Abstract
Objectives
To report the clinical outcome and safety profile of repeated B cell depletion in seven patients with refractory systemic lupus erythematosus (SLE).
Methods
Since June 2000, seven patients with refractory SLE had repeated cycles of B cell depletion (18 cycles in total, up to three cycles per patient) because of disease relapse. The clinical response (assessed by the British Isles Lupus Activity Guide (BILAG) activity index), duration of B cell depletion, and adverse events in these patients was reviewed.
Results
Four patients (Nos 1, 2, 3, 6) had three cycles of treatment and three (Nos 4, 5, 7) had two cycles. Four of the seven patients (Nos 1, 3, 5, 6) improved. The mean global BILAG scores dropped from 15 to 6 at 5–7 months. The median duration of clinical response and B cell depletion was 13 months and 6 months, respectively. After the third cycle, 2/4 patients (Nos 1 and 2) improved. The median duration of clinical benefit was 12 months. Most patients tolerate re‐treatment very well.
Conclusion
Re‐treatment with B cell depletion of patients with severe SLE is safe and may be effective for 6–12 months on average.
Keywords: systemic lupus erythematosus, rituximab, re‐treatment, B cell depletion
Systemic lupus erythematosus (SLE) is an autoimmune rheumatic disease characterised serologically by the production of numerous autoantibodies. Rituximab is a chimeric monoclonal antibody specific for human CD20 with variable regions of a murine antihuman CD20 B cell hybridoma fused to human IgG antibody. Recently, there have been encouraging preliminary clinical outcome reports about the use of B cell depletion therapy in patients with SLE.
Our centre reported significant clinical improvement in six patients with active, refractory SLE with a combination protocol of rituximab and cyclophosphamide.1 Subsequently, we have reported 6 month follow up data on 24 patients using a combination protocol.2 Looney et al showed that rituximab alone reduced global lupus activity measured by the Systemic Lupus Activity Measure (SLAM) index at 3 months in patients with relatively mild lupus.3
There may be a role for repeated B cell depletion in patients with severe SLE who relapse after one cycle of rituximab. However, the safety and clinical efficacy of this is unknown. This study reports the clinical outcome in seven lupus patients treated with repeated B cell depletion at our centre.
Patients and methods
Patients
Since June 2000, seven of 24 patients with refractory SLE receiving B cell depletion therapy in our centre have had repeated cycles of treatment. All patients fulfilled at least four of the revised American College of Rheumatology criteria4 for the classification of SLE and gave informed consent to re‐treatment. Patients were re‐treated if a relapse of disease occurred. Relapse was defined as the appearance of a new British Isles Lupus Activity Guide (BILAG) “A” or two new “B”s (except for one patient who had a single new B) from a previous record of BILAG “C”, “D” or “E” in any organ system. Conventional immunosuppressive treatment, including intravenous cyclophosphamide, had already failed for these patients. Clinical response was defined as a loss of BILAG “A” or “B” after treatment.
Assessment
Patients were assessed at 1–3 monthly intervals. At each visit, activity of disease was measured using the BILAG index. Antibodies to double stranded DNA (anti‐dsDNA) were measured by enzyme linked immunosorbent assay (ELISA; Shield Diagnostics, Dundee, UK) (normal <50 IU/ml) and serum C3 by laser nephelometry (normal 0.90–1.80 g/l) at each assessment. Patients with lupus nephritis also had the protein/creatinine ratio (normal <13 mg/mmol) measured from a random urine sample.
Serum immunoglobulins levels were measured by immunoturbidometry (IgA normal 0.7–4.0 g/l, IgG normal 7.0–16.0 g/l, IgM normal 0.4–2.3 g/l) and B cell depletion monitored by circulating CD19+ cell count (<0.005×109/l in peripheral blood).
Treatment regimen
The treatment regimen for each cycle was two infusions of rituximab and intravenous cyclophosphamide, each given 2 weeks apart (table 1). Patient 4 had no cyclophosphamide owing to a previous allergy. Steroid cover was given with each cycle.
Table 1 Patients' demographics, treatment regimens, and duration of B cell depletion.
| Patient No | Sex/age (years)/ethnicity | Disease duration (years) | Regimen for each cycle | Duration since previous cycle (months) | Main clinical indication for each cycle | Previous treatments | Duration B cell depletion (months) |
|---|---|---|---|---|---|---|---|
| 1 | F/45/E | 16 | 2×500 mg RTX 2×750 mg CYC 2×60 mg Pred | Arthritis, pleuritis, shrinking lung | Steroids, HCQ, AZA, CYC, MMF, MTX, CyA | 4 | |
| 2×1000 mg RTX 2×750 mg CYC 2×60 mg Pred | 7 | Arthritis, fever, rash | 6 | ||||
| 2×1000 mg RTX 2×750 mg CYC 2×100 mg MP | 31 | Arthritis, pleuritis | 7 | ||||
| 2 | F/21/A | 8 | 2×500 mg RTX 2×750 mg CYC 2×60 mg Pred | Nephritis (IV), arthritis | Steroids, HCQ, AZA, CYC, MMF | 4 | |
| 2×1000 mg RTX 2×250 mg MP | 10 | Nephritis, arthritis, pleuritis | Nil | ||||
| 3×hA20 (375 mg/m2) 1×500 mg CYC 3×100 mg MP | 25 | Nephritis, haemolytic anaemia, thrombocytopenia | Partial depletion at 2 months | ||||
| 3 | F/52/A | 15 | 2×1000 mg RTX 2×750 mg CYC 2×60 mg Pred | Nephritis (IV), arthritis | Steroids, HCQ, AZA, CYC, MTX, CyA | 6 | |
| 2×1000 mg RTX 2×250 mg MP | 7 | Arthritis | 9 | ||||
| 1×1000 mg RTX 1×750 mg CYC 1×100 mg MP | 9 | Nephritis | 2 | ||||
| 4 | F/41/A | 11 | 2×1000 mg RTX 2×250 mg MP | Nephritis (IV) | Steroids, HCQ, CYC, AZA, MMF, MTX | 9 | |
| 2×1000 mg RTX 2×100 mg MP | 13 | Arthritis | Depleted at 2 months, then lost to follow up | ||||
| 5 | F/33/E | 14 | 2×1000 mg RTX 1×750 mg CYC 2×100 mg MP | Nephritis (IV) | Steroids, HCQ, AZA, CYC, MMF | 3 | |
| 2×1000 mg RTX 2×750 mg CYC 2×100 mg MP | 3 | Arthritis, rash, fever | 6 | ||||
| 6 | F/33/A | 18 | 2×1000 mg RTX 2×750 mg CYC 2×250 mg MP | Nephritis (V) | Steroids, HCQ, AZA, MMF, CYC | 4 | |
| 2×1000 mg RTX 2×750 mg CYC 2×100 mg MP | 11 | Nephritis | Depleted at 2 months | ||||
| 2×1000 mg RTX 2×750 mg CYC 2×250 mg MP | 12 | Nephritis (IV) | Partial depletion at 3 months | ||||
| 7 | F/42/A | 5 | 2×1000 mg RTX 2×750 mg CYC 2×100 mg MP | Arthritis | Steroids, HCQ, AZA, CYC, MTX, MMF | 3 | |
| 2×1000 mg RTX 2×100 mg MP | 12 | Arthritis | Partial depletion at 6 months |
E, European; A, Afro‐Caribbean; RTX, rituximab; CYC, cyclophosphamide; Pred, oral prednisolone; MP, methylprednisolone; hA20, humanised anti‐CD20 monoclonal antibody; HCQ, hydroxychloroquine; AZA, azathioprine; MMF, mycophenolate; MTX, methotrexate; CyA, ciclosporin A.
Routine immunosuppressive drugs were stopped before the first cycle except for hydroxychloroquine and oral steroids. However, mycophenolate was added in three patients (Nos 2, 3, and 6) after the third treatment cycle. Mycophenolate was introduced in patient 5 seven months after the second cycle. Patient 7 had methotrexate added 7 months after the initial cycle.
Results
A total of 18 cycles of treatment and up to three treatment cycles per patient were given. Table 1 shows the patient demographics and clinical indication for re‐treatment. Four patients (Nos 1, 2, 3, 6) had three cycles of treatment, whereas three patients (Nos 4, 5, 7) had two cycles. The mean time to re‐treatment was 13 months (range 3–31).
Clinical outcome after second treatment cycle
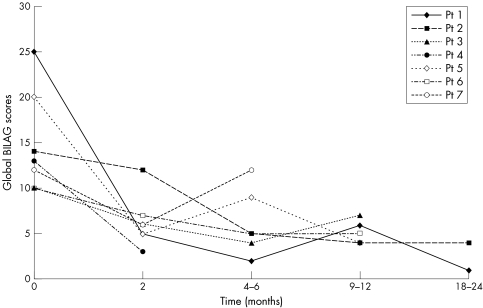
At 4–6 months, four of seven patients (Nos 1, 3, 5, 6) improved clinically. The mean BILAG global scores for all patients dropped from 15 to 6. The mean duration of response after this cycle was 13 months, with patient 3 relapsing at 9 months (fig 1). Patient 6 with nephritis improved with a decrease of the urinary protein/creatinine ratio from 1058 to 214 mg/mmol at 6 months. Patient 2 developed human chimeric antibodies but improved at 6 months with azathioprine and one pulse dose of cyclophosphamide. Patient 4 was lost to follow up at 3 months and patient 7 with severe polyarthritis did not improve with evidence of partial B cell depletion (CD19+ count 0.02×109/l) at 6 months.
Figure 1 Global BILAG scores after cycle 2 rituximab.
Laboratory data after second treatment cycle
In those patients who improved, there was a corresponding decrease of median anti‐dsDNA from 567 IU/l to 466 IU/l and increase of C3 from 0.65 g/l to 0.78 g/l at 6–8 months. From the available data of two patients who responded, serum immunoglobulins decreased but remained above the lower limit of normal at 6–8 months (mean IgG from 39 g/l to 36 g/l, mean IgA from 5.15 g/l to 4.7 g/l and mean IgM from 1.55 g/l to 1.15 g/l).
The mean duration of B cell depletion was 6 months (range 5–7) from available data in three patients.
Clinical outcome after third treatment cycle
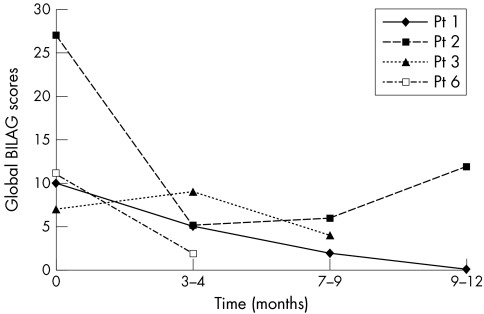
Two of four patients (Nos 1 and 2) improved, with global BILAG scores dropping from 10 to 2 and 27 to 6, respectively, at 7 months (fig 2). Patient 2 with human chimeric antibodies after the second cycle responded to the third cycle of a humanised anti CD20 antibody5 but relapsed a year later. Patient 1 has remained well without any immunosuppressive drugs 13 months later. Patient 3, with biopsy evidence of renal damage, progressed to renal failure shortly after her third cycle. Patient 6 with lupus nephritis improved with a decrease of the protein/creatinine ratio from 589 to 287 mg/mmol after mycophenolate at the last follow up at 4 months.
Figure 2 Global BILAG scores after cycle 3 rituximab.
Laboratory data after third treatment cycle
Data were available in one patient (No 1) at 4 and 12 months. At 4 months, anti‐dsDNA titres decreased from baseline 280 IU/l to 115 IU/l and C3 increased from 0.15 g/l to 0.5 g/l. Baseline serum IgA and IgG dropped from 2.5 g/l to 2.22 g/l and 12.1 g/l to 8.09 g/l, respectively. Serum IgM decreased below the normal range to 0.13 g/l at 4 months and remained low at 0.15 g/l at 12 months. This patient repopulated her B cells at 7 months.
Adverse events
Most patients tolerated re‐treatment very well. Patient 1 had a urinary tract infection after the second cycle, which resolved with antibiotics. Patient 2 had thoracic shingles during her third cycle (humanised anti‐CD20), which settled with intravenous aciclovir. Only one patient (No 3) had a mild serum sickness‐like reaction at the third cycle and refused to proceed with the second infusion.
Discussion
This study has demonstrated that repeated cycles of rituximab in combination with cyclophosphamide may provide a viable therapeutic option in managing lupus patients who are difficult to treat. Previous studies using rituximab with or without cyclophosphamide have provided very encouraging short term clinical improvement.1,3 These data have supported the use of rituximab in randomised controlled lupus trials which have started recently.
Clinical benefit was seen in at least 50% of patients after re‐treatment in this study. The lupus features that improved were heterogeneous with a corresponding serological benefit of decrease in anti‐dsDNA titres and rise of C3 levels. Serum immunoglobulins levels were well preserved except for a mild reduction of IgM with the third cycle in patient 1.
The duration of clinical benefit after each cycle was frequently longer than the period of B cell depletion. With the second cycle of rituximab, the mean duration of clinical response and B cell depletion was 13 months and 6 months, respectively. This response is also seen in patient 1 after the third cycle. This prolonged period of improvement has been seen in several patients with rheumatoid arthritis after one cycle of rituximab.6
Interestingly, of the four patients who improved after cycle 2 in our study, the mean duration of response was 13 months compared with an initial response of 7 months, suggesting that re‐treatment may have additional benefit.
Re‐treatment may be undertaken on relapse or as maintenance therapy. Maintenance therapy with full dose rituximab every 3 months was beneficial in two lupus patients with cerebral involvement as described in a case report.7 The long term effects of B cell depletion in lupus are unknown with particular concern of immunosuppression. Furthermore, a maintenance regimen may not be necessary if there is an extended period of benefit with each cycle.
The effects of rituximab in SLE appear to differ from those in patients with lymphoma, with a notably shorter period of B cell depletion. B cell repopulation is usually seen between 6 and 9 months in patients with lymphoma8 and in lupus, this can be as early as 3 months.1 This effect may be due to inherited or acquired deficiencies of complement9,10 and different pharmacokinetics of rituximab in patients with SLE.
Re‐treatment with B cell depletion in patients with lupus appears to be safe, with no significant infections seen in this study. A mild serum sickness‐like reaction was seen in patient 3. This occurrence has been reported in patients with primary Sjögren's syndrome treated with rituximab.11
B cell depletion re‐treatment may be an attractive option for patients with refractory lupus who relapse. This possibility will need to be clarified in future studies.
Acknowledgements
Dr Kristine Ng is a recipient of the Rose Hellaby scholarship.
Abbreviations
BILAG - British Isles Lupus Activity Guide
SLE - systemic lupus erythematosus
Footnotes
Competing interest: None.
References
- 1.Leandro M J, Edwards J C, Cambridge G, Ehrenstein M R, Isenberg D A. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum 2002462673–2677. [DOI] [PubMed] [Google Scholar]
- 2.Leandro M J, Cambridge G, Edwards J C, Ehrenstein M R, Isenberg D A. B cell depletion in the treatment of patients with systemic lupus erythematosus ‐ a longitudinal analysis of 24 patients. Rheumatology (Oxford) 2005441542–1545. [DOI] [PubMed] [Google Scholar]
- 3.Looney R J, Anolik J H, Campbell D, Felgar R E, Young F, Arend L J.et al B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose‐escalation trial of rituximab. Arthritis Rheum 2004502580–2589. [DOI] [PubMed] [Google Scholar]
- 4.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 5.Tahir H, Rohrer J, Bhatia A, Wegener W A, Isenberg D A. Humanized anti‐CD20 monoclonal antibody in the treatment of severe resistant systemic lupus erythematosus in a patient with antibodies against rituximab. Rheumatology (Oxford) 200544561–562. [DOI] [PubMed] [Google Scholar]
- 6.Leandro M J, Edwards J C, Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis 200261883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weide R, Heymanns J, Pandorf A, Koppler H. Successful long‐term treatment of systemic lupus erythematosus with rituximab maintenance therapy. Lupus 200312779–782. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin P, Grillo‐Lopez A J, Link B K, Levy R, Czuczman M S, Williams M E. Rituximab chimeric anti‐CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four‐dose treatment program. J Clin Oncol 1998162825–2833. [DOI] [PubMed] [Google Scholar]
- 9.Cartron G, Watier H, Golay J, Solal‐Celigny P. From the bench to the bedside: ways to improve rituximab. Blood 20041042635–2642. [DOI] [PubMed] [Google Scholar]
- 10.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E.et al Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol 20031711581–1587. [DOI] [PubMed] [Google Scholar]
- 11.Pijpe J, van Imhoff G W, Spijkervet F K, Roodenburg J L, Wolbink G J, Mansour K.et al Rituximab treatment in patients with primary Sjögren's syndrome: an open‐label phase II study. Arthritis Rheum 2005522740–2750. [DOI] [PubMed] [Google Scholar]