Abstract
Background
Anti‐neutrophil cytoplasmic antibodies (ANCA) directed against proteinase 3 (PR3) are highly specific for Wegener's granulomatosis (WG). Evidence for a pivotal role of PR3‐ANCA in the induction of vasculitis has been demonstrated. B cell clusters have been observed within endonasal biopsy specimens.
Objectives
To determine whether B cell selection and maturation take place in granulomatous lesions of WG.
Methods
Granulomatous lesions and the immunoglobulin (VH) gene repertoire from nasal tissue of six WG patients—two active and two smouldering localised WG (ANCA negative, restricted to respiratory tract), plus one active and one smouldering PR3‐ANCA positive generalised WG—were characterised by immunohistochemistry, polymerase chain reaction, cloning, DNA sequencing and database comparison.
Results
B lymphocyte‐rich, follicle‐like areas were observed proximal to PR3 positive cells and plasma cells in granulomatous lesions; 184 VH genes from these granulomatous lesions were compared with 84 VH genes from peripheral blood of a healthy donor. The mutational pattern of VH genes from active WG resembled memory B cells. Structural homologies of VH genes from granulomatous lesions to PR3‐ANCA encoding genes were detected. Significantly more genes (55%, 45%, and 53%, respectively) from active WG compared with the healthy repertoire carried mutations to negatively charged amino acids within the binding site coding regions, favouring affinity to the positively charged PR3.
Conclusions
Selection and affinity maturation of potentially PR3‐ANCA producing autoreactive B cells may start in granulomatous lesions, thereby contributing to disease progression from ANCA negative localised to PR3‐ANCA positive generalised WG.
Keywords: B lymphocyte, Wegener's granulomatosis, PR3, PR3‐ANCA, VH genes
Key issues in understanding human autoimmune diseases concern questions about where, why, and how autoantibody production takes place and is maintained. Recently, animal models have underscored the importance of inflammation of the target organ and persistent autoantigen presentation in organised lymphoid tissue in autoimmune disease.1,2 Wegener's granulomatosis (WG) can be regarded as a model disease for research on autoimmunity as it is characterised by chronic inflammation and an autoimmune response targeting one key autoantigen—that is, “Wegener's autoantigen” proteinase 3 (PR3).3 WG is a disease of unknown aetiology characterised by necrotising granulomatous inflammation and systemic vasculitis predominantly affecting small vessels.4 Antineutrophil cytoplasmic autoantibodies against proteinase 3 (PR3‐ANCA) are highly specific for WG and are detected in 95% of WG patients with generalised disease.5 Interestingly, PR3‐ANCA are transiently induced in infectious diseases such as bacterial endocarditis.6 Experimental data from in vitro studies and animal models provide evidence for a pivotal role of ANCA in the induction of autoimmune vasculitis in WG.7,8,9 Recently, a murine model suggested that PR3‐ANCA are pathogenic in vivo.9
It has been suggested that WG may start as granulomatous disease of the respiratory tract (so called “localised WG”10) before generalisation with manifestations of small vessel vasculitis. Moreover, as underlined in a recent editorial, relapses are linked to persistent granulomatous inflammation of the respiratory tract.11 In patients with generalised WG refractory to standard cyclophosphamide and steroid treatment or with severe renal manifestations, elimination of circulating PR3‐ANCA either by plasma separation12 or by targeting B cells with the monoclonal anti‐CD20 antibody rituximab13 resulted in complete remission.
Although the pathogenic role of PR3‐ANCA in the induction of vasculitis has been demonstrated as outlined above, up to now little is known about the autoantibody's origin and the process of B cell selection and maturation involved in its formation. Previously, we found B cell clusters within granulomatous lesions from endonasal biopsy specimen.14 Thus we hypothesised that PR3‐ANCA formation by B cells starts in granulomatous lesions in the early disease phase, when patients are often still PR3‐ANCA seronegative (localised WG). Thereafter, the disease may proceed to PR3‐ANCA seropositive generalised WG.15
We analysed the antibody encoding heavy chain (VH) gene repertoire of six different WG cases. A non‐WG tissue sample (conchal hyperplasia) and a representative study of the peripheral VH gene repertoire from a healthy donor16 served as controls.
Methods
Patients
Endonasal biopsy specimens were obtained after written consent from six patients with biopsy proven, WG specific granulomatous inflammation and one control with conchal hyperplasia without WG. Four patients representing different clinical courses of localised WG were compared with two generalised WG patients (table 1) and with a control (a 29 year old woman suffering of septal deviation and conchal hyperplasia, at the time of surgery for conchotomia and septum reconstruction). The biopsy specimens were independently evaluated by KH‐U and ACF, who confirmed the diagnosis of WG.
Table 1 Patient characteristics.
| Patient No | WG manifestation* | WG disease activity | WG disease duration | cANCA | Staph | Treatment before biopsy | |
|---|---|---|---|---|---|---|---|
| (age at biopsy (y)) | at biopsy | (anti‐PR3) | |||||
| 1 (61) | Generalised (E,L,K,A,B) | Active | 7 years | 1:2048 (>80 U/Ml) | No | No | |
| 2 (61) | Localised (E) | Active | 6 years | Negative | No | No | |
| 3 (55) | Localised (E) | Smouldering | 30 years | Negative | Yes | Methotrexate/steroids | |
| 4 (64) | Generalised (E,P,C) | Smouldering | 1 year | 1:512 (80 U/Ml) | Yes | No | |
| 5 (82) | Localised (E) | Smouldering | 3 months | Negative | Yes | No | |
| 6 (72) | Localised (E) | Active | 4 years | 1:4 (negative) | No | Cotrimoxazole/steroids |
*WG manifestations are as follows: A, arthritis; B, systemic inflammation; C, central nervous system involvement; E, ear, nose and throat; K, kidney; L, lung; P, peripheral nervous system.
cANCA immunofluorescence test results are given in titres, anti‐PR3 antibodies (standard enzyme linked immunosorbent assay) in units per ml.
Staph, Staphylococcus aureus in endonasal swabs; WG, Wegener's granulomatosis; y, years.
Tissue staining
The biopsy specimens from patients Nos 1–3 were snap frozen in liquid nitrogen immediately after extraction and stored at −80°C. The biopsy specimens from cases Nos 4–6 were paraffin embedded. Cryosections (5–7 µm thick) and paraffin sections (4 µm thick after deparaffinisation) were fixed with acetone and chloroform. Sections were stained with anti‐CD20 monoclonal antibody (mAb) (L26, DakoCytomation, Hamburg, Germany) for B cells, with anti‐CD38 for plasma cells (DakoCytomation), with anti‐PR3 (WGM2), and with an appropriate isotype control to substitute for the primary antibody (mouse universal negative control, DakoCytomation) and developed using either alkaline phosphatase (PR3) or peroxidase (CD20, CD38) detection systems.14,17
Genomic DNA preparation
Paraffin embedded sections were deparaffinised twice using xylol and precipitated with ethanol. Thereafter, DNA from paraffinised tissue and from frozen tissue was prepared as described.18
PCR and cloning
A 250 ng sample of each DNA was subjected to six different polymerase chain reactions (PCR) of 35 cycles, both individual VH specific and with a mix of the JH Intron oligonucleotides (table 2). PCR product identification and bacterial subcloning was undertaken under the conditions described previously.18 For patients Nos 3–6 and the control, 1 μl of each PCR product was subjected to a second PCR under the same conditions with 5′ primers like in the first round and a mix of internal JH oligonucleotides as 3′ primers (table 2) (semi‐nested PCR).
Table 2 The VH PCR oligonucleotide primers.
| (1) 5′ primers | ||||
| VH1 | 5′CTACGTCGAC CCTCAGTGAA GGTYTCCTGC AAGGC | (16–24) | ||
| VH2 | 5′GCACGTCGAC GTCCTGCGCT GGTGAAASCC ACACA | (16–24) | ||
| VH3 | 5′GTACGTCGAC GGGGTCCCTG AGCTCTCCTG TGCAG | (15–24) | ||
| VH4 | 5′CGTCGTCGAC CCTGTCCCTC ACCTGCRCTG TC | (16–24) | ||
| VH5 | 5′CGACGTCGAC AAAAAGCCCG GGGAGTCTCT GARGA | (12–20) | ||
| VH6 | 5′CGTCGTCGAC CTGTGCCATC TCCGGGGACA GTG | (21–29) | ||
| (2) External 3′ primers | ||||
| JH Intron 1,2–4,5 | 5′GACTCACCTG AGGAGACGGT GACC | |||
| JH Intron 3,6 | 5′TCTTGCCTGA GGAGACGGTG ACCRT | |||
| (3) Internal 3′ primers | ||||
| JH1 | 5′CGTCGTCGAC CAGGGTGCCC TGGCCCCAGT GC | (101–109) | ||
| JH2 | 5′CGTCGTCGAC CAGGGTGCCC TGGCCCCAGT GC | (101–109) | ||
| JH3 | 5′CGTCGTCGAC ATTGTCCCTT GGCCCCAGAC ATCA | (100–108) | ||
| JH4 | 5′CGTCGTCAAC CACGGTTCCT TGGCCCCAGT AG | (101–109) | ||
| JH5 | 5′CGTCGTCGAC GTGACCAGGG TTCCTTGGCC CCAGG | (102–110) | ||
| JH6 | 5′CGTCGTCGAC GTGGTCCCTT GCCCCCAGAC GTCC | (100–108) | ||
Amino acid positions in parentheses.
Sequencing
Approximately 5 ng DNA were precipitated with ammonium acetate and ethanol, air dried, and subjected to automatic sequencing (Lycor, Lincoln, Nebraska, USA) using the M13 rev −29 primer. Sequences were analysed with the IMGT/V‐Quest database and DNASIS (Hitachi, Berlin, Germany). All sequences reported here are accessible on EMBL database under accession numbers AJ550996−AJ551088 and AM050894−AM051008.
Data from peripheral VH genes of healthy volunteers
The sequences of 84 peripheral VH genes from a healthy volunteer16 as well as of 340 peripheral VH gene transcripts from another healthy donor19 were taken from the NCBI nucleotide database. Each gene sequence was analysed using the same approach as for the genes from our study.
Statistical analysis
Only functional genes—that is, genes with a reading frame that potentially encodes functional immunoglobulin heavy chains—were analysed. All statistical analyses were done using SPSS statistical software (SPSS Inc, Chicago, Illinois, USA). Because of the non‐normal distribution, non‐parametric tests were used in the analysis. Each case was compared with the healthy control for the mutation frequency (homology), the ratio of amino acid replacement to silent mutations (R:S ratio) in the complementarity determining regions (CDR) and in the framework regions, for the number of genes that contained mutations to negatively charged amino acids, and for the number of genes that contained mutations to positively charged amino acids. For the comparison of R:S ratios, we used Fisher's exact test. To analyse the mutation frequency and the mutations to negatively/positively charged amino acids, the Mann–Whitney U test was employed. To compare the mutations to negatively and positively charged amino acid exchanges for each patient/control we used the Wilcoxon signed rank test.
Results
B lymphocyte infiltrates in endonasal granulomatous lesions of WG
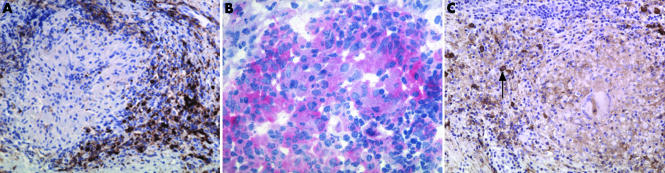
B lymphocyte containing infiltrates were detected in all six WG patients. Figure 1 is a representative illustration of an endonasal granuloma which harbours an infiltrate of mononuclear cells. At least 30% of the cells in this follicle‐like structure were CD20+ B lymphocytes (fig 1A), distinguishing this formation from mononuclear infiltrates with diffusely dispersed B lymphocytes. Moreover, neutrophils and monocytes expressing Wegener's autoantigen PR3 (fig 1B) were detected within granulomatous lesions as well as CD38+ plasma cells (fig 1C). The tissue used as disease control (conchal hyperplasia) did not show a significant number of B cells or PR3+ cells.
Figure 1 (A) CD20 expression in an endonasal lesion from a case of Wegener's granulomatosis (WG). A deparaffinised serial section (4 µm) of an endonasal biopsy specimen (from patient No 2) stained positive for B lymphocytes by anti‐CD20 peroxidase staining (brown) and was counterstained by haematoxylin (blue). In direct proximity to an ill defined Wegener's granuloma there is a follicle‐like infiltrate with a B lymphocyte content of more than 30%. Original magnification ×200. (B) PR3 expression in a WG endonasal lesion. A serial cryosection (7 µm) of an endonasal biopsy specimen (from patient No 2) stained positive for PR3 by anti‐PR3 APAAP (red) and was counterstained by haematoxylin (blue). Original magnification ×400. (C) CD38 expression in a WG endonasal lesion. A deparaffinised serial section (4 µm) of an endonasal biopsy specimen (from patient No 2) stained positive for plasma cells (depicted by a black arrow) by anti‐CD38 peroxidase staining (brown) and was counterstained by haematoxylin (blue). Original magnification ×200.
Immunoglobulin gene characterisation
One hundred and eighty four individual WG tissue derived VH genes from six different cases were compared with peripheral blood derived gene data from two studies of healthy VH gene repertoires.16,19 The approach of Brezinschek et al16 was similar to ours in analysing the VH‐DNA irrespective of immunoglobulin isotype and productivity. We therefore chose that study as the primary reference for statistical analyses (table 4). In general, we observed differences with respect to the number of mutated genes and the mutational load, the ratio of replacement to silent mutations, and the number of mutations leading to exchanges towards negatively charged amino acids and with regard to the represented VH genes between patients and controls. Nearly all (95%) WG tissue‐derived VH genes were mutated. Moreover, differently mutated genes from patients Nos 1, 4, 5, and 6 carried identical rearrangements and consecutively represent offspring of one clone which were diversified by antigen driven affinity maturation (table 3). Interestingly, we found that endonasal B cells from patients with active WG showed significantly more features of selection and maturation and, because of an accumulation of negatively charged residues, a potentially higher binding affinity to PR3 than those of the patients with smouldering WG. A summary along with individual numbers and characteristics of the genes are shown in table 4 and as supplementary material (table 5A and 5B; this table can be viewed on the journal website: http://www.annrheumdis.com/supplemental).
Table 4 Characterisation of VH genes.
| Case 1: | Case 2: | Case 3: | Case 4: | Case 5: | Case 6: | Healthy control16 | ||
|---|---|---|---|---|---|---|---|---|
| Generalised WG | Localised WG | Localised WG | Generalised WG | Localised WG | Localised WG | |||
| Individual genes | 35 | 30 | 24 | 29 | 28 | 38 | 84 | |
| Functionally rearranged* | 31 (89%) | 22 (73%) | 24 (100%) | 25 (86%) | 27 (96%) | 34 (89%) | 71 (84%) | |
| Mean mutation frequency (p value)† | 8% (<0.001)a | 7% (<0.001)a | 4% (0.011)a | 6% (<0.001)a | 6% (<0.001)a | 5% (<0.001)a | 3%a | |
| 8% (<0.001)b | 7% (<0.001)b | 5% (NS)b | 7% (<0.001)b | 6% (<0.01)b | 5% (<0.05)b | 4%b | ||
| Mean R:S ratio‡, CDR (p value) | 3.8 (NS) | 6.5 (0.04) | 4 (NS) | 2.6 (0.09) | 3.3 (NS) | 6.4 (0.04) | 3.3 | |
| Mean R:S ratio‡, FR (p value) | 1.49 (NS) | 1.6 (NS) | 2 (NS) | 1.5 (NS) | 1.7 (NS) | 1.4 (0.07) | 1.9 | |
| Genes with aa mutations to E/D: CDR1+2 (% of genes, p value) | 17 (55%, 0.00002) | 10 (45%, 0.002) | 7 (29%, NS) | 6 (24%, NS) | 8 (30%, 0.08) | 18 (53%, 0.00003) | 10 (14%) | |
| Mean CDR3 length (aa) | 13 | 13 | 14 | 16 | 14 | 15 | 12 |
Comparison between VH genes derived from endonasal tissues of patients with Wegener's granulomatosis and VH genes derived from peripheral blood of a healthy volunteer.16 Numbers (percentage) of functionally rearranged genes that carry amino acid (aa) exchanges to aspartate (D) or glutamate (E) were compared between patients and the healthy control (p values are given in parentheses).
*Number (percentage) of genes that potentially encode functional antibodies.
†The mean mutation frequency of all functionally rearranged genes (a) as well as of mutated genes only (b) was compared between patients and healthy controls.
‡Mean R:S ratio represents mutations that lead to amino acid (aa) replacement divided by mutations that do not affect the aa sequence within both the CDR and the FR.
aa, amino acid; CDR, complementarity determining region; FR, framework region.
Another healthy control of 340 exclusively functional somatically mutated genes19 showed a mean mutation frequency of 6%, mean R:S ratios of 3.7 in the complementarity determining region and 1.8 in the framework region, and 122 (36%) genes carrying mutations to E/D. When compared with the VH genes from the WG patients, a significant increase in the number of mutated genes and the mutational load, as well as in mutations towards E/D, remained only for patients with active disease—that is, for patients Nos 1 and 2 and for Nos 1 and 6 in the latter case.
In detail, patient No 1 (generalised WG with a high cytoplasmic ANCA titre) displayed a VH gene repertoire of exclusively VH3 and VH4 genes (table 5a and 5b, supplementary). Sequencing of 50 bacterial colonies yielded 35 distinct B lymphocyte clones, of which 31 were functionally rearranged (table 4). Comparison of these functional genes with known VH genes16 revealed a predominance of rearrangements (numbers in parentheses) for VH3‐30 (7), VH4‐34 (5), VH3‐23 (4), and VH4‐59 (3) genes (table 5a, supplementary). Significantly more genes from patient No 1 compared with the healthy repertoire carried exchange mutations to the negatively charged amino acids aspartate or glutamate within the CDR1+2 (55% v 14%, p = 0.00002). Moreover, exchange mutations to the positively charged amino acids arginine, histidine, or lysin were less frequent than to the negatively charged amino acids (29% v 55%, p = 0.03). The genes J2‐8 and J2‐12 were clonally related (identical CDR3) and diversified by mutations (table 3). In comparison to the respective germline gene (VH4‐30), J2‐8 and J2‐12 varied by different point mutations within the CDR1 that led to distinctive amino acids exchanges. Interestingly, one differently placed mutation within the CDR1 of J2‐8 and J2‐12 resulted in an exchange towards aspartate.
Table 3 Short table of intraclonal diversification.
| ‐‐CDR1‐‐ | ‐‐CDR2 | ‐‐CDR3 | |
| VH4‐30 | GGSISSGSYY... | YIYYSGST... | |
| J2_8 | ‐‐‐‐‐‐‐D‐‐... | FM‐‐‐‐‐M... | VRDLGLGSD |
| J2_12 | ‐D‐‐‐‐‐NF‐... | FM‐‐‐‐‐M... | VRDLGLGSD |
| ‐‐CDR1‐‐ | ‐‐CDR2‐‐ | ‐‐‐‐‐‐CDR3‐‐‐‐‐‐ | |
| VH1‐46: | GYTFTSYY | INPSGGST | ................ |
| Ro85: | ‐‐‐‐‐‐H‐ | ‐‐‐R‐‐RP | ARVAGLAGHDNYYMDV |
| Ro89: | ‐‐‐‐I‐HD | ‐‐‐R‐‐RP | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
| ‐‐CDR1‐‐ | ‐‐CDR2‐‐ | ‐‐‐‐‐‐‐‐‐‐‐CDR3‐‐‐‐‐‐‐‐‐‐‐ | |
| VH1‐8: | GYTFTSYD | MNPNSGNT | .......................... |
| Ro101: | ‐‐‐‐I‐H‐ | ID‐‐‐‐K‐ | ARDGKAISLTRGFHLSVEADYPGLDV |
| Ro151: | ‐‐S‐ITH‐ | ‐D‐S‐‐K‐ | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
| ‐‐CDR1‐‐ | FR2‐>‐‐CDR2‐‐ | ‐‐‐‐‐‐‐CDR3‐‐‐‐‐‐‐ | |
| VH3‐23: | GFTFSSYA | EWVSAISGSGGST | .................. |
| Ro113: | ‐‐‐‐‐‐‐‐ | ‐‐‐‐D‐‐‐‐‐‐‐‐ | AKFQYYYGSRTPSNWFDP |
| Ro112: | ‐‐S‐‐TFG | ‐‐‐‐D‐‐‐‐‐‐‐‐ | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
| ‐‐‐CDR1‐‐‐ | FR2‐>‐CDR2‐‐ | ‐‐‐‐CDR3‐‐‐‐ | |
| VH4‐30: | GGSISSGGYS | EWIGYIYHSGST | ............ |
| Ko152: | ‐‐‐‐‐‐‐L‐‐ | ‐‐‐‐‐‐‐‐‐‐‐‐ | ARGEGGSYDFDS |
| Ko155: | ‐‐‐‐‐‐‐L‐‐ | ‐‐‐‐E‐N‐‐‐‐‐ | ‐‐‐‐‐‐‐‐‐‐‐‐ |
| ‐‐CDR1‐‐ | ‐‐CDR2‐‐ | ‐‐‐‐‐CDR3‐‐‐‐‐ | |
| VH3‐23: | GFTFSSYA | ISGSGGST | .............. |
| Scy07: | ‐L‐‐‐T‐‐ | ‐R‐‐‐DR‐ | AKGTHSGSFSELDS |
| Scy64: | ‐‐‐‐‐D‐W | ‐NKD‐SV‐ | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
Alignment of amino acid sequences of differently mutated offspring from six B lymphocyte clones of patients Nos 1, 4, 5, and 6 to their respective germline sequences. In the first line FR, CDR, and the respective amino acid positions are indicated. Amino acids that are identical to the germline sequence are symbolised by dashes.
CDR, complementarity determining region; FR, framework region.
For patient No 2 (localised WG, ANCA negative) we observed that from 50 bacterial colonies sequenced, 30 represented distinct B lymphocyte clones and 22 were functionally rearranged (table 4). Except for VH6, all VH families were represented, and no dominance of particular genes was observed (Tables 5a and 5b, supplementary). Significantly more genes from patient No 2 compared with the healthy repertoire carried exchange mutations to the negatively charged amino acids aspartate or glutamate within the CDR1+2 (45% v 14%, p = 0.002). However, a similar number of genes carried exchanges to positively charged amino acids arginine, histidine, and lysin (54%).
Patients Nos 3–5 showed smouldering courses of WG. PCR products of the expected length were detected for all VH families (tables 5a and 5b, supplementary). As with case No 1, genes using the segments VH3‐30/31, VH4‐34, and VH4‐59 dominated the repertoire. In contrast to the active disease in patients Nos 1 and 2, the VH repertoire of the longstanding localised disease of patient No 3 revealed fewer features of active selection: the difference in mutation frequency became insignificant when only mutated genes were compared, and the R:S ratios within complementarity determining and framework regions were similar to the healthy repertoire (table 4). Several clonally related genes were found for patients Nos 4 and 5, diversified by mutations (table 3).
Patient No 6 was similar to No 2, with an aggressive clinical course of localized WG and a predominance of somatically mutated genes: the high R:S ratio of 6.4 within the CDR1+2 was significantly higher than the healthy control, and counterselection of replacement mutations within the framework region (R:S ratio of 1.4) also indicated maturation towards memory cells. Further, 53% of exchange mutations led to the negatively charged amino acids aspartate and glutamate and differed significantly from the healthy control (p = 0.00003). In contrast, significantly fewer exchanges to the positively charged amino acids were seen when compared with the negatively charged amino acid exchange mutations (18% v 53%, p = 0.003). This patient also had two clonally related genes showing mutations towards aspartate (table 3).
No VH genes were detected in a biopsy from conchal hyperplasia after two rounds of VH‐PCR using the same amount of DNA as for the WG biopsy specimens. The same was true for the negative controls that accompanied each PCR.
Discussion
Inflammatory lesions in autoimmune diseases such as rheumatoid arthritis, Sjögren's syndrome, and Hashimoto thyroiditis contain distinct B cell areas.20 B cell clusters have been demonstrated within granulomatous lesions of WG.14 So far, neither the origin of PR3‐ANCA has been shown nor has it been linked to B cell clusters in granulomatous lesions before. Here we observed B cell‐rich follicle‐like aggregates in endonasal granulomatous lesions in vicinity to PR3+ cells and plasma cells, indicating a local antigen driven affinity maturation and differentiation. We therefore analysed VH genes from six endonasal tissues, and our findings suggest that potentially PR3‐ANCA producing B cells might be selected in PR3+ granulomatous lesions starting in localised WG and continuing in generalised WG. Further, the presence of B cells maturing on PR3 in granulomatous lesions of (still) ANCA seronegative localised WG may provide the missing link between localised and generalised PR3‐ANCA+ WG.
Overall, the sequences of the WG tissue derived functional immunoglobulin genes showed significantly more mutated genes and a significantly higher mutational load than the healthy repertoire (table 4), supportive of a selection process. Further, high R:S ratios within the complementarity determining region, as for patients Nos 2 and 6, and low R:S ratios within the framework region, indicate selection by an antigen.21 The mean R:S values of 1.5, 1.6, and 1.4, respectively within the framework region of the WG derived genes from patients Nos 1, 2, and 6 reflect counterselection of replacement mutations within the immunoglobulin gene framework region, which is typical for memory B cells.21 Intriguingly, granuloma derived B cell genes from active WG (patients Nos 1, 2, and 6) showed more signs of antigen selection, while B cell genes from the smouldering cases Nos 3–5 were similar to the healthy control. This observation points to an association between disease activity and selection of antibody specificities. Furthermore, differently mutated offspring of six B lymphocyte clones indicated diversification by antigen driven hypermutation as a means of affinity maturation (table 3). Similar processes of B cell selection in inflamed tissue have been demonstrated for the synovial membrane of rheumatoid arthritis, in which autoantibody (that is, rheumatoid factor) producing B cells have been detected in ectopic lymphoid‐like tissue formation within inflammatory lesions.18,22,23
For comparison we took data from single cell analyses of a healthy donor's peripheral B lymphocytes published by Brezinschek and co‐workers.16 Their work was based on the analysis of genomic DNA irrespective of idiotype and functionality of immunoglobulin genes, as with our approach. In addition, we compared our results to 340 sequences of heavy chain IgG+ transcripts from single cells of another healthy donor19 representing a selected, mature repertoire. In comparison with this study the mutational pattern converged.
The VH4‐34 gene is highly associated with autoreactive antibodies.24 In our study it was the most frequently used segment of somatically mutated genes from WG tissues followed by mutated VH3‐23 genes. Sibilia and coworkers characterised the immunoglobulin genes from five peripheral B cell lines that produced anti‐PR3 IgM antibodies and concluded it was an antigen driven process.25 The granuloma derived genes from our study and the five peripheral PR3 antibody encoding genes from the study be Sibilia et al25 share the overrepresentation of similarly mutated VH3‐23 genes and the accumulation of negatively charged residues within the complementarity determining region. In active longstanding WG (patients Nos 1 and 6), a significant bias of exchange mutations towards negatively charged residues in comparison with mutations towards positively charged residues was observed. Thus the driving force of affinity maturation in these tissues favours a negative charge of the antibody binding site. The high affinity of PR3 to ANCA is determined by amino acids that distinguish PR3 from similar serine proteases such as cathepsin G.26 As positively charged amino acids determine the binding site of PR3, negatively charged residues within the binding site of ANCA might increase PR3 affinity.27 Thus a driving force of PR3‐ANCA formation might be present within these inflamed granulomatous lesions, starting during localised WG and expanding in generalised WG. For patient No 2 the VH genes were unbiased and exchange mutations towards positively as well as towards negatively charged amino acids were both very similar. This patient had an acute and aggressive disease exacerbation immediately before biopsy and therefore the biopsy may reflect an ongoing affinity maturation. Patients Nos 3−5 were endonasal carriers of Staphylococcus aureus and consecutively treated with anti‐staphylococcal agents. Staphylococcus aureus B cell superantigens SED and SpA stimulate B cells through conserved segments of VH4 and VH3 genes.28,29 Interestingly, VH4‐30/31, VH4‐34, and VH4‐59 gene segments were represented in the repertoire of patient No 3 with differently mutated genes. A participation of Staphylococcus aureus B lymphocyte superantigen stimulation in the WG immune response has been discussed before.30 Our data suggest an induction of PR3‐ANCA by autoreactive B cells takes place in the granulomatous lesions, starting during early disease phases when patients might still be ANCA seronegative. Against this background, the question arises why three WG patients (Nos 2, 3, and 5) have remained ANCA seronegative up to now. The most important factor may have been the imposition of immunosuppressive treatment following the diagnosis, inhibiting disease progression as well as expansion of autoreactive B cells.
In summary, our findings indicate that selection and affinity maturation of potentially PR3‐ANCA producing autoreactive B cells may start in granulomatous lesions, thereby contributing to disease progression from ANCA negative localised to PR3‐ANCA positive generalised WG.
Supplementary table 5 can be seen on the journal website (http://www.annrheumdis.com/supplemental).
Supplementary Material
Acknowledgements
We thank Dr Elena Csernok for undertaking the ANCA testing and for critical reading of the manuscript, as well as Petra Zander for technical assistance in immunostaining and Dr Kai Kalies (Institute of Biology, University of Lübeck, Germany) for assistance in sequencing.
The study was supported by grants from the Deutsche Forschungsgemeinschaft (DFG): SFB 367/A11 (to JV) and SFB367/A8 (to PL, AM, WLG), and a grant from the University of Lübeck: FUL 3501 (to JV).
Abbreviations
ANCA - antineutrophil cytoplasmic antibodies
CDR - complementarity determining region
PR3 - proteinase 3
WG - Wegener's granulomatosis
Footnotes
Supplementary table 5 can be seen on the journal website (http://www.annrheumdis.com/supplemental).
References
- 1.Lang K S, Recher M, Junt T, Navarini A A, Harris N L, Freigang S.et al Toll‐like receptor engagement converts T‐cell autoreactivity into overt autoimmune disease. Nat Med 200511138–145. [DOI] [PubMed] [Google Scholar]
- 2.Zinkernagel R M, Hengartner H. Regulation of the immune response by antigen. Science 2001293251–253. [DOI] [PubMed] [Google Scholar]
- 3.Luedemann J, Utecht B, Gross W L. Anti‐neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med 1990171357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennette J C, Falk R J, Andrassy K, Bacon P A, Churg J, Gross W L.et al Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 199437187–192. [DOI] [PubMed] [Google Scholar]
- 5.Hagen E C, Daha M R, Hermans J, Andrassy K, Csernok E, Gaskin G.et al Diagnostic value of standardized assays for anti‐neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. Kidney Int 199853743–753. [DOI] [PubMed] [Google Scholar]
- 6.Choi H K, Lamprecht P, Niles J L, Gross W L, Merkel P A. Subacute bacterial endocarditis with positive cytoplasmic antineutrophil cytoplasmic antibodies and anti‐proteinase 3 antibodies. Arthritis Rheum 200043226–231. [DOI] [PubMed] [Google Scholar]
- 7.Falk R J, Terrell R S, Charles L A, Jennette J C. Anti‐neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA 1990874115–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y.et al Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002110955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfister H, Ollert M, Froehlich L F, Quintanilla‐Martinez L, Colby T V, Specks U.et al Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood 20041041411–1418. [DOI] [PubMed] [Google Scholar]
- 10.Jayne D. Update on the European Vasculitis Study Group trials. Curr Opin Rheumatol 20011348–55. [DOI] [PubMed] [Google Scholar]
- 11.Bacon P. The spectrum of Wegener's granulomatosis and disease relapse. N Engl J Med 2005352330–332. [DOI] [PubMed] [Google Scholar]
- 12.Stegeman C A, Cohen Tervaert J W, De Jong P E, Kallenberg C G. Trimethoprim‐sulfamethoxazole (Co‐Trimoxazole) for the prevention of relapses of Wegener's granulomatosis. N Engl J Med 199633516–20. [DOI] [PubMed] [Google Scholar]
- 13.Keogh K A, Wylam M E, Stone J H, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody‐ associated vasculitis. Arthritis Rheum 200552262–268. [DOI] [PubMed] [Google Scholar]
- 14.Mueller A, Trabandt A, Gloeckner‐Hofmann K, Seitzer U, Schoenermarck U, Csernok E.et al Localized Wegener's granulomatosis: predominance of CD26 and IFN‐gamma expression. J Pathol 2000192113–120. [DOI] [PubMed] [Google Scholar]
- 15.Mueller A, Holl‐Ullrich K, Feller A C, Gross W L, Lamprecht P. Immune phenomena in localized and generalized Wegener's granulomatosis. Clin Exp Rheumatol 20032149–54. [PubMed] [Google Scholar]
- 16.Brezinschek H P, Brezinschek R I, Lipsky P E. Analysis of the heavy chain repertoire of human peripheral B cells using single‐cell polymerase chain reaction. J Immunol 1995155190–202. [PubMed] [Google Scholar]
- 17.Braun M G, Csernok E, Gross W L, Mueller‐Hermelink H K. Proteinase 3, the target antigen of anticytoplasmic antibodies circulating in Wegener's granulomatosis. Immunolocalization in normal and pathologic tissues. Am J Pathol 1991139831–838. [PMC free article] [PubMed] [Google Scholar]
- 18.Voswinkel J, Weisgerber K, Pfreundschuh M, Gause A. The B lymphocyte in rheumatoid arthritis: recirculation of B lymphocytes between different joints and blood. Autoimmunity 19993125–34. [DOI] [PubMed] [Google Scholar]
- 19.de Wildt R M, Hoet R M, van Venrooij W J, Tomlinson I M, Winter G. Analysis of heavy and light chain pairings indicates that receptor editing shapes the human antibody repertoire. J Mol Biol 1999285895–901. [DOI] [PubMed] [Google Scholar]
- 20.Mebius R E. Organogenesis of lymphoid tissues. Nat Rev Immunol 20033292–303. [DOI] [PubMed] [Google Scholar]
- 21.Klein U, Goossens T, Fischer M, Kanzler H, Braeuninger A, Rajewsky K.et al Somatic hypermutation in normal and transformed human B cells. Immunol Rev 1998162261–280. [DOI] [PubMed] [Google Scholar]
- 22.Voswinkel J, Trümper L, Carbon G, Hopf T, Pfreundschuh M, Gause A. Evidence for a selected humoral immune response encoded by VH4 family genes in the synovial membrane of a patient with rheumatoid arthritis. Clin Exp Immunol 19961065–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voswinkel J, Gause A. From immunoglobulin gene fingerprinting to motif‐specific hybridization: advances in the analysis of B lymphoid clonality in rheumatic diseases. Arthritis Res 200241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual V, Capra J D. VH4‐21, a human VH gene segment overrepresented in the autoimmune repertoire. Arthritis Rheum 19923511–18. [DOI] [PubMed] [Google Scholar]
- 25.Sibilia J, Benlagha K, Vanhille P, Ronco P, Brouet J C, Mariette X. Structural analysis of human antibodies to proteinase 3 from patients with Wegener granulomatosis. J Immunol 1997159712–719. [PubMed] [Google Scholar]
- 26.Williams R C, Staud R, Malone C C, Payabyab J, Byres L, Underwood D. Epitopes on proteinase‐3 recognized by antibodies from patients with Wegener's granulomatosis. J Immunol 19941524722–4737. [PubMed] [Google Scholar]
- 27.Peen E, Williams R C. What you should know about PR3‐ANCA. Structural aspects of antibodies to proteinase 3 (PR3). Arthritis Res 20002255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domiati‐Saad R, Attrep J F, Brezinschek H P, Cherrie A H, Karp D R, Lipsky P E. Staphylococcal enterotoxin D functions as a human B cell superantigen by rescuing VH4‐expressing B cells from apoptosis. J Immunol 19961563608–3620. [PubMed] [Google Scholar]
- 29.Hakoda M, Kamatani N, Hayashimoto‐Kurumada S, Silverman G J, Yamanaka H, Terai C.et al Differential binding avidities of human IgM for staphylococcal protein A derived from specific germ‐line VH3 gene usage. J Immunol 19961572976–2981. [PubMed] [Google Scholar]
- 30.Popa E R, Stegeman C A, Kallenberg C G M, Cohen Tervaert J W. Staphylococcus aureus and Wegener's granulomatosis. Arthritis Res 2002477–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.