Abstract
Background
Primary Sjögren's syndrome (SS) is associated with an increased frequency of non‐Hodgkin's lymphomas (NHLs), mainly of low histological grade. However, aggressive diffuse large B cell lymphomas (DLBCL) characterised by poor treatment outcome can also be encountered in SS. It has recently been shown that rituxan has significant therapeutic activity in this type of lymphoma.
Objective
To evaluate the efficacy of CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone) in combination with rituxan in SS patients with DLBCL, and to determine the outcome in such patients.
Methods
In an open, single case trial, six SS patients with DLBCL were assigned to receive eight cycles of CHOP every three weeks plus rituxan given on day 1 of each cycle. In a retrospective study, conducted by the European Concerted Action for SS, nine cases were diagnosed as DLBCL, all of whom had been treated with CHOP alone. These patients were used as historical controls.
Results
The difference in the overall survival between the two treatment groups was significant. The group treated with rituxan plus CHOP had a 100% two year overall survival rate, while the historical controls had only a 37% survival rate. Extraglandular manifestations serving as predictors for lymphoma development such as palpable purpura and peripheral neuropathy disappeared. The remission of these signs was accompanied by a decrease in both circulating monoclonal cryoglobulins and rheumatoid factor activity and an increase in C4 levels. Clinically relevant toxicity was not detected.
Conclusions
The addition of rituxan to standard CHOP chemotherapy results in improved treatment outcome in SS patients with aggressive DLBCL, without increasing toxicity.
Keywords: Sjögren's syndrome, non‐Hodgkin's lymphoma, diffuse large B cell lymphoma
The risk of non‐Hodgkin's lymphomas (NHL) is 44 times greater in patients with primary Sjögren's syndrome than in the general population.1 Recent studies have shown that patients with Sjögren's syndrome who develop lymphoma present with specific predictor factors such as palpable purpura, low C4 levels, and mixed monoclonal cryoglobulinaemia.2
Lymphomas in Sjögren's syndrome fall into two main categories, the first relating to the majority of patients who develop an indolent extranodal marginal zone B cell type lymphoma, characterised by a prolonged overall survival of 6.4 years.3 The second category includes histologically aggressive lymphomas, such as diffuse large B cell lymphomas (DLBCL), which are only occasionally encountered in patients with Sjögren's syndrome. In a multicentre analysis conducted by the European Concerted Action on Sjögren's syndrome, nine of 33 patients developing lymphoid neoplasms which were classified as high grade had a comparatively poor overall survival of 1.8 years despite treatment with an anthracycline containing regimen.3 Data showing that combined treatment with anti‐CD20 monoclonal antibody (rituxan) plus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) had a significant therapeutic effect on patients with DLBCL4 prompted us to use this combination on Sjögren's syndrome patients with aggressive NHL. After a mean follow up period of 15 months, this proved to be both effective in achieving remission of lymphoma and safe in four patients with aggressive Sjögren's syndrome associated NHL.5 In this report, we provide further long term data on the continued follow up of these four patients as well as data obtained from a further two patients not previously included.
Methods
The study consisted of a single treatment group. Six female patients with Sjögren's syndrome who developed a DLBCL at a median of 5.5 years after the primary diagnosis, were included in the study. The median age of the patients was 52.5 years (range 37 to 74). All patients received a total of eight intravenous infusions of rituxan 375 mg/m2 and eight cycles of CHOP given every 21 days (cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 (maximum 2 mg) intravenously on day 1 and prednisone 100 mg on days 1 to 5). Methotrexate, 15 mg, was also given intrathecally for CNS prophylaxis. Aggressive DLBCL was diagnosed according to the newly proposed revised European‐American classification of lymphoid neoplasms.6 The following data was recorded: patients' age, sex, and performance status according to Eastern Cooperative Oncology Group (ECOG), disease stage according to the Ann Arbor criteria, location of extranodal disease, presence of B symptoms, serum lactate dehydrogenase, β2‐microglobulin levels, hepatitis C virus (HCV) infection serology, and International Prognostic Index (IPI) score.7 The IPI score is a widely accepted prognostic classification scheme based on five independent risk factors including age, stage, serum LDH, performance status, and the number of the extranodal sites involved. According to this, index patients are categorised into four groups with varying risk of death and five year overall survival rates: low, low‐intermediate, high‐intermediate, and high risk groups. Generalised symptoms such as fever over 38°C, night sweats, and weight loss of over 10% of body weight in the six months preceding diagnosis are classified as B symptoms.
The staging procedures included chest and abdominal imaging investigations, digestive tract endoscopic investigations, and bone marrow biopsy. Restaging was carried out before to the fifth cycle of treatment, at the end of treatment, and thereafter every six months. Complete remission of lymphoma was defined as the disappearance of all disease clinical evidence for at least six weeks, or more specifically, the normalisation of all laboratory values and radiographs that were abnormal at presentation and, if initially involved, the normalisation of bone marrow pathology. Overall survival was defined as the time from lymphoma diagnosis to death or last follow up. Sjögren's syndrome related variables including exocrine and systemic manifestations were recorded before and after treatment. Values of haematological, immunological, biochemical, and urine protein immunofixation assays, as well as serum levels of cryoglobulins, were similarly recorded. Response for Sjögren's syndrome was defined as subjective improvement in sicca symptoms, clinical features (parotid gland enlargement, extraglandular manifestations), and immunological features (monoclonal bands, anti‐SSA, anti‐SSB, rheumatoid factor (RF), cryoglobulins), as well as reduction in the degree of the lymphocytic infiltrates in the labial minor salivary glands. Neuropathic symptoms (pain and paraesthesiae) were graded according to a patient scored visual analogue scale (range 0–10), whereas neurological assessment also included strength evaluation.
Results
Tables 1 and 2 show the main clinical, serological and histopathological features of the six patients included in the study.
Table 1 Main clinical, serological, and histopathological features of Sjögren's syndrome associated lymphoma at diagnosis.
| Case 1† | Case 2† | Case 3† | Case 4 | Case 5 | Case 6† | |
|---|---|---|---|---|---|---|
| Age (y) | 62 | 37 | 51 | 45 | 74 | 54 |
| Type of lymphoma | Diffuse large B cell | Nodal marginal zone | Diffuse large B cell | Nodal marginal zone | Diffuse large B cell | Diffuse large B cell |
| Ann Arbor stage | IV | II | IV | II | II | IV |
| Performance status | 0 | 0 | 0 | 0 | 1 | 0 |
| B symptoms | − | + | − | − | − | + |
| Lymphadenopathy | + | + | − | + | + | + |
| Splenomegaly | + | − | − | − | + | + |
| β2‐Microglobulin (mg/l)* | 8.9 | 5 | 8.2 | 12 | 4.6 | 7.4 |
| Serum LDH level (U/l)‡ | 670 | 520 | 938 | 250 | 520 | 750 |
| Extranodal involvement | Salivary glands | Salivary glands | Lung, salivary glands | − | − | Salivary glands |
| Bone marrow involvement | + | − | + | − | − | + |
| IPI score | 4 | 1 | 3 | 0 | 2 | 3 |
*Increased β2‐microglobulin >3 mg/l.
†Previously reported cases.
‡Normal lactate dehydrogenase level: 200–475 U/l.
IPI, international prognostic index; y, years.
Table 2 Exocrine and systemic manifestations and serological profile of the study patients.
| Case 1† | Case 2† | Case 3† | Case 4 | Case 5 | Case 6† | |
|---|---|---|---|---|---|---|
| Age (y) | 62 | 37 | 51 | 45 | 74 | 54 |
| Exocrine signs/symptoms | BPGE, ED | BPGE, ED, OD | UPGE, ED, OD | ED, OD | ED, OD | BPGE, ED, OD |
| Systemic manifestations | Arthralgia, purpura, peripheral oedema, lymphadenopathy | Arthralgia, purpura, neuropathy, anaemia, lymphadenopathy | Arthralgia, anaemia | Purpura, lymphadenopathy, proteinuria, chronic renal failure | Arthralgia, lymphadenopathy, dyspnoea | Arthralgia, anaemia, purpura, neuropathy, lymphadenopathy |
| ANA titre | 1/2560 | 1/640 | 1/160 | 1/1280 | 1/320 | 1/160 |
| Anti‐Ro/La positivity | +/− | +/− | +/+ | +/+ | +/+ | +/− |
| RF (IU) | 640 | 640 | 80 | 640 | Negative | 80 |
| C3/C4 (mg/dl) | 102/2.8 | 87/16 | 126/24 | 86.6/12.7 | 82/30 | 107/4 |
| Cryoglobulinaemia (type, mg/dl) | Type II IgMk 150 | Type II IgMk 320 | Negative | Negative | Negative | Type II IgMk 40 |
| Previous immunosuppressive steroid therapy* | Steroid | Steroid, cyclophosphamide | Steroid | Steroid, cyclophosphamide | Steroid | Steroid, MTX |
*Given during the disease course for the extraglandular manifestations before lymphoma development.
†Previously reported cases.
ANA, antinuclear antibody; BPGE, bilateral parotid gland enlargement; ED, eye dryness; MTX, methotrexate; OD, oral dryness; RF, rheumatoid factor; UPGE, unilateral parotid gland enlargement; y, years.
Three patients displayed bilateral parotid enlargement but bulky disease (tumour diameter ⩾10 cm) was not documented. All patients had extraglandular systemic manifestations of Sjögren's syndrome, including arthralgia, lymphadenopathy, purpura, peripheral neuropathy, anaemia, and low C4 levels. Two patients also had B symptoms associated with lymphoma development. A serum monoclonal component and mixed monoclonal cryoglobulinaemia containing an IgMk monoclonal component was detected in two and three patients, respectively. HCV serology was negative in all cases.
All patients were previously treated for Sjögren's syndrome manifestations as follows. All six patients had received low dose prednisone. Two patients received four pulses of intravenous cyclophosphamide (750 mg/m2) for the treatment of cryoglobulinaemia type II presenting with peripheral neuropathy and membranoproliferative glomerulonephritis. One patient with severe arthritis had been treated with methotrexate (12.5 mg/week for two years). Three patients were classified as high or high to intermediate risk according to the IPI score. In three patients, the lymphoma involved loco‐regional nodes adjacent to the primary extranodal site (stage II), while the remaining three presented with stage IV disseminated disease. The serum LDH level was raised in five of six patients. Tissue biopsies showed DLBCL in all patients—an aggressive lymphoma which is often characterised by resistance to conventional treatment. Histological evidence was compatible with a high grade transformation of a mucosa associated lymphoid tissue (MALT) lymphoma in three cases (1, 3, 6) and of a nodal marginal zone B cell lymphoma in two (2, 4). The high grade transformation in these patients was documented by the presence of solid clusters (>20 cells) or sheet‐like proliferation of large lymphoma cells. One patient displayed a de novo DLBCL (case 5). CD20 was strongly expressed by tumour cells in all cases. The lymphoma cells were also bcl‐2 positive. Cytogenetic analysis of fresh tumour sample for evaluation of chromosomal abnormalities such as t(11,18) or t(14,18) was not undertaken.
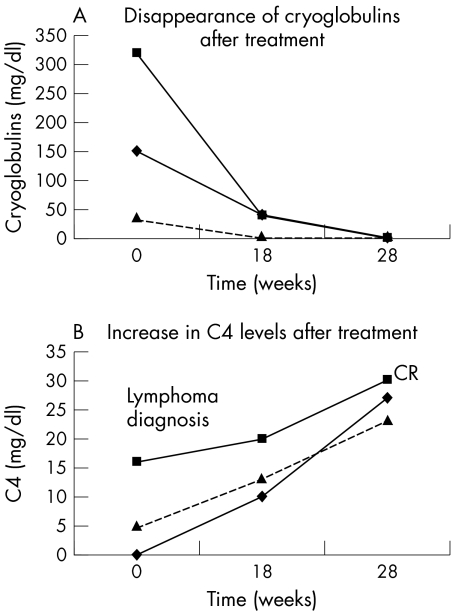
All patients experienced a sustained complete remission for 10 to 35 months (median 23 months) after treatment. Extraglandular manifestations such as peripheral neuropathy (case 2, 6) and cutaneous vasculitis (case 1, 2, 6) showed a gradual improvement after three to five courses of chemotherapy, and by the end of treatment had finally disappeared. Noticeably, peripheral neuropathy improved in spite of the use of the potentially neurotoxic agent vincristine. The remission of these signs was accompanied by both decreased circulating monoclonal cryoglobulins and RF activity and increased C4 levels (fig 1, panels A and B). RF levels were increased in five patients (cases 1, 2, 3, 4, and 6). Three of these five had reduction of the RF levels after treatment: cases 1 and 2 showed a reduction from 640 IU to 160 IU and case 6 a reduction from 80 IU to 40 IU. Sicca symptoms tested by a specific questionnaire before and after treatment showed no improvement.8 Twelve months after the end of treatment, labial minor salivary gland biopsies were repeated in two patients. No significant alterations from the pretreatment histological characteristics were noted. Lymphoepithelial lesions were present. Focal lymphocytic infiltrates and plasma cells were present in the interfollicular areas. Reactive follicles were also detected without expansion of the marginal zones. Autoantibody profile including antinuclear, anti‐SSA, and anti‐SSB antibodies remained unchanged. Two episodes of fever and shivers were noted during rituxan infusions and resolved without interrupting the infusion. Significant post‐infusion reactions, such as Stevens–Johnson syndrome, serum sickness, skin vasculitis, bronchospasm, or hypotension, were not observed in our patients. The absence of these severe adverse reactions can be attributed to the co‐ administration of high doses of corticosteroids. CHOP toxicity was manifested by alopecia (six patients), grade III neutropenia (neutrophils ⩽1000/mm3) (five patients), and neutropenic fever (two patients).
Figure 1 Reduction of serum cryoglobulins (A) and normalisation of C4 levels (B) during treatment with rituxan/CHOP in three patients with Sjögren's syndrome and diffuse large B cell lymphoma (cases 1, 2, and 6). CR, complete remission of the lymphoma.
Discussion
This report shows the safety and efficacy of B cell depletion treatment (rituxan) in combination with standard dose CHOP in the management of high risk aggressive DLBCL in Sjögren's syndrome. Owing to the rarity of this type of lymphoma in Sjögren's syndrome, the number of patients in our study was limited. Nonetheless the ability of anti‐CD20/CHOP to achieve sustained remissions is evident and most promising. Two large phase III studies in elderly non‐Sjögren's syndrome patients with DLBCL showed improved survival resulting from the addition of rituxan to CHOP.4,9 Furthermore, a recent study in younger patients suggested an equally beneficial effect.10 As a result, rituxan/CHOP has been adopted as standard treatment for patients with advanced stage DLBCL. The precise mechanism by which rituxan induces anti‐tumour or anti‐B‐cell effects is not clearly understood. It has been shown that accelerate apoptosis, complement mediated cell lysis, and antibody dependent cell mediated cytotoxicity are participants in this effect.11 The chronic antigenic stimulation in exocrine glands, and most importantly the high B cell activating factor levels which are especially associated with Sjögren's syndrome, have occasionally been implicated in lymphoma development.12 In addition, Sjögren's syndrome associated NHLs are characterised by several genetic aberrations which may lead to disturbances of the apoptotic pathway. In this regard, it was interesting that in patients with Sjögren's syndrome, rituxan plus CHOP appeared effective, despite the fact that apoptosis inhibition could have impaired the pro‐apoptotic effect of rituxan.
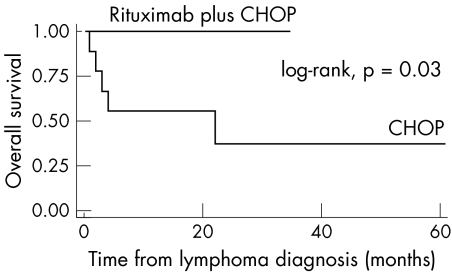
In 1999 the European Concerted Action on Sjögren's syndrome assessed the characteristics, clinical course, and evolution of 33 Sjögren's syndrome patients with NHL registered in nine European medical centres.3 In this study, nine patients with aggressive DLBCL were detected, all of whom had been treated with CHOP. During a median follow up of 6.33 years, seven events were recorded (two relapses and five deaths) with a median overall survival estimated at only 1.83 years. Using these patients as historical controls, we compared the efficacy of rituxan addition to standard CHOP regimen. The difference in overall survival between the two treatment groups is statistically significant (p = 0.03) (fig 2). The two year overall survival rate of the group treated with rituxan plus CHOP is 100%, while historical controls displayed an overall survival of only 37%. It is well accepted by the International Lymphoma Committees that in DLBCL, the clinical, prognostic, and molecular heterogeneity in patients is a limiting factor for the evaluation of specific treatment protocols. This problem has now been partially solved with the use of the IPI. The lack of certain clinical indices in the previous multicentre, retrospective study prevented us from categorising the historical controls according to the IPI. Therefore, the heterogeneity of ethnicity, clinical presentation, and response to treatment of historic controls and our patients is a drawback in this non‐randomised study. However, all our patients were categorised according to IPI. The fact that half of these patients belong to the poor‐risk IPI indicates that their favourable event‐free and overall survival could not be attributed to the absence of adverse prognostic factors.
Figure 2 Overall survival: Sjögren's syndrome patients with diffuse large B cell lymphoma treated with CHOP or with CHOP plus rituxan.
Our department suggested a predictive classification of Sjögren's syndrome into two distinct categories, each carrying a varying risk of lymphoma development.2,13,14 Patients with low C4 levels or palpable purpura, or both, are classified as high risk (“type I SS”), while those without (80% of all primary Sjögren's syndrome diagnoses) are classified as low risk (“type II SS”) with negligible potential for lymphoma development. The B cell CD20 antigen possesses certain characteristics that make it an appealing target for monoclonal antibody treatment. It is present on the surface of RF positive mature B cells which produce monoclonal cryoglobulins with RF activity, responsible for manifestations such as palpable purpura, peripheral neuropathy, and low serum C4 levels. However, it is neither present on B cell precursors, thus allowing B cell repopulation after B cell depletion, nor on long lived plasma cells which constitute the source of autoantibody production. In our study, “type I” manifestations (purpura, neuropathy, low C4 levels, and serum cryoglobulins) evident before lymphoma development remitted following rituxan plus CHOP treatment.
The efficacy of B cell depletion therapy against RF positive B cell clones composing the neoplastic counterpart of lymphoma development explains the remission of these “type I” characteristics in our patients.15 Conversely, antinuclear, anti‐SSA, and anti‐SSB remained unaffected. These autoantibodies are produced by short lived CD20 positive plasmablasts and long lived CD20 negative plasma cells, both of which are considered responsible for long term autoimmunity and “type II” profile. While anti‐CD20 antibody depletes short lived plasmablasts, long lived plasma cells survive and continue to produce these autoantibodies. According to recent experimental evidence, the homeostasis and life time of long lived plasma cells is determined by their molecular microenvironment and is regulated by their competition with early B stage cells for specific survival niches such as bone marrow, spleen, lamina propria, and inflamed tissues.16 This competition provides another mechanism for the regulation of humoral immune responses. In this context, the depletion of the CD20 positive B cells—which are the source of both plasmablasts and plasma cells—by rituxan may result in even better survival conditions for autoreactive plasma cells that already exist, by minimising their competition for survival signals. Consequently, the plasma cells formed last, producing autoreactive antibodies, suffer less competitive pressure to find survival niches and can maintain long term autoimmunity. This appealing theory coincides with the persistence of the autoantibodies in our patients. In addition, it may also explain the persistence of scattered plasma cells in the infiltrate of the salivary glands of two of our patients who were re‐subjected to biopsy after rituxan treatment.
Our results do not coincide with a previous report in which Sjögren's syndrome associated histopathological and functional manifestations improved after rituxan monotherapy. These reports included recently diagnosed Sjögren's syndrome patients with or without low grade MALT lymphomas.17,18 Nevertheless, our patients were characterised by longstanding Sjögren's syndrome and suffered from high grade lymphomas. Furthermore, the four weekly doses of rituxan in these studies differ from our one dose of rituxan every three weeks (eight doses in all).
The lack of efficacy of rituxan plus CHOP in improving the minor salivary gland histopathological lesions and subjective sicca symptoms implies that these lesions constitute a sanctuary site from rituxan penetration.19 The efficacy of rituxan may also be diminished when the pathogenesis of the condition provides B cells with additional stimulatory and prosurvival signals.20 The in vivo susceptibility to rituxan of B cells in Sjögren's syndrome may be influenced by their environmental cells, such as local activated T cells, as cytokine and subsequent “second signals” will potentially oppose the proapoptotic effects of rituxan.
In conclusion, this study reconfirms the two “faces” of Sjögren's syndrome and indicates that the rituxan plus CHOP regimen significantly improves overall survival of patients with this disease who develop poor risk aggressive DLBCL in comparison with those treated with CHOP alone.
Abbreviations
CHOP - cyclophosphamide, doxorubicin, vincristine, prednisone
DLBCL - diffuse large B cell lymphoma
IPI - International Prognostic Index
NHL - non‐Hodgkin's lymphoma
RF - rheumatoid factor
SS - Sjögren's syndrome
References
- 1.Kassan S S, Thomas T L, Moutsopoulos H M, Hoover R, Kimberly R P, Budman D R.et al Increased risk of lymphoma in sicca syndrome. Ann Intern Med 197889888–892. [DOI] [PubMed] [Google Scholar]
- 2.Ioannidis J P, Vassiliou V A, Moutsopoulos H M. Long‐term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren's syndrome. Arthritis Rheum 200246741–747. [DOI] [PubMed] [Google Scholar]
- 3.Voulgarelis M, Dafni U G, Isenberg D A, Moutsopoulos H M. Malignant lymphoma in primary Sjögren's syndrome. Arthritis Rheum 1999421765–1772. [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R.et al CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med 2002346235–242. [DOI] [PubMed] [Google Scholar]
- 5.Voulgarelis M, Giannouli S, Anagnostou D, Tzioufas A G. Combined therapy with rituximab plus cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) for Sjögren's syndrome‐associated B‐cell aggressive non‐Hodgkin's lymphomas. Rheumatology (Oxford) 2004431050–1053. [DOI] [PubMed] [Google Scholar]
- 6.Harris N L, Jaffe E S, Stein H, Banks P M, Chan J K, Cleary M L.et al A revised European–American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994841361–1392. [PubMed] [Google Scholar]
- 7. A predictive model for aggressive non‐Hodgkin's lymphoma. The International Non‐Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993329987–994. [DOI] [PubMed] [Google Scholar]
- 8.Vitali C, Bombardieri S, Moutsopoulos H M, Bolestrieri G, Bencivelli W, Bernstein R M.et al Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 199336340–347. [DOI] [PubMed] [Google Scholar]
- 9.Vose J M, Link B K, Grossbard M L, Czuczman M, Grillo‐Lopez A, Gilman P.et al Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non‐Hodgkin's lymphoma. J Clin Oncol 200119389–397. [DOI] [PubMed] [Google Scholar]
- 10.Pfreundschuh M G, Trumper L, Ma D.et al Randomized intergroup trial of first line treatment for patients ⩽60 years with diffuse large B‐cell non‐Hodgkin's lymphoma with a CHOP‐like regimen with or without the anti‐CD20 antibody rituximab—early stopping after the first interim analysis. Proc ASCO; 2004556a
- 11.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri G M, Bernasconi S.et al Biologic response of B lymphoma cells to anti‐CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement‐mediated cell lysis. Blood 2000953900–3908. [PubMed] [Google Scholar]
- 12.Groom J, Kalled S L, Cutler A H, Olson C, Woodcock S A, Schneider P.et al Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J Clin Invest 200210959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzioufas A G, Boumba D S, Skopouli F N, Moutsopoulos H M. Mixed monoclonal cryoglobulinemia and monoclonal rheumatoid factor cross‐reactive idiotypes as predictive factors for the development of lymphoma in primary Sjögren's syndrome. Arthritis Rheum 199639767–772. [DOI] [PubMed] [Google Scholar]
- 14.Skopouli F N, Dafni U, Ioannidis J P A, Moutsopoulos H M. Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin Arthritis Rheum 200029296–304. [DOI] [PubMed] [Google Scholar]
- 15.Zaja F, De Vita S, Mazzaro C, Sacco S, Damiani D, De Marchi G.et al Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood 20031013827–3834. [DOI] [PubMed] [Google Scholar]
- 16.Manz R A, Arce S, Cassese G, Hauser A E, Hiepe F, Radbruch A. Humoral immunity and long‐lived plasma cells. Curr Opin Immunol 200214517–521. [DOI] [PubMed] [Google Scholar]
- 17.Pijpe J, van Imhoff G W, Vissink A, van der Wal J E, Kluin P M, Spijkervet F K.et al Changes in salivary gland immunohistology and function after rituximab monotherapy in a patient with Sjögren's syndrome and associated MALT lymphoma. Ann Rheum Dis 200564958–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pijpe J, van Imhoff G W, Spijkervet F K, Roodenburg J L, Wolbink G J, Mansour K.et al Rituximab treatment in patients with primary Sjögren's syndrome: an open‐label phase II study. Arthritis Rheum 2005522740–2750. [DOI] [PubMed] [Google Scholar]
- 19.Raderer M, Jager G, Brugger S, Puspok A, Fiebiger W, Drach J.et al Rituximab for treatment of advanced extranodal marginal zone B cell lymphoma of the mucosa‐associated lymphoid tissue lymphoma. Oncology 200365306–310. [DOI] [PubMed] [Google Scholar]
- 20.Silverman G J, Weisman S. Rituximab therapy and autoimmune disorders: prospects for anti‐B cell therapy. Arthritis Rheum 2003481484–1492. [DOI] [PubMed] [Google Scholar]