Abstract
Objective
To evaluate the two generations of anti‐cyclic citrullinated protein (CCP) antibodies as a diagnostic marker of rheumatoid arthritis (RA) and as a predictor of future development of RA in healthy subjects and in patients with early undifferentiated arthritis.
Methods
A systematic analysis of the literature published between 1999 and February 2006 was conducted. Data were collected on the sensitivity and specificity of the two generations of anti‐CCP antibodies for diagnosing RA and predicting future development of the disease.
Results
Among 107 studies initially identified, 68 had interpretable data and were analysed. Diagnostic properties were assessed in 58 studies: mean (SD) sensitivity was 53 (10)% (range 41–68) and 68 (15)% (range 39–94) for anti‐CCP1 and anti‐CCP2, respectively; mean (SD) specificity was 96 (3)% (range 90–99) and 95 (5)% (range 81–100) for anti‐CCP1 and anti‐CCP2, respectively. Predictive properties were assessed in 14 studies; odds ratio (95% confidence interval) of anti‐CCP1 and anti‐CCP2 for the future development of RA were 20 (14 to 31) and 25 (18 to 35), respectively, among patients with early undifferentiated arthritis and 64.5 (8.5 to 489) and 28 (8 to 95), respectively, among healthy subjects.
Conclusion
Sensitivity of the second generation of anti‐CCP is close to that of rheumatoid factor, with a higher specificity, for distinguishing RA from other rheumatic diseases. Moreover, anti‐CCP antibodies appear to be highly predictive of the future development of RA in both healthy subjects and patients with undifferentiated arthritis.
Keywords: rheumatoid arthritis, anti‐CCP antibodies, diagnosis, predictive marker, serological marker
Rheumatoid arthritis (RA) is a systemic autoimmune disease with a prevalence of 1% world wide.1 It is characterised by chronic inflammation of the synovial joints, which leads to progressive joint erosions and eventually to disability and loss of quality of life. This poor prognosis has led to an emphasis on early diagnosis and aggressive treatment. However, the American College of Rheumatology (ACR) classification criteria2 are not very well suited to diagnosing RA at an early stage because non‐clinical measures are often not fulfilled.3,4 Therefore, a reliable and specific test early in the disease would be very useful for identifying patients with RA before the occurrence of joint damage, and enable targeting the use of potentially toxic and expensive drugs at those patients where the benefits clearly outweigh the risks.5
Over the past few years, several new antibodies have been described in patients with RA (antiperinuclear factor antibodies, antikeratin antibodies, anti‐RA33), but not all have been successfully incorporated into routine clinical practice.6 A new group of autoantibodies that have generated particular interest are the anti‐cyclic citrullinated peptide (anti‐CCP) antibodies, which appear to be of value for the diagnosis of RA.7 However, data about the diagnostic value of these antibodies are somewhat conflicting and it is difficult to apprehend the potential usefulness of anti‐CCP for clinical practice because of the multiplicity of trials using them. A systematic review would be useful to clarify and emphasise the diagnostic value of these antibodies.
This article presents a systematic review of published studies with two objectives: to evaluate the properties of anti‐CCP antibodies in making an accurate diagnosis of RA and to assess their ability to predict the future development of RA in healthy subjects or in patients with undifferentiated early arthritis.
Methods
A systematic review of the published literature following the methods of evidence based medicine was performed.
Literature review
Type of participants
The analysis was restricted to adults over 16 years; studies concerning juvenile arthritis were not taken into account.
The analysis to determine the diagnostic properties of anti‐CCP antibodies concerned patients with confirmed RA according to the ACR 1987 revised criteria,2 control populations of healthy subjects, and patients with other rheumatic diseases.
The analysis to determine the predictive value of anti‐CCP antibodies concerned patients with early undifferentiated arthritis and patients who had donated blood samples before the development of RA.
Search strategy
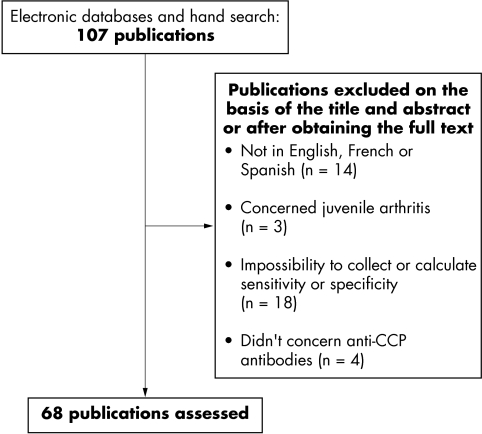
The search was conducted using electronic databases (Medline and Embase), with no limitations by type of publication. This systematic literature analysis was restricted to English, French, and Spanish language articles published between 1999 (first studies in which anti‐CCP antibodies have been used for the diagnosis of RA) and February 2006. Free text search was conducted using the following combination: CCP or anti‐citrullinated peptide antibodies and rheumatoid arthritis and diagnosis. In addition, references of the papers initially detected were hand searched to identify additional relevant reports. Figure 1 reports the results of the article selection process.
Figure 1 Articles reporting the diagnostic value of anti‐CCP antibodies in RA. Screening process.
Article analysis
The analysis of each manuscript was standardised.
Test characteristics
Studies involving the first or second generation of anti‐CCP antibodies were distinguished. The nature of the kit used for the detection of antibodies and the cut off value for a positive test were collected from each manuscript.
Clinical application of anti‐CCP
The studies which investigated the diagnostic performance of anti‐CCP and those for anti‐CCP as a predictive marker of future development of RA were evaluated separately. The association between anti‐CCP and x ray damage or changes due to treatment was not taken into account.
Patients' characteristics
The following data were collected in all studies (if available): percentage of female patients, age, disease duration, and nature of RA diagnosis criteria. Moreover, in manuscripts concerning undifferentiated early arthritis or healthy subjects, patients' follow up duration and the positive anti‐CCP ratio at baseline and at the time of diagnosis were collected.
Statistical analysis
Diagnostic test properties (sensitivity defined by the proportion of people with RA who have a positive test result and specificity defined by the proportion of people without RA who have a negative test result) were collected or calculated in all the analysed studies. In each manuscript related to the ability of anti‐CCP to predict the future development of RA in healthy subjects or in undifferentiated early arthritis, the odds ratio (defined by the ratio of probability of an event in one group to probability of the event in a compared group) was calculated.
Results
Of the 107 publications identified, 68 had interpretable data and were included in the analysis (fig 1). Among these 68 manuscripts, 50 (74%) concerned the second generation of anti‐CCP (introduced at the beginning of 2002), 16 (23%) the first generation, and in two (3%) studies, the type of anti‐CCP test was not specified. The standard test for the detection of IgG antibodies to CCP was a solid phase immunoassay, usually referred to as an enzyme linked immunosorbent assay (ELISA) in all studies. Most studies (85%) used commercially prepared kits, containing plates coated with the CCP antigen. The anti‐CCP kits used for the detection of antibodies were mainly provided by four different manufacturers: Euro‐Diagnostica, The Netherlands (33 studies), Axis‐Shield, UK (16 studies), Inova Diagnostics, USA (8 studies), and Euroimmun, Germany (4 studies). The cut off point to define a positive test varied from 21.4 IU to 1000 IU and from 3.8 IU to 50 IU for anti‐CCP1 and anti‐CCP2, respectively.
Diagnostic performance of anti‐CCP
Fifty eight manuscripts8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65 dealt with the usefulness of anti‐CCP in the diagnosis of RA: 13 (22.4%) concerning anti‐CCP1, 42 (72.4%) concerning anti‐CCP2, 1 (1.7%) concerning both the first and second generation, and 2 (3.4%) where it was not specified. Seven studies66,67,68,69,70,71,72 specifically evaluated the prevalence and use of anti‐CCP in other rheumatic diseases.
Study population
The total number of patients with RA included in all the analysed studies was 8206, all of whom satisfied the ACR 1987 criteria for RA.2 The mean (SD) age of patients with RA was 55.5 (6) years (median 55.5, range 46–66) and the percentage of female patients ranged from 55% to 95%. Publications included in their samples both patients with early and established RA with mean disease duration of 4 (4) years (median 2, range 3 months–15 years).
In total, 6495 patients and 1885 healthy subjects were included as controls in order to assess the specificity of anti‐CCP antibodies or their prevalence in other rheumatic diseases. The control group included both normal subjects and patients with rheumatic diseases in 16/58 (28%) studies. Some of these rheumatic diseases were taken into account for the present analysis: systemic lupus erythematosus, Sjögren's syndrome, hepatitis C virus infection, Wegener's granulomatosis, ankylosing spondylitis, psoriatic arthritis, polymyalgia rheumatica, and palindromic rheumatism.
Table 1 describes the characteristics of patients with RA and controls included in the study populations and separated according to the generation of the CCP test.
Table 1 Diagnostic value of anti‐CCP and rheumatoid factor for RA.
| Reference | Patients with RA | Healthy subjects | Other rheumatic diseases | |||||
|---|---|---|---|---|---|---|---|---|
| No | Age (years) | DD (years) | No | No | Sensitivity | Specificity | ||
| Anti‐CCP1 | 21, 53–65 | 2234 | 55 (7) | 1.5 (1) | 324 | 1465 | 53 (10) | 96 (3) |
| 54 (46–65) | 1 (0.3–3) | 54 (41–68) | 97 (90–99) | |||||
| Anti‐CCP2 | 8–52 | 6125 | 55 (5) | 5 (4.5) | 1561 | 4646 | 68 (15) | 95 (5) |
| 55 (46–66) | 4 (0.2–14.5) | 68.5 (39–94) | 97 (81–100) | |||||
| RF | 8–65 | 8206 | 55.5 (6) | 4 (4) | 1865 | 5797 | 60 (18) | 79 (15) |
| 55.5 (46–66) | 2 (0.2–15) | 65 (25–95) | 81 (31–95) | |||||
DD, disease duration; RA, rheumatoid arthritis; RF, rheumatoid factor.
Unless otherwise mentioned, results are presented as mean (SD), median (range).
Determination of sensitivity and specificity
Patients with RA
Table 1 shows the cumulative analysis for each CCP test. The mean (SD) sensitivities of anti‐CCP1 and anti‐CCP2 tests reported for the whole population of patients with RA were 53 (10)% (median 54%, range 41–68%) and 68 (15)% (median 68.5%, range 39–94%), respectively.
The specificity values of anti‐CCP1 and anti‐CCP2 tests for the whole population of patients with RA were 96 (3)% (median 97%, range 90–99%) and 95 (5)% (median 97%, range 81–100%) respectively.
The mean (SD) sensitivities of the anti‐CCP1 test reported for patients with RA with disease <12 months and >24 months were, respectively, 49 (9)% (median 47%, range 41–66%) and 53 (7)% (median 55%, range 44–60%).
The mean (SD) sensitivities of the anti‐CCP2 test reported for patients with RA with disease <6 months, <12 months, and >24 months were, respectively, 48 (7)% (median 48%, range 39%–58%), 51 (9)% (median 54%, range 41–54%), and 71 (15)% (median 77%, range 44–97%).
The mean (SD) sensitivity and specificity of rheumatoid factor for the whole population of RA was 60 (18)% (median 65%, range 25–95%) and 79 (15)% (median 81%, range 31–95%) respectively.
Healthy controls
Of the 1885 normal healthy subjects, 6/1561 had positive anti‐CCP2 antibodies (prevalence 0.4%) and 5/324 had positive anti‐CCP1 antibodies (prevalence 1.5%).
Other rheumatic diseases
Table 2 shows the prevalence of anti‐CCP1 and anti‐CCP2 antibodies in other rheumatic diseases collected in each study.
Table 2 Prevalence of anti‐CCP antibodies in other rheumatic diseases.
| Disease | Anti‐CCP1 | Anti‐CCP2 | ||||
|---|---|---|---|---|---|---|
| References | Patients (No) | Positive anti‐CCP test No (%) | References | Patients (No) | Positive anti‐CCP test No (%) | |
| Systemic lupus erythematosus | 21, 53, 55, 61, 72 | 89 | 2 (2) | 17, 18, 21, 23, 24, 30, 33, 34, 43, 45, 48, 49, 50, 52 | 567 | 49 (9) |
| Sjögren's syndrome | 53, 55, 61 | 39 | 1 (3) | 17, 19, 23, 30, 33, 45, 46 48, 49, 52, 68, 69 | 521 | 27 (5) |
| Hepatitis C virus | 61 | 16 | 1 (6) | 17, 41, 42, 70 | 219 | 3 (1) |
| Wegener's granulomatosis | 0 | 0 | 19, 24, 34, 48, 52 | 67 | 1 (1) | |
| Ankylosing spondylitis | 21, 53, 55, 56 | 147 | 2 (1) | 18, 21, 23, 24, 34,43, 45, 48, 50, 52 | 181 | 5 (3) |
| Psoriatic arthritis | 21, 61, 62 | 48 | 1 (2) | 14, 21, 34, 46,48, 50, 52, 66, 67 | 424 | 36 (8) |
| Polymyalgia rheumatica | 0 | 0 | 47 | 49 | 0 | |
| Palindromic rheumatism | 0 | 0 | 71 | 63 | 28 (44) | |
Comparison of test results using kits from different manufacturers
Four studies tested the same serum samples using kits from different manufacturers: three publications reported anti‐CCP2 as a diagnostic test in 46, 53, and 87 patients, respectively, and compared the results from different manufacturers (Axis Shield (AS), Euro Diagnostica (ED) Inova Diagnostics (INOVA), and EUROIMMUN).12,16,33 The sensitivity and specificity obtained from the four providers were very similar in each study (table 3). Another study22 compared the test results of three of the same manufacturers in 87 patients. In this study the sensitivity of the AS test was lower than that of the two others (43% v ED 74% and INOVA 77%) and specificity was the same (table 3).
Table 3 Comparison of test results using kits from different manufacturers.
| Author (date) | Patients with RA/controls | Test for anti‐CCP2 | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Dubucquoi (2004)33 | 46/22 | ED | 85 | 91 |
| AS | 82 | 91 | ||
| INOVA | 85 | 95.5 | ||
| Garcia‐Berrocal (2005)22 | 87/46 | ED | 74 | 85 |
| AS | 43 | 85 | ||
| INOVA | 77 | 85 | ||
| Fernandez‐Suarez (2005)12 | 53/75 | ED | 52.8 | 100 |
| AS | 52.8 | 100 | ||
| INOVA | 58.5 | 100 | ||
| Greiner (2005)16 | 87/246 | ED | 81 | 98 |
| INOVA | 80 | 97 | ||
| EUROIMMUN | 81 | 98 |
ED, Euro‐Diagnostica; AS, Axis‐Shield.
Predictive performance of anti‐CCP
Fourteen studies examined the value of anti‐CCP as a potential predictor of future development of RA. Eleven concerned early undifferentiated arthritis and three concerned patients with RA who had donated blood samples before the development of RA.73,74,75
Early undifferentiated arthritis
Among the 11 studies, the mean (SD) symptom duration at baseline was <9.5 (10) months. The 11 studies included a total of 2877 patients with a mean (SD) follow up of 17 (8) months (median 12; range 12–36). Of these 2877 patients, 1476 (51%) were classified as having RA at the end of the follow up. Respectively, 23 (5)% (median 21.5%, range 16–33%) and 23 (6)% (median 22%, range 16–32%) patients with early arthritis had positive anti‐CCP2 and anti‐CCP1 antibodies at baseline. Respectively, 51 (8)% (median 53%, range 39–62%) and 46 (6)% (median 44.5%, range 41–55%) patients classified as having RA had positive anti‐CCP2 and anti‐CCP1 antibodies at the time of the diagnosis. Thus, the mean odds ratio (which characterises the risk of developing RA in those patients with undifferentiated arthritis) was 25 (95% confidence interval (CI) 18 to 35) and 20 (95% CI 14 to 31) for anti‐CCP2 and anti‐CCP1, respectively. Table 4 provides detailed results for patients with early arthritis separated among the generation of the CCP test.
Table 4 Predictive value of anti‐CCP in early undifferentiated arthritis.
| Test | References | EA (No) | Follow up (months)† | RA (n) diagnosis after follow up | DD at baseline (months)‡ | CCP at baseline (%)† | CCP at diagnosis of RA (%)† | Odds ratio* (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Anti‐CCP1 | 21, 53, 59, 62 | 1327 | 15 (5) 12 (12–24) | 603 | <16 (12) | 23 (6) 22 (16–32) | 46 (6) 44.5 (41–53) | 20 (14 to 31) |
| Anti‐CCP2 | 12, 20, 21, 24, 25, 29, 44, 48 | 2017 | 18 (9) 12 (12–36) | 1026 | <5 (3) | 23 (5) 21.5 (16–33) | 51 (8) 53 (39–62) | 25 (18 to 35) |
CI, confidence interval; EA, early arthritis; RA, rheumatoid arthritis; DD, disease duration.
*Odds ratios were calculated by dividing the odds of the RA group by the odds of the non‐RA group; †results shown as mean (SD) and median (range); ‡results shown as mean (SD).
Blood donor cohorts
Three studies concerned patients with RA who had donated blood samples before the development of RA.
Rantapaa‐Dahlqvist et al investigated two different Swedish cohorts, and identified 83 future patients with RA.73 In samples examined 9 years and more than 1.5 years before symptom onset, anti‐CCP2 predicted the development of the disease with a sensitivity of 4% and 25%, respectively, and a specificity of 98%. The sensitivity increased to 52% in samples examined within 1.5 years of the disease onset and the specificity was 98%, while the sensitivity of IgM rheumatoid factor was 30%. In a subanalysis of the same cohort, Berglin et al74 analysed the presence of shared epitope and anti‐CCP2. Two years before symptom onset, the sensitivity and specificity of anti‐CCP2 antibodies and shared epitope as predictors of future development of RA were 37% and 98%, respectively. In a multivariate analysis with logistic regression test, anti‐CCP2 had the highest predictive value with an odds ratio of 15.9 (v 6.8 and 2.35 for IgA rheumatoid factor and shared epitope, respectively).
Nielen et al investigated 79 patients.75 In the 5 years before symptom onset, the sensitivity and specificity of anti‐CCP1 to predict the occurrence of RA were respectively 29% and 99.5%. Table 5 provides detailed results.
Table 5 Predictive value of anti‐CCP in healthy blood donors.
| Author (date) | Nature of the test | Sample/ design | Follow up | Cohort | Sensitivity* (%) | Specificity (%) | Odds ratio† (95% CI) |
|---|---|---|---|---|---|---|---|
| Rantapaa‐Dahlqvist (2003)73 | Anti‐CCP2 | Case‐control study | Retrospective analysis of blood samples collected at onset | 83 Blood donors (before RA) | 4 (9 y before) 25 (>1.5 y before) 52 (<1.5 y before | 98 | 28 (8 to 95) |
| Berglin (2004)74 | Anti‐CCP2 | Same group as above | Same as above | 59 Blood donors (before RA) | 37 (<2 y before) | 98 | 15.9 |
| Nielen (2004) 75 | Anti‐CCP1 | Case‐control study | Retrospective analysis of blood samples collected at onset | 79 | 29 (<5 y before) | 99.5 | 64.5 (8.5 to 489) |
CI, confidence interval; RA, rheumatoid arthritis.
*Sensitivity was defined by the proportion of people with RA who had a positive anti‐CCP test result before symptom onset; †odds ratio was defined by the ratio of the probability of developing RA in the group of blood donors with positive anti‐CCP to the probability of developing RA in the group of blood donors with negative anti‐CCP antibodies.
Discussion
This systematic review of 68 articles confirmed the value of anti‐CCP antibodies as a diagnostic and predictive marker of RA. These results are consistent with those of previous relevant reviews.76 This analysis highlighted the fact that the anti‐CCP2 test had a higher sensitivity than the anti‐CCP1 test (68% v 53%) and similar specificity (95% v 96%) for the diagnosis of RA. For this purpose, the higher sensitivity of the CCP2 clearly puts this test at an advantage over CCP1. However, it is not yet clear if this advantage is retained when CCP antibodies are tested in combination with IgM rheumatoid factor,21 and this point was not evaluated in our analysis. Moreover, the commercially available CCP2 has been optimised by the manufacturer for use on human blood and does not make use of non‐citrullinated control peptides. The remaining major advantage of the CCP1 test is that the substrates are in the public domain and therefore costs are easier to control.21
Important differences were seen in the characteristics of patients evaluated as well as the cut off value used to define a positive test. These differences may explain the wide range of sensitivity results reported. These results indicate that the sensitivity of anti‐CCP appears to be higher in established RA than in patients with recent onset RA, for whom the ACR classification criteria are not very well suited. The cut off value used to define a positive result varied significantly in articles conducted either with the first or second generation anti‐CCP antibodies, even if provided by the same manufacturer. Some standardisation would appear desirable, with determination for each manufacturer of the optimum cut off point. Differences in sensitivity in the same serum samples between manufacturers, observed by Garcia‐Berrocal,22 emphasised this need for standardisation. In addition, it seems to be necessary to study if the presence of raised levels of anti‐CCP antibodies is strongly associated with RA (“dose effect”). Indeed, even when published data suggested that a strongly positive test is associated with RA, no specific comparison of levels of anti‐CCP antibodies in RA and other rheumatic diseases was made in the analysed studies. Thus we cannot define, on the basis of this analysis, a standardised cut off value which would allow RA to be distinguished precisely from other rheumatic diseases.
On the other hand, the specificity of the two generations of anti‐CCP antibodies was homogeneous, and ranged from 81% to 100%. These data and the weak prevalence of anti‐CCP in healthy subjects and in other rheumatic diseases confirm their high propensity to be associated with RA. However, the highest specificity was seen in studies in which the control group was rather small42,47 and included only patients with polymyalgia rheumatica and healthy subjects47 or patients with chronic hepatitis C infection.42
Anti‐CCP antibodies appear to be indicative of the future development of RA in patients with early undifferentiated arthritis with an odds ratio of 25 (95% CI 18 to 35) for anti‐CCP2 and 20 (95% CI 14 to 31) for anti‐CCP1. These tests appear to be better than rheumatoid factor tests in predicting which patients with recent synovitis will develop RA.21,25,48 The usefulness of repeated determination of anti‐CCP antibodies in patients with early RA when a first negative result has been obtained remains to be defined. Nevertheless, the detection of these autoantibodies very early in the disease may help the rheumatologist in reaching decisions on the optimal treatment strategies and thus, joint erosion may already be inhibited at the very early stages.
Major information is that anti‐CCP antibodies precede the onset of RA by years and their presence seems to be associated with future development of RA, with an odds ratio of 15.9,74 higher than shared epitope and rheumatoid factors (odds ratio of 2.35 and 6.8, respectively).
Thus, a normal subject with a positive anti‐CCP antibody test has a substantial risk of future development of RA. This suggests that the initial trigger for the development of RA may occur long before the appearance of symptoms. Monitoring anti‐CCP in people who may have an increased risk for the development of RA (for instance, shared epitope or other genetic factors) might allow earlier treatment of anti‐CCP positive subjects in whom the antibody titres are increasing.77 As a consequence, the lag time between the first visit to the rheumatology centre and a therapeutic intervention may be markedly reduced.
Detection of antibodies to citrullinated peptides as in the anti‐CCP assay, provides an additional serological test to assist in the diagnosis of RA. Sensitivity of the second generation of anti‐CCP is close to that of rheumatoid factor, with a higher specificity for distinguishing between RA and other rheumatic diseases. Thus anti‐CCP represents a better serological marker for the diagnosis of RA.
The presence of anti‐CCP in healthy subjects and in patients with early and undifferentiated arthritis predicts with high probability that they will develop RA. Future developments which should be discussed include a consideration of the usefulness of adding the presence of anti‐CCP to the ACR classification criteria for RA, and whether anti‐CCP antibodies might potentially be useful as a screening test for RA.
Abbreviations
ACR - American College of Rheumatology
CCP - cyclic citrullinated protein
CI - confidence interval
RA - rheumatoid arthritis
References
- 1.Feldmann M, Brennan F M, Maini R N. Rheumatoid arthritis. Cell 199685307–310. [DOI] [PubMed] [Google Scholar]
- 2.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 3.Kaarela K, Kauppi M J, Lehtinen K E. The value of the ACR 1987 criteria in very early rheumatoid arthritis. Scand J Rheumatol 199524279–281. [DOI] [PubMed] [Google Scholar]
- 4.Saraux A, Berthelot J M, Chales G, Le Henaff C, Thorel J B, Hoang S.et al Ability of the American College of Rheumatology 1987 criteria to predict rheumatoid arthritis in patients with early arthritis and classification of these patients two years later. Arthritis Rheum 2001442485–2491. [DOI] [PubMed] [Google Scholar]
- 5.Kirwan J R, Quilty B. Prognostic criteria in rheumatoid arthritis: can we predict which patients will require specific anti‐rheumatoid treatment? Clin Exp Rheumatol 199715(suppl 17)S15–S25. [PubMed] [Google Scholar]
- 6.van Boekel M A, Vossenaar E R, van den Hoogen F H, van Venrooij W J. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res 2002487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schellekens G A, de Jong B A, van den Hoogen F H, van de Putte L B, van Venrooij W J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis‐specific autoantibodies. J Clin Invest 1998101273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H A, Lin K C, Chen C H, Liao H T, Wang H P, Chang H N.et al The effect of etanercept on anti‐cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Ann Rheum Dis 20066535–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limaye S, Carr V, Kirkpatrick P, Williams A, Adelstein S. Antibodies to cyclic citrullinated peptide in patients with chronic arthritis attending an arthritis‐monitoring clinic. J Clin Rheumatol 200511150–152. [DOI] [PubMed] [Google Scholar]
- 10.Shovman O, Gilburd B, Zandman‐Goddard G, Sherer Y, Orbach H, Gerli R.et al The diagnostic utility of anti‐cyclic citrullinated peptide antibodies, matrix metalloproteinase‐3, rheumatoid factor, erythrocyte sedimentation rate, and C‐reactive protein in patients with erosive and non‐erosive rheumatoid arthritis. Clin Dev Immunol 200512197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwok J S, Hui K H, Lee T L, Wong W, Lau Y L, Wong R W.et al Anti‐cyclic citrullinated peptide: diagnostic and prognostic values in juvenile idiopathic arthritis and rheumatoid arthritis in a Chinese population. Scand J Rheumatol 200534359–366. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez‐Suarez A, Reneses S, Wichmann I, Criado R, Nunez A. Efficacy of three ELISA measurements of anti‐cyclic citrullinated peptide antibodies in the early diagnosis of rheumatoid arthritis. Clin Chem Lab Med 2005431234–1239. [DOI] [PubMed] [Google Scholar]
- 13.Samanci N, Ozdem S, Akbas H, Mutlu D, Gultekin M, Arman M.et al Diagnostic value and clinical significance of anti‐CCP in patients with advanced rheumatoid arthritis. J Natl Med Assoc 2005971120–1126. [PMC free article] [PubMed] [Google Scholar]
- 14.Gao I K, Haas‐Wohrle A, Mueller K G, Lorenz H M, Fiehn C. Determination of anti‐CCP antibodies in patients with suspected rheumatoid arthritis: does it help to predict the diagnosis before referral to a rheumatologist? Ann Rheum Dis 2005641516–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aotsuka S, Okawa‐Takatsuji M, Nagatani K, Nagashio C, Kano T, Nakajima K.et al A retrospective study of the fluctuation in serum levels of anti‐cyclic citrullinated peptide antibody in patients with rheumatoid arthritis. Clin Exp Rheumatol 200523475–481. [PubMed] [Google Scholar]
- 16.Greiner A, Plischke H, Kellner H, Gruber R. Association of anti‐cyclic citrullinated peptide antibodies, anti‐citrullin antibodies, and IgM and IgA rheumatoid factors with serological parameters of disease activity in rheumatoid arthritis. Ann N Y Acad Sci 20051050295–303. [DOI] [PubMed] [Google Scholar]
- 17.Sauerland U, Becker H, Seidel M, Schotte H, Willeke P, Schorat A.et al Clinical utility of the anti‐CCP assay: experiences with 700 patients. Ann N Y Acad Sci 20051050314–318. [DOI] [PubMed] [Google Scholar]
- 18.Choi S W, Lim M K, Shin D H, Park J J, Shim S C. Diagnostic performances of anti‐cyclic citrullinated peptides antibody and antifilaggrin antibody in Korean patients with rheumatoid arthritis. J Korean Med Sci 200520473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamali S, Polat N G, Kasapoglu E, Gul A, Ocal L, Aral O.et al Anti‐CCP and antikeratin antibodies in rheumatoid arthritis, primary Sjögren's syndrome, and Wegener's granulomatosis. Clin Rheumatol 200524673–676. [DOI] [PubMed] [Google Scholar]
- 20.Nell V P, Machold K P, Stamm T A, Eberl G, Heinzl H, Uffmann M.et al Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis 2005641731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Gaalen F A, Visser H, Huizinga T W. A comparison of the diagnostic accuracy and prognostic value of the first and second anti‐cyclic citrullinated peptides (CCP1 and CCP2) autoantibody tests for rheumatoid arthritis. Ann Rheum Dis 2005641510–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia‐Berrocal B, Gonzalez C, Perez M, Navajo J A, Moreta I, Davila C.et al Anti‐cyclic citrullinated peptide autoantibodies in IgM rheumatoid factor‐positive patients. Clin Chim Acta 2005354123–130. [DOI] [PubMed] [Google Scholar]
- 23.Dubrous P, Gardet V, Hugard L. Value of anti‐cyclic citrullinated peptides antibodies in comparison with rheumatoid factor for rheumatoid arthritis diagnosis. Pathol Biol (Paris) 20055363–67. [DOI] [PubMed] [Google Scholar]
- 24.Raza K, Breese M, Nightingale P, Kumar K, Potter T, Carruthers D M.et al Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis. J Rheumatol 200532231–238. [PMC free article] [PubMed] [Google Scholar]
- 25.Nielen M M, van der Horst A R, van Schaardenburg D, van der Horst‐Bruinsma I E, van de Stadt R J, Aarden L.et al Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann Rheum Dis 2005641199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronnelid J, Wick M C, Lampa J, Lindblad S, Nordmark B, Klareskog L.et al Longitudinal analysis of citrullinated protein/peptide antibodies (anti‐CP) during 5 year follow up in early rheumatoid arthritis: anti‐CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 2005641744–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caramaschi P, Biasi D, Tonolli E, Pieropan S, Martinelli N, Carletto A.et al Antibodies against cyclic citrullinated peptides in patients affected by rheumatoid arthritis before and after infliximab treatment. Rheumatol Int 20052658–62. [DOI] [PubMed] [Google Scholar]
- 28.Herold M, Boeser V, Russe E, Klotz W. Anti‐CCP: history and its usefulness. Clin Dev Immunol 200512131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boire G, Cossette P, de Brum‐Fernandes A J, Liang P, Niyonsenga T, Zhou Z J.et al Anti‐Sa antibodies and antibodies against cyclic citrullinated peptide are not equivalent as predictors of severe outcomes in patients with recent‐onset polyarthritis. Arthritis Res Ther 20057R592–R603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bongi S M, Manetti R, Melchiorre D, Turchini S, Boccaccini P, Vanni L.et al Anti‐cyclic citrullinated peptide antibodies are highly associated with severe bone lesions in rheumatoid arthritis anti‐CCP and bone damage in RA. Autoimmunity 200437495–501. [DOI] [PubMed] [Google Scholar]
- 31.Mikuls T R, O'Dell J R, Stoner J A, Parrish L A, Arend W P, Norris J M.et al Association of rheumatoid arthritis treatment response and disease duration with declines in serum levels of IgM rheumatoid factor and anti‐cyclic citrullinated peptide antibody. Arthritis Rheum 2004503776–3782. [DOI] [PubMed] [Google Scholar]
- 32.Bobbio‐Pallavicini F, Alpini C, Caporali R, Avalle S, Bugatti S, Montecucco C. Autoantibody profile in rheumatoid arthritis during long‐term infliximab treatment. Arthritis Res Ther 20046R264–R272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubucquoi S, Solau‐Gervais E, Lefranc D, Marguerie L, Sibilia J, Goetz J.et al Evaluation of anti‐citrullinated filaggrin antibodies as hallmarks for the diagnosis of rheumatic diseases. Ann Rheum Dis 200463415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solanki K, Spellerberg M, Chapman P, Moller P, O'Donnell J. Anti‐cyclic citrullinated antibodies: complementary to IgM rheumatoid factor in the early diagnosis of rheumatoid arthritis. N Z Med J 2004117U1097. [PubMed] [Google Scholar]
- 35.Lindqvist E, Eberhardt K, Bendtzen K, Heinegard D, Saxne T. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 200564196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alessandri C, Bombardieri M, Papa N, Cinquini M, Magrini L, Tincani A.et al Decrease of anti‐cyclic citrullinated peptide antibodies and rheumatoid factor following anti‐TNFalpha therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement. Ann Rheum Dis 2004631218–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forslind K, Ahlmen M, Eberhardt K, Hafstrom I, Svensson B, BARFOT Study Group Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti‐CCP). Ann Rheum Dis 2004631090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti‐CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 2004631085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallbracht I, Rieber J, Oppermann M, Forger F, Siebert U, Helmke K. Diagnostic and clinical value of anti‐cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis 2004631079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderlin M K, Kastbom A, Kautiainen H, Leirisalo‐Repo M, Strandberg G, Skogh T. Antibodies against cyclic citrullinated peptide (CCP) and levels of cartilage oligomeric matrix protein (COMP) in very early arthritis: relation to diagnosis and disease activity. Scand J Rheumatol 200433185–188. [DOI] [PubMed] [Google Scholar]
- 41.Girelli F, Foschi F G, Bedeschi E, Calderoni V, Stefanini G F, Martinelli M G. Is anti cyclic citrullinated peptide a useful laboratory test for the diagnosis of rheumatoid arthritis? Allerg Immunol (Paris) 200436127–130. [PubMed] [Google Scholar]
- 42.Bombardieri M, Alessandri C, Labbadia G, Iannuccelli C, Carlucci F, Riccieri V.et al Role of anti‐cyclic citrullinated peptide antibodies in discriminating patients with rheumatoid arthritis from patients with chronic hepatitis C infection‐associated polyarticular involvement. Arthritis Res Ther 20046R137–R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Rycke L, Peene I, Hoffman I E, Kruithof E, Union A, Meheus L.et al Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra‐articular manifestations. Ann Rheum Dis 2004631587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Gaalen F A, Linn‐Rasker S P, van Venrooij W J, de Jong B A, Breedveld F C, Verweij C L.et al Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum 200450709–715. [DOI] [PubMed] [Google Scholar]
- 45.Correa P A, Tobon G J, Citera G, Cadena J, Schneeberger E, Camargo J F.et al Anti‐cyclic citrullinated peptide antibodies in rheumatoid arthritis: relation with clinical features, cytokines and HLA‐DRB1. Biomedica 200424140–152. [PubMed] [Google Scholar]
- 46.Grootenboer‐Mignot S, Nicaise‐Roland P, Delaunay C, Meyer O, Chollet‐Martin S, Labarre C. Second generation anti‐cyclic citrullinated peptide (anti‐CCP2) antibodies can replace other anti‐filaggrin antibodies and improve rheumatoid arthritis diagnosis. Scand J Rheumatol 200433218–220. [DOI] [PubMed] [Google Scholar]
- 47.Lopez‐Hoyos M, Ruiz de Alegria C, Blanco R, Crespo J, Pena M.et al Clinical utility of anti‐CCP antibodies in the differential diagnosis of elderly‐onset rheumatoid arthritis and polymyalgia rheumatica. Rheumatology (Oxford) 200443655–657. [DOI] [PubMed] [Google Scholar]
- 48.Vittecoq O, Incaurgarat B, Jouen‐Beades F, Legoedec J, Letourneur O, Rolland D.et al Autoantibodies recognizing citrullinated rat filaggrin in an ELISA using citrullinated and non‐citrullinated recombinant proteins as antigens are highly diagnostic for rheumatoid arthritis. Clin Exp Immunol 2004135173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki K, Sawada T, Murakami A, Matsui T, Tohma S, Nakazono K.et al High diagnostic performance of ELISA detection of antibodies to citrullinated antigens in rheumatoid arthritis. Scand J Rheumatol 200332197–204. [DOI] [PubMed] [Google Scholar]
- 50.Lee D M, Schur P H. Clinical utility of the anti‐CCP assay in patients with rheumatic diseases. Ann Rheum Dis 200362870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinheiro G C, Scheinberg M A, Aparecida da Silva M, Maciel S. Anti‐cyclic citrullinated peptide antibodies in advanced rheumatoid arthritis. Ann Intern Med 2003139234–235. [DOI] [PubMed] [Google Scholar]
- 52.Vasishta A. Diagnosing early‐onset rheumatoid arthritis: the role of anti‐CCP antibodies. Am Clin Lab 20022134–36. [PubMed] [Google Scholar]
- 53.Saraux A, Berthelot J M, Devauchelle V, Bendaoud B, Chales G, Le Henaff C.et al Value of antibodies to citrulline‐containing peptides for diagnosing early rheumatoid arthritis. J Rheumatol 2003302535–2539. [PubMed] [Google Scholar]
- 54.Jansen L M, van Schaardenburg D, van der Horst‐Bruinsma I, van der Stadt R J, de Koning M H, Dijkmans B A. The predictive value of anti‐cyclic citrullinated peptide antibodies in early arthritis. J Rheumatol 2003301691–1695. [PubMed] [Google Scholar]
- 55.Zeng X, Ai M, Tian X, Gan X, Shi Y, Song Q.et al Diagnostic value of anti‐cyclic citrullinated peptide antibody in patients with rheumatoid arthritis. J Rheumatol 2003301451–1455. [PubMed] [Google Scholar]
- 56.Bas S, Genevay S, Meyer O, Gabay C. Anti‐cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology (Oxford) 200342677–680. [DOI] [PubMed] [Google Scholar]
- 57.Vencovsky J, Machacek S, Sedova L, Kafkova J, Gatterova J, Pesakova V.et al Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis 200362427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A.et al Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 200362120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jansen A L, van der Horst‐Bruinsma I, van Schaardenburg D, van de Stadt R J, de Koning M H, Dijkmans B A. Rheumatoid factor and antibodies to cyclic citrullinated peptide differentiate rheumatoid arthritis from undifferentiated polyarthritis in patients with early arthritis. J Rheumatol 2002292074–2076. [PubMed] [Google Scholar]
- 60.Bas S, Perneger T V, Seitz M, Tiercy J M, Roux‐Lombard P, Guerne P A. Diagnostic tests for rheumatoid arthritis: comparison of anti‐cyclic citrullinated peptide antibodies, anti‐keratin antibodies and IgM rheumatoid factors. Rheumatology (Oxford) 200241809–814. [DOI] [PubMed] [Google Scholar]
- 61.Bizzaro N, Mazzanti G, Tonutti E, Villalta D, Tozzoli R. Diagnostic accuracy of the anti‐citrulline antibody assay for rheumatoid arthritis. Clin Chem 2001471089–1093. [PubMed] [Google Scholar]
- 62.Goldbach‐Mansky R, Lee J, McCoy A, Hoxworth J, Yarboro C, Smolen J S.et al Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res 20002236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kroot E J, de Jong B A, van Leeuwen M A, Swinkels H, van den Hoogen F H, van't Hof M.et al The prognostic value of anti‐cyclic citrullinated peptide antibody in patients with recent‐onset rheumatoid arthritis. Arthritis Rheum 2000431831–1835. [DOI] [PubMed] [Google Scholar]
- 64.Schellekens G A, Visser H, de Jong B A, van den Hoogen F H, Hazes J M, Breedveld F C.et al The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 200043155–163. [DOI] [PubMed] [Google Scholar]
- 65.van Jaarsveld C H, ter Borg E J, Jacobs J W, Schellekens G A, Gmelig‐Meyling F H, van Booma‐Frankfort et al The prognostic value of the antiperinuclear factor, anti‐citrullinated peptide antibodies and rheumatoid factor in early rheumatoid arthritis. Clin Exp Rheumatol 199917689–697. [PubMed] [Google Scholar]
- 66.Vander Cruyssen B, Hoffman I E, Zmierczak H, Van den Berghe M, Kruithof E, De Rycke L. Anti‐citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis 2005641145–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bogliolo L, Alpini C, Caporali R, Scire C A, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol 200532511–515. [PubMed] [Google Scholar]
- 68.Tobon G J, Correa P A, Anaya J M. Anti‐cyclic citrullinated peptide antibodies in patients with primary Sjogren's syndrome. Ann Rheum Dis 200564791–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gottenberg J E, Mignot S, Nicaise‐Rolland P, Cohen‐Solal J, Aucouturier F, Goetz J.et al Prevalence of anti‐cyclic citrullinated peptide and anti‐keratin antibodies in patients with primary Sjögren's syndrome. Ann Rheum Dis 200564114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wener M H, Hutchinson K, Morishima C, Gretch D R. Absence of antibodies to cyclic citrullinated peptide in sera of patients with hepatitis C virus infection and cryoglobulinemia. Arthritis Rheum 2004502305–2308. [DOI] [PubMed] [Google Scholar]
- 71.Salvador G, Gomez A, Vinas O, Ercilla G, Canete J D, Munoz‐Gomez J.et al Prevalence and clinical significance of anti‐cyclic citrullinated peptide and antikeratin antibodies in palindromic rheumatism. An abortive form of rheumatoid arthritis? Rheumatology (Oxford) 200342972–975. [DOI] [PubMed] [Google Scholar]
- 72.Mediwake R, Isenberg D A, Schellekens G A, van Venrooij W J. Use of anti‐citrullinated peptide and anti‐RA33 antibodies in distinguishing erosive arthritis in patients with systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis 20016067–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rantapaa‐Dahlqvist S, de Jong B A, Berglin E, Hallmans G, Wadell G, Stenlund H.et al Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003482741–2749. [DOI] [PubMed] [Google Scholar]
- 74.Berglin E, Padyukov L, Sundin U, Hallmans G, Stenlund H, Van Venrooij W J. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA‐DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res Ther 20046R303–R308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nielen M M, van Schaardenburg D, Reesink H W, van de Stadt R J, van der Horst‐Bruinsma I E, de Koning M H.et al Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 200450380–386. [DOI] [PubMed] [Google Scholar]
- 76.Riedemann J P, Munoz S, Kavanaugh A. The use of second generation anti‐CCP antibody (anti‐CCP2) testing in rheumatoid arthritis—a systematic review. Clin Exp Rheumatol 200523S69–S76. [PubMed] [Google Scholar]
- 77.Pruijn G J, Vossenaar E R, Drijfhout J W, van Venrooij W J, Zendman J W. Anti‐CCP antibody detection facilitates early diagnosis and prognosis of rheumatoid arthritis. Curr Rheumatol Rev 200511–7. [Google Scholar]