The most frequent comorbid conditions were hypertension, depression, peptic ulcer disease, and respiratory disease.
Conclusions
In routine use, patients treated with biological agents have high levels of baseline comorbidity, which should influence the interpretation of reported adverse events.
Keywords: biological agent, DMARD, comorbidity, rheumatoid arthritis
Rheumatoid arthritis is a chronic inflammatory disease affecting the synovial joints and other tissues. It is also considered a systemic disease and as such is associated with an increase in mortality, predominantly from non‐arthritis‐related causes.1,2,3 Although there have been few studies, patients with rheumatoid arthritis also report a high level of associated comorbidity. Thus Berkanovic et al found that 54% of 288 randomly selected patients reported at least one additional chronic disease.4 Gabriel et al reported that almost 60% of a rheumatoid cohort (n = 450) had at least one other medical condition, compared with only 49% of age and sex matched non‐rheumatoid controls.5 The majority of these conditions were represented by cardiovascular disease, malignancy, peptic ulcer disease, and chronic lung disease. These increases in mortality and morbidity may be related to the rheumatoid arthritis itself, to shared risk factors between rheumatoid arthritis and the associated comorbidities, or to the adverse effects of antirheumatic treatments.
The introduction of biological agents into the treatment armamentarium has revolutionised the modern management of rheumatoid arthritis. There is, however, a theoretical concern that this particular group of drugs may increase the risk of various morbidities. Reassuringly, though, there was no increase in serious adverse events in patients receiving biological treatments in the so called “pivotal” randomised controlled trials (RCTs) compared with placebo. However, it is possible that, owing to strict entry criteria, such RCTs selectively excluded patients with significant comorbidity risk. It is therefore difficult to use these data to draw any firm conclusions about the safety of biological treatments in patients who receive the drugs during routine practice.
There remain limited data on the safety of these agents in routine use. There have been reports of a possible increase of certain serious adverse events, including serious infections,6,7,8 tuberculosis,9,10 demyelination,11 congestive heart failure,12 and malignancies.13 Most of these data have been in the form of spontaneous adverse event reports. Before events can be attributed to these new treatments, however, it is important to ascertain the level of coexisting baseline disease among a treated cohort. Patients who are selected for biological treatment are likely to represent those with the most severe disease, who may already have a high level of comorbidity. This leads to the specific question: What is the level of comorbidity among patients starting biological therapy during routine clinical use?
To address this question, we set out to identify the prevalence and types of comorbidity in a large national register of rheumatoid arthritis patients receiving biological drugs during routine practice in the United Kingdom.
Methods
Study design
As described elsewhere,14 the British Society for Rheumatology Biologics Register (BSRBR) is a national prospective observational study, established in October 2001, to monitor carefully the long term effects of biological treatments. Data are gathered in a standardised format on all patients aged 16 years or older starting biological drugs for a rheumatic disease throughout the UK. Unlike an RCT, the decision to start or change disease modifying antirheumatic drugs (DMARDs) and biological treatments was solely at the discretion of the treating rheumatologist, although national guidelines currently limit the use of the latter to those patients with active rheumatoid arthritis (defined as a 28 joint count disease activity score (DAS28) of >5.1 despite previous treatment with at least two DMARDs, one of which should have been methotrexate15). This current analysis is limited to patients who fulfilled the 1987 ACR classification criteria for rheumatoid arthritis16 and were registered with the BSRBR before 1 October 2004.
Baseline information
Baseline information on all patients was obtained from the treating rheumatologist through standard questionnaires. Information included demographic variables, disease characteristics, and past and present antirheumatic treatment. In addition, all the necessary clinical and laboratory data to calculate DAS2817 were collected at the time the patient was starting their new treatment. Patients were also asked to complete the health assessment questionnaire (HAQ)18 and the 36 item short form health survey (SF‐36)19.
Identification of comorbidity
Comorbidity was assessed at the time of starting treatment, using a prespecified list of coexisting conditions on the baseline questionnaire (table 1). These data were obtained from the patient's medical records. In addition, physicians were asked to supply a complete list of the patient's current drugs. This list was used to identify any further comorbid conditions—for example, a prescription of thyroxine was considered to represent underlying hypothyroidism. It was also used to confirm other diagnoses. Thus patients were only considered to have a diagnosis of hypertension if they were receiving antihypertensive drug treatment. Comorbidities were recorded as present (ever) or absent. Further details on individual comorbid disease severity were not collected. For each patient, the presence of any comorbidity and the number of comorbidities was determined.
Table 1 Baseline comorbidities recorded on register questionnaire.
| Hypertension |
| Ischaemic heart disease (angina pectoris and/or myocardial infarction) |
| Asthma |
| Chronic obstructive pulmonary disease |
| Pulmonary fibrosis |
| Renal disease |
| Liver disease |
| Peptic ulcer disease |
| Thyroid disease |
| Depression |
| Cerebrovascular accident |
| Demyelinating disease |
| Epilepsy |
| Diabetes |
| Tuberculosis |
| Malignancy |
Ethics approval
Ethics approval for this study was obtained from the UK NHS Central Office for Research ethics committee.
Results
Biological cohort
In all, 7818 persons with rheumatoid arthritis who had started a biological drug had been registered with the BSRBR to October 1, 2004. The majority of the patients were receiving either etanercept (n = 3302) or infliximab (n = 3332). An additional 1059 subjects were receiving adalimumab and 132 were receiving anakinra. Baseline characteristics are presented in table 2. Sixty nine per cent were receiving a concurrent DMARD (table 3), 49% were receiving corticosteroids, and 65% were receiving non‐steroidal anti‐inflammatory drugs (NSAIDs).
Table 2 Baseline characteristics.
| Number of cases | 7818 |
| Age (years) | 56 (12) |
| Female | 6012 (77%) |
| Current smokers | 1676 (21%) |
| Ever smoked | 4602 (59%) |
| Disease duration (years) | 14 (9) |
| Steroids | 3793 (49%) |
| DAS28 | 6.6 (1.0) |
| HAQ | 2.1 (0.6) |
| SF‐36 mental component scale | 42 (8) |
| SF‐36 physical component scale | 26 (6) |
Values are mean (SD) or n (%).
DAS28, 28 joint disease activity score; HAQ, health assessment questionnaire score; SF‐36, 36 item short form health survey score.
Table 3 Details of non‐biological disease modifying antirheumatic drug (DMARD) treatment.
| Current DMARD | 5378 (69) |
| Current combination DMARD treatment | 1238 (16) |
| Methotrexate | 4316 (55) |
| Sulfsalazine | 926 (12) |
| Leflunomide | 564 (7) |
| Hydroxychloroquine | 530 (7) |
| Azathioprine | 192 (3) |
| Ciclosporine | 146 (2) |
| Gold | 119 (2) |
| Penicillamine | 57 (1) |
| Other | 17 |
| No of previous DMARDs* | 4 (3 to 5) |
Values are n (%) unless specified otherwise.
*Median (interquartile range).
Comorbidity
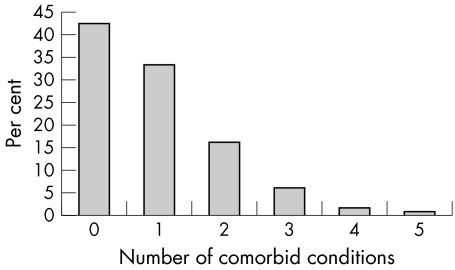
In all, 58% of patients starting a biological agent reported at least one comorbid condition, and 25% reported more than one (fig 1). The most common comorbid conditions identified (table 4) was cardiovascular disease, including hypertension (22%) and ischaemic heart disease (6%). Five per cent of patients were reported to be diabetic. There was also a high prevalence of pulmonary disease including asthma (10%) and chronic obstructive pulmonary disease (5%). Of specific interest, 152 patients (2%) were reported to have a past exposure or infection with Mycobacterium tuberculosis. One fifth of the cohort were reported to have depression. Previous malignancy was uncommon (n = 152, 3%) the most frequent being basal cell carcinomas (n = 73), breast cancer (n = 47), and malignancy of the female reproductive tract (n = 35). Two patients were reported to have had a history of lymphoproliferative disease.
Figure 1 Numbers of comorbid conditions.
Table 4 Details of comorbid disease in patients treated with biological agents*.
| Comorbidity | n (%) |
|---|---|
| Any comorbidity | 4505 (58) |
| Hypertension | 1710 (22) |
| Ischaemic heart disease† | 470 (6) |
| Cerebrovascular accident | 150 (2) |
| Asthma | 736 (10) |
| COPD | 365 (5) |
| Pulmonary fibrosis | 249 (3) |
| Past tuberculosis | 152 (2) |
| Diabetes | 414 (5) |
| Diabetes receiving oral therapy | 164 (2) |
| Diabetes receiving insulin | 119 (2) |
| Hypothyroidism | 573 (7) |
| Depression | 1491 (19) |
| Peptic ulcer disease | 671 (9) |
| Liver disease | 174 (2) |
| Renal disease | 218 (3) |
| Demyelination | 14 (0.2) |
| Epilepsy | 91 (1) |
| Past malignancy | 231 (3) |
*Adjusted for age, sex, disease duration, and smoking history.
†Includes either angina pectoris or myocardial infarction.
COPD, chronic obstructive pulmonary disease.
Discussion
The burden of comorbid diseases was high among this large national cohort of patients starting biological treatments, with 58% having at least one comorbid condition. The most frequent comorbid conditions were cardiovascular and respiratory disease and depression. Interpretation of the occurrence of morbidity diagnosed following the start of biological therapy needs to take into account this baseline burden.
The ascertainment of the comorbidities was based on the patient clinical interview, documented comorbidity in the rheumatological case records and the nature of current treatment. Although this approach to gathering comorbidity data in large scale studies is widespread, there has to be a level of uncertainty in the accuracy of these in terms of both false positives and false negatives. The interpretation of the different comorbidities needs to take this into account. Thus the data on current treated hypertension and diabetes are likely to be substantially more accurate than, for example, the self or physician reported previous diagnosis of peptic ulcer. However, the cohort was a nationally recruited one and thus there were unlikely to be biases caused by the selection of specific groups of patients or rheumatological centres.
One interesting issue is whether the comorbidity in this cohort represents selection factors in those eligible for treatment or whether it is an indication of the comorbidity levels in a severe rheumatoid arthritis patient population. Indeed in order to interpret data on the incidence of new adverse events in the anti‐TNF treated group, the BSRBR is also concurrently recruiting, as a comparison group from over 20 major rheumatological centres, a nationwide cohort of rheumatoid arthritis patients with active disease who have started on a new non‐biological DMARD treatment within the previous six months. It was also possible, therefore, to use that dataset to address whether there are major differences between anti‐TNF and standard DMARD treated patients in their comorbidity. Baseline data are collected in an identical fashion on these patients. By 1 October 2004, 969 patients had been recruited to this cohort. Although they have a shorter mean disease duration (9 years v 14 years), they were similar in age (mean 59 years) and sex (70% female). The level of baseline comorbidity in this cohort was, however, similar to that in our biological cohort (62%). Again, the most common conditions were hypertension (25%), asthma (15%), and depression (18%). There was not a higher rate of previous tuberculosis (2%) although there was a slightly higher rate of previous malignancy (5%), which would not be unexpected given the contraindications for anti‐TNF drugs. Thus these data would suggest that the high baseline comorbidity in the anti‐TNF treated cohort reflects that found in a population of rheumatoid patients under current active treatment
The strengths of the BSRBR lie in its size and inclusive nature. In the United Kingdom, mandatory registration of all patients (with consent) with the BSRBR forms part of the national guidelines for the use of these agents in rheumatoid arthritis. It is therefore likely that the patients in this study are a true reflection of all patients with rheumatoid arthritis receiving biological treatment in the United Kingdom. The prevalence of comorbidity does not appear to be higher than that reported before the widespread use of anti‐TNF agents.4,5,20 Although it is difficult to compare directly across studies, because of the lack of standardisation in comorbidity assessment between studies and the differences between the cohorts in terms of disease duration and severity, the results suggest that physicians are not avoiding these drugs in patients with comorbid disease.
The findings of this study confirm that the baseline rate of coexistent conditions among patients starting biological treatments is high. As a consequence, reports of, for example, new episode of comorbidity such as an exacerbation of congestive heart failure following the use of anti‐TNFα treatment must take into account the high baseline rate of cardiovascular disease in this population. Similarly, a high rate of pulmonary disease, including chronic obstructive pulmonary disease and asthma before the start of biological therapy may already place these patients at an increased risk of respiratory infection, regardless of the use of a biological agent. The occurrence of adverse events in patients with rheumatoid arthritis receiving biological agents must be interpreted in the light of these findings of a high background level of comorbidity
Acknowledgements
The British Society for Rheumatology (BSR) receives financial support from the following companies marketing biological agents in the UK: Schering Plough, Wyeth Laboratories, Abbott Laboratories, and Amgen. These resources are used by the BSR to provide a research grant to the University of Manchester to fund the BSRBR. KH is an Arthritis Society of Canada Research Fellow.
We acknowledge the enthusiastic collaboration of all consultant rheumatologists and their specialist nurses in the UK in providing the data used in this report. The substantial contribution of Andy Tracey, Katie McGrother, and Mark Lunt in database design and manipulation is acknowledged. We also acknowledge the support from Dr Ian Griffiths, Chairman of the BSRBR Management Committee, Professors Gabriel Panayi, David GI Scott, and David Isenberg, Presidents of the BSR during the period of data collection, for their active role in enabling the register to undertake its tasks, and Samantha Peters and Mervyn Hogg of the BSR.
Abbreviations
BSRBR - British Society for Rheumatology Biologics Register
DAS28 - 28 joint disease activity score
DMARD - disease modifying antirheumatic drug
Appendix
THE MEMBERS OF THE BRITISH SOCIETY FOR RHEUMATOLOGY BIOLOGICS REGISTER (BSRBR)
Musgrave Park Hospital, Belfast (Dr Allister Taggart); Cannock Chase Hospital, Cannock Chase (Dr Tom Price); Christchurch Hospital, Christchurch (Dr Neil Hopkinson); Derbyshire Royal Infirmary, Derby (Dr Sheila O'Reilly); Russells Hall Hospital, Dudley (Dr George Kitas); Gartnavel General Hospital, Glasgow (Dr Duncan Porter); Glasgow Royal Infirmary, Glasgow (Dr Hilary Capell); Leeds General Infirmary, Leeds (Prof Paul Emery); King's College Hospital, London (Dr Ernest Choy); Macclesfield District General Hospital, Macclesfield (Prof Deborah Symmons); Manchester Royal Infirmary, Manchester (Dr Ian Bruce); Freeman Hospital, Newcastle‐upon‐Tyne (Dr Ian Griffiths); Norfolk and Norwich University Hospital, Norwich (Prof David Scott); Poole General Hospital, Poole (Dr Paul Thompson); Queen Alexandra Hospital, Portsmouth (Dr Fiona McCrae); Hope Hospital, Salford (Dr Romela Benitha); Selly Oak Hospital, Selly Oak (Dr Ronald Jubb); St Helens Hospital, St Helens (Dr Rikki Abernethy); Haywood Hospital, Stoke‐on‐Trent (Dr Andy Hassell); Kings Mill Centre, Sutton‐In Ashfield (Dr David Walsh).
References
- 1.Wolfe F, Mitchell D M, Sibley J T, Fries J F, Bloch D A, Williams C A.et al The mortality of rheumatoid arthritis. Arthritis Rheum 199437481–494. [DOI] [PubMed] [Google Scholar]
- 2.Gordon P, West J, Jones H, Gibson T. A 10 year prospective follow‐up of patients with rheumatoid arthritis 1986–96. J Rheumatol 2001282409–2415. [PubMed] [Google Scholar]
- 3.Goodson N J, Wiles N J, Lunt M, Barrett E M, Silman A J, Symmons D P. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum 2002462010–2019. [DOI] [PubMed] [Google Scholar]
- 4.Berkanovic E, Hurwicz M L. Rheumatoid arthritis and comorbidity. J Rheumatol 199017888–892. [PubMed] [Google Scholar]
- 5.Gabriel S E, Crowson C S, O'Fallon W M. Comorbidity in arthritis. J Rheumatol 1999262475–2479. [PubMed] [Google Scholar]
- 6.Kroesen S, Widmer A F, Tyndall A, Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti‐TNF‐alpha therapy. Rheumatology (Oxford) 200342617–621. [DOI] [PubMed] [Google Scholar]
- 7.Lee J H, Slifman N R, Gershon S K, Edwards E T, Schwieterman W D, Siegel J N.et al Life‐threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002462565–2570. [DOI] [PubMed] [Google Scholar]
- 8.Slifman N R, Gershon S K, Lee J ‐ H, Edwards E T, Braun M M. Listeria monocytogenes infection as a complication of treatment with tumor necrosis factor a‐neutralizing agents. Arthritis Rheum 200348319–324. [DOI] [PubMed] [Google Scholar]
- 9.Keane J, Gershon S, Wise R P, Mirabile‐Levens E, Kasznica J, Schwieterman W D.et al Tuberculosis associated with infliximab, a tumor necrosis factor a‐neutralizing agent. N Engl J Med 20013451098–1104. [DOI] [PubMed] [Google Scholar]
- 10.Gomez‐Reino J J, Carmona L, Valverde V R, Mola E M, Montero M D. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active‐surveillance report. Arthritis Rheum 2003482122–2127. [DOI] [PubMed] [Google Scholar]
- 11.Mohan N, Edwards E T, Cupps T R, Oliverio P J, Sandberg G, Crayton H.et al Demyelination occurring during anti‐tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001442862–2869. [DOI] [PubMed] [Google Scholar]
- 12.Kwon H J, Cote T R, Cuffe M S, Kramer J M, Braun M M. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med 2003138807–811. [DOI] [PubMed] [Google Scholar]
- 13.Brown S L, Greene M H, Gershon S K, Edwards E T, Braun M M. Tumor necrosis factor antagonist therapy and lymphoma development: twenty‐six cases reported to the Food and Drug Administration. Arthritis Rheum 2002463151–3158. [DOI] [PubMed] [Google Scholar]
- 14.Silman A, Symmons D, Scott D G, Griffiths I. British Society for Rheumatology Biologics Register. Ann Rheum Dis 200362(suppl 2)ii28–ii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.British Society for Rheumatology Update of BSR guidelines for prescribing TNF blockers in adults with rheumatoid arthritis: https://www.msecportal.org/portal/editorial/PublicPages/bsr/536883013/FinalTNFforRAguideline.pdf 2004
- 16.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 17.Prevoo M L, 't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 18.Fries J F, Spitz P W, Young D Y. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 19829789–793. [PubMed] [Google Scholar]
- 19.Ware J E, Sherbourne C D. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 199230473–483. [PubMed] [Google Scholar]
- 20.Sany J, Bourgeois P, Saraux A, Durieux S, Lafuma A, Daures J P.et al Characteristics of patients with rheumatoid arthritis in France: a study of 1109 patients managed by hospital based rheumatologists. Ann Rheum Dis 2004631235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]