Abstract
Objectives
To determine the impact on synovial histopathology of changes in clinical disease activity in the absence of effective treatment.
Methods
Twelve patients with active RA not receiving effective treatment were studied over a 14 week period. Synovial biopsy specimens obtained at baseline and week 14 were analysed by histology and immunohistochemistry.
Results
Over the course of 14 weeks, there was a trend towards a decrease of the DAS28, with 7/12 patients being good or moderate DAS28 responders despite the absence of effective treatment. Patients' assessment of global disease activity and swollen joint count both decreased significantly. Histologically, there was a decrease of lining layer hyperplasia and lymphoid aggregates, a similar trend for vascularity, but there was no effect on global synovial infiltration. Accordingly, there was no decrease of the cellular infiltration with T lymphocytes (CD3, CD4, CD8), B lymphocytes (CD20), plasma cells (CD38), dendritic cells (CD1a, CD83), and even an increase of CD163+ sublining macrophages, with a similar trend for CD68+ sublining macrophages. The changes in DAS28 scores in these patients did not correlate with changes in histological variables, with the exception of an inverse correlation with plasma cells. Remarkably, even in the DAS28 responders, no significant changes in synovial inflammatory infiltration were noted.
Conclusions
Despite variations in global disease activity, synovial inflammatory infiltration did not change significantly in the absence of effective treatment. The lack of a placebo effect on synovial markers of treatment response such as sublining macrophages can facilitate conclusive early phase trials with small numbers of patients with RA.
Keywords: synovitis, rheumatoid arthritis, histopathology, biomarker, response to treatment
Over the past couple of years, a number of highly effective targeted treatments have become available for the treatment of rheumatoid arthritis (RA) as exemplified by the different tumour necrosis factor α blocking agents. Despite the major impact of these treatments on signs and symptoms, quality of life, and disease progression, the incomplete response and potential adverse events in a subset of patients warrant further exploration and validation of new therapeutic approaches. The efficacy and widespread availability of biological agents, however, have made it medically and ethically difficult to evaluate newer drugs in large, placebo controlled, long term trials.1 One possible way to deal with this new challenge, especially in the case of targeted treatments, is to include biological markers of response in early phase trials in order to obtain a proof of principle in small patient cohorts treated for a short period of time before going on to larger, placebo controlled trials.2
Sequential synovial biopsy sampling has made it possible to study treatment response directly at the primary site of inflammation in RA: the synovial membrane. A large number of studies have now convincingly demonstrated that effective treatment in RA is associated with a rapid and significant decrease of global synovial inflammation as assessed by infiltrating leucocytes and expression of adhesion molecules, cytokines, chemokines, and matrix metalloproteinases.3 Recently, Gerlag et al demonstrated in a 2 week prednisolone study that a variety of synovial features were down regulated by effective treatment; sublining macrophages appeared to show the most pronounced changes.4 Subsequently, an analysis of 111 patients with RA confirmed that changes in sublining macrophages consistently reflected changes in the Disease Activity Score (DAS) across 10 different therapeutic regimens.5
A crucial aspect of the use of sequential synovial analysis as biomarker in proof of principle trials is its ability to discriminate between genuine biological effects and placebo responses or natural variations of disease activity during the disease course in small to intermediate patient cohorts. Several studies including between four and 12 placebo treated patients2,4,6,7 or patients treated with ineffective therapeutic regimens8 showed the absence of consistent synovial changes during stable treatment with disease modifying antirheumatic drugs (DMARDs). Moreover, in the previously mentioned analysis of sublining macrophages as synovial biomarkers,5 the standardised response means suggested that changes in sublining macrophages were less sensitive to placebo effects than changes in the DAS28.
We aimed at investigating in more detail if synovial histopathology is better than clinical assessments in discriminating between placebo responses and genuine immunomodulation and may thus contribute to size reduction of early phase clinical trials. Therefore, the present study investigated the relationship between clinical and local histopathological variables of disease activity occurring over time in the absence of effective treatment or DMARD. We demonstrate here that changes in DAS28 do not correlate with changes in synovial biomarkers in the absence of effective treatment, even in those patients having a spontaneous good or moderate response according to the EULAR response criteria; this is in contrast with the correlation seen during effective treatment. The lack of placebo effect on synovial markers of treatment response such as sublining macrophages can facilitate conclusive early phase trials with small numbers of patients with RA.
Patients and methods
Patients
Twelve patients were enrolled in the study after written informed consent, as approved by the ethics committee of Ghent University Hospital. The patients were participating in a large double blind, placebo controlled phase II trial which failed to show that the experimental compound was better than placebo for reducing both disease activity and radiographic joint damage.9 The main efficacy assessments used in the phase II trial were the seven measures of the American College of Rheumatology (ACR) core set of disease activity measures for RA clinical trials.10 The DAS28 served as the primary efficacy measure. Secondary efficacy measures included the individual measures of the ACR core set and the ACR composite RA response measures: ACR20, ACR50, and ACR70.11 The DAS28 scores and the percentages of ACR responders at the end point were comparable for all treatment groups, including placebo, and no notable effects on clinical RA variables and potential surrogate measures were seen.
The study cohort of the histological substudy comprised eight men and four women fulfilling the ACR classification criteria for RA,12 of whom, nine were treated with the ineffective compound and three received placebo. The median duration of symptoms was 3 years (range 1–8) and the median disease duration since diagnosis was 1 year (range 0.2–7). Nine patients were rheumatoid factor positive. At baseline, the median DAS28 was 5.8 (range 3.9–7.2), the median patient's global assessment of disease activity on a 100 mm visual analogue scale was 67 (range 31–88), the median swollen joint count (SJC) was 14.5 (range 6–22), the median tender joint count (TJC) was 10 (range 5–18), and the median erythrocyte sedimentation rate (ESR) was 20 mm/1st h (range 4–56). All patients had at least one swollen knee joint. None of the patients was being treated with corticosteroids or DMARDs 4 weeks before and during the study period; six patients were treated with stable doses of non‐steroidal anti‐inflammatory drugs.
Synovial histopathology
In all patients, synovial tissue biopsy specimens (16 in each patient at each time point) were obtained by needle arthroscopy from the clinically affected knee joint at baseline and from the same joint after 14 weeks.13 Joint lavage during the arthroscopic procedure was restricted to a minimum (<150 ml) and no intra‐articular steroids were injected in order not to influence the local disease activity. Eight biopsy specimens were embedded in paraffin, cut to 5 μm sections, and used for classical histological evaluation as extensively described previously.13,14,15 The following synovial features were assessed: synovial lining layer thickness, vascularity of the sublining layer, infiltration of the sublining layer, lymphoid aggregates, plasma cells, and polymorphonuclear cells. Because the lining layer thickness is not homogeneous but shows large variations from one region of the synovium to another, we used the mean of three scores obtained from different biopsies.
Immunohistochemistry
The remaining eight biopsy specimens were embedded in tissue freezing medium and stained as extensively described previously.13,14,15 Frozen sections (5 μm) were used for immunohistochemistry with the following commercially available monoclonal antibodies (mAbs): anti‐CD3 (T cells, clone UCHT1, Dako, Glostrup, Denmark), anti‐CD4 (T helper cells, clone MT310, Dako), anti‐CD8 (T cytotoxic cells, clone DK25, Dako), anti‐CD20 (B cells, clone L26, Dako), anti‐CD38 (plasma cells, clone AT13/5, Dako), anti‐CD68 (pan‐macrophage marker, clone PG‐M1, Dako), anti‐CD163 (mature macrophage marker, clone Ber‐MAC3, Dako), anti‐CD83 (dendritic cells, clone HB15A, Immunotech SA, Marseille, France), anti‐CD1a (interdigitating dendritic cells, clone NA1/34, Dako), anti‐E‐selectin (CD62E, endothelial leucocyte adhesion molecule 1 mainly expressed on activated endothelial cells, clone 1.2B6, Dako), anti‐ICAM‐1 (CD54, intercellular adhesion molecule 1, clone 6.5B5, Dako), and anti‐VCAM‐1 (CD106, vascular cell adhesion molecule 1, clone 1.4C3, Dako). Intracellular citrullinated proteins were detected with a polyclonal rabbit antibody to l‐citrulline (Biogenesis, Poole, UK) as described previously.15,16 The RA autoantigen human cartilage glycoprotein‐39 (HC gp‐39) and the complex of the immunodominant epitope of HC gp‐39, peptide 263–275 with HLA‐DR4 molecules (major histocompatibility complex/peptide complexes as detected by mAb 12A) were detected with mAbs provided by NV Organon (Oss, The Netherlands), as described previously.17,18,19 Parallel sections were incubated with irrelevant, isotype and concentration matched mAb as negative control.
Semiquantitative scoring
Stained sections were coded and analysed by two independent observers, who were unaware of the patient data, time of biopsy sampling (baseline or week 14), and patient code (sections were not scored in pairs). The analysis included all areas of the biopsy specimens, and a global score was given for each variable, using a semiquantitative four point scale14,15,20,21: 0 represented the lowest and 3 the highest level of expression. As some histological markers were more abundant than others, the scoring system was calibrated for each marker separately by examining a representative number of samples. When the observers disagreed about the scores, the mean of the two scores was used. For expression in the lining layer, the number of positive cells was compared with the total number of cells in the lining layer. Semiquantitative scoring was previously shown to generate results similar to those obtained by manual counting and digital image analysis.22,23
Statistics
Results are expressed as medians (range). Comparisons between baseline and week 14 were performed with the paired, non‐parametric Wilcoxon's signed ranks test. Correlations were calculated with Spearman's correlation (rs) test. A value of p<0.05 was considered significant.
Results
Changes in clinical disease activity in the absence of effective treatment
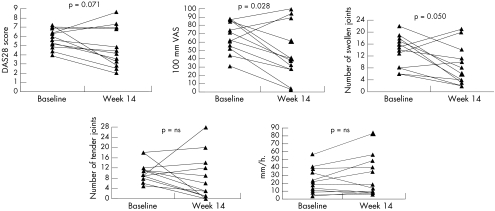
The 12 patients with RA included in the study were evaluated at baseline and week 14 (fig 1). Over this study period, there was a trend towards decrease of the DAS28 from 5.8 (3.9 to 7.2) at baseline to 4.3 (2.0 to 8.6) at week 14 (p = 0.071). The median change in DAS28 was −0.9 (−4.3 to 2.3), with a decrease in 10 patients and an increase in two patients. According to the EULAR response criteria based on the DAS28,24 three patients were good responders (improvement >1.2 and DAS28 at week 14 ⩽3.2), four patients were moderate responders (improvement >0.6 and ⩽1.2 and DAS28 at week 14 ⩽5.1, or improvement >1.2 and DAS28 at week 14 >3.2), and five patients were non‐responders.
Figure 1 Changes in disease activity in 12 patients with RA over the 14 week study period as assessed by the DAS28 and its individual components: the patient's global assessment of disease activity (scored on a 100 mm visual analogue scale), the swollen joint count, the tender joint count, and the erythrocyte sedimentation rate. A significant decrease was seen for the patient's global assessment of disease activity and the swollen joint count, with a similar trend for the DAS28. The p values were calculated with Wilcoxon's signed ranks test. NS = non‐significant.
For the individual components of the DAS28 score (fig 1), there was a clear decrease of the patient's global assessment of disease activity from 67 (31 to 88) at baseline to 39 (3 to 100) at week 14 (p = 0.028), with a similar decrease in SJC from 14.5 (6 to 22) at baseline to 7 (2 to 21) at week 14 (p = 0.050). The TJC did not change significantly, with a score of 10 (5 to 18) at baseline and 4.5 (0 to 28) at week 14 (p = 0.180). There was no change in the ESR from 20 mm/1st h (4 to 56) at baseline to 16.5 mm/1st h (6 to 83) at the end of the study (p = 0.194). Additionally, neither changes in the DAS28 score nor changes in the individual components showed any trend towards difference between the patients treated with the ineffective compound (n = 9) and patients treated with placebo (n = 3).
Changes in synovial histopathology
Synovial biopsy specimens obtained from a single, clinically affected knee joint at baseline and week 14 were compared in pairs (table 1). Histologically, there was a decrease of lining layer thickness (p = 0.010) and of the lymphoid aggregates (p = 0.040) between baseline and week 14, with a similar trend for the degree of vascularity (p = 0.064). In contrast, there was no change in the global degree of inflammatory infiltration, the number of plasma cells, and the number of polymorphonuclear cells as assessed by classical histological analysis (fig 2).
Table 1 Histological and immunohistochemical characterisation of the inflamed synovium in 12 patients with RA at baseline and at week 14.
| Variable | Baseline | Week 14 | p Value |
|---|---|---|---|
| Lining layer thickness | 2 (1–3) | 1 (1–1.5) | 0.010 |
| Vascularity | 2 (1–3) | 1.25 (0.5–2.5) | 0.064 |
| Lymphoid aggregates | 0.5 (0–3) | 0 (0–1) | 0.040 |
| Inflammatory infiltration | 1.5 (0–3) | 1 (0–3) | 0.254 |
| Plasma cells | 0 (0–2.5) | 0.5 (0–2.5) | 0.518 |
| Polymorphonuclear cells | 0 (0–1) | 0 (0–15) | 0.157 |
| CD3+ T lymphocytes | 1 (0–3) | 1.5 (0–2) | 1.000 |
| CD4+ T lymphocytes | 0.5 (0–2.5) | 1 (0–1.5) | 0.773 |
| CD8+ T lymphocytes | 1 (0–2.5) | 1 (0–2.5) | 0.732 |
| CD20+ B lymphocytes | 1.5 (0–3) | 1.5 (0–2.5) | 0.226 |
| CD38+ plasma cells | 1 (0–3) | 1 (0–3) | 1.000 |
| Lining CD68+ macrophages | 1 (0.5–2) | 1 (0.5–2.5) | 0.332 |
| Sublining CD68+ macrophages | 1 (0.5–3) | 2 (0.5–3) | 0.066 |
| Lining CD163+ macrophages | 1.5 (0.5–3) | 1 (0.5–2.5) | 0.524 |
| Sublining CD163+ macrophages | 1 (0–2) | 1.25 (0–2.5) | 0.021 |
| CD1a+ dendritic cells | 1 (0–2.5) | 0.5 (0–3) | 0.832 |
| CD83+ dendritic cells | 0 (0–3) | 0 (0–2) | 0.131 |
| Lining ICAM‐1 | 2 (1–3) | 2 (0.5–3) | 0.590 |
| Sublining ICAM‐1 | 0.5 (0–3) | 1 (0–3) | 0.798 |
| Endothelial ICAM‐1 | 2 (1.5–3) | 2.5 (0.5–3) | 0.952 |
| Endothelial E‐selectin | 2 (2–3) | 2 (0–3) | 0.203 |
| Lining VCAM‐1 | 1.5 (1–3) | 1.5 (0–2.5) | 0.298 |
| Sublining VCAM‐1 | 0.5 (0–2) | 0.5 (0–2) | 0.831 |
| Endothelial VCAM‐1 | 0 (0–1) | 0 (0–0.5) | 0.480 |
| Intracellular citrullinated proteins | 0 (0–2.5) | 0 (0–3) | 0.180 |
| HC gp‐39 | 1 (0–2.5) | 0 (0–2.5) | 0.301 |
| mAb 12A staining | 0 (0–2) | 0 (0–3) | 0.276 |
The synovial features were scored semiquantitatively on a 0–3 scale (0 is the lowest score and 3 is the highest score) and data are represented as median (range). The p values were calculated with Wilcoxon's signed ranks test.
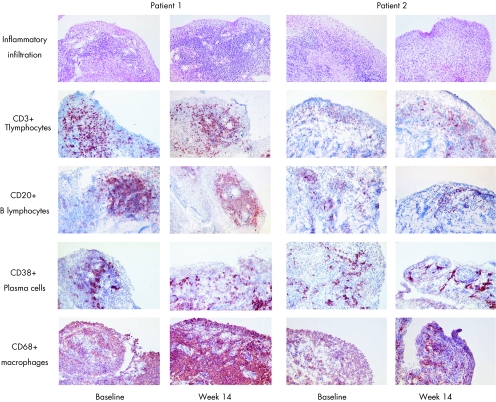
Figure 2 Changes in synovial inflammatory infiltration over a 14 week period. Representative pictures are shown from two patients with RA at baseline and week 14 for global inflammatory infiltration as assessed on haematoxylin and eosin staining and immunohistochemistry (positive cells are stained red‐brown) for CD3+ T lymphocytes, CD20+ B lymphocytes, CD38+ plasma cells, and CD68+ macrophages.
Further characterisation of the infiltrating cells by immunohistochemistry confirmed this finding (table 1, fig 2). For the lymphocyte populations, there were no significant changes in CD3+ T lymphocytes, CD4+ T lymphocytes, CD8+ T lymphocytes, CD20+ B lymphocytes, and CD38+ plasma cells. Macrophages, detected either with the pan‐macrophage marker CD68 or with the CD163 scavenger receptor expressed on resident tissue macrophages,24,25 did not change in the synovial lining layer and even increased in the sublining layer (p = 0.066 for CD68 and p = 0.021 for CD163). There were no alterations in the number of dendritic cells as detected by CD1a and CD83 staining.
For the adhesion molecules ICAM‐1, E‐selectin, and VCAM‐1, there were no significant changes over the 14 week study period. Finally, there was no alteration of the expression of the candidate RA autoantigen intracellular citrullinated proteins and HC gp‐39, or of the presentation of the immunodominant peptide of HC gp‐39 by HLA‐DR4 as detected by mAb 12A staining.
As for the clinical assessments, none of the changes in histopathological measures between baseline and week 14 differed between the patients treated with the ineffective compound (n = 9) and the patients treated with placebo (n = 3). Moreover, when the data of the synovial histopathology were analysed more conservatively with the application of a Bonferroni correction for multiple testing, no difference at all was seen between baseline and week 14.
Correlations between changes in synovial measures and clinical disease activity
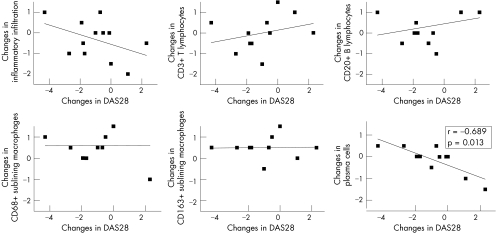
Because changes in markers of synovial inflammatory infiltration were previously shown to correlate with changes in clinical disease activity upon effective treatment,3,4,5 we analysed if these synovial changes also paralleled changes in DAS28 occurring in the absence of effective treatment. The changes in the investigated synovial measures did not correlate with changes in DAS28, as illustrated in fig 3 for global inflammatory infiltration, CD3+ T lymphocytes, CD20+ B lymphocytes, and CD68+ and CD163+ sublining macrophages. The only significant finding was an inverse correlation with synovial plasma cells (fig 3): when DAS28 decreased there was an increase of synovial plasma cells as assessed by histology (rs = 0.689; p = 0.013). A similar trend was found for plasma cell detection using CD38 staining for identification of plasma cells (rs = −0.524; p = 0.098). For the individual components of the DAS28, the same inverse correlation with synovial plasma cells was found for patient's global assessment of disease activity (rs = −0.855; p<0.001) and ESR (rs = −0.825; p = 0.001), with a similar trend for SJC (rs = −0.568; p = 0.054). No other significant correlations were found between changes in the individual components of the DAS28, including ESR, and changes in synovial features.
Figure 3 Correlations between changes in clinical disease activity and synovial histopathology in 12 patients with RA over a 14 week period. The plots illustrate the correlations between changes in the DAS28 score (x axis) and changes in the following synovial measure (scored on a semiquantitative scale; y axis): inflammatory infiltration, CD3+ T lymphocytes, CD20+ B lymphocytes, CD68+ sublining macrophages, CD163+ sublining macrophages, and plasma cells. Correlation coefficients (rs) and significance (p) were assessed with the non‐parametric Spearman's correlation test. As indicated, only the inverse correlation between changes in DAS28 and changes in plasma cells reached significance.
Analysis of synovial changes in DAS28 responders
Because the previous data indicated that the decrease in clinical disease activity in the absence of effective treatment was not paralleled by a significant change in synovial inflammatory infiltration, we further analysed the histological changes in the patients with a clear clinical amelioration. The seven patients defined as clinical responders according to the DAS28 showed a decrease in patient's assessment of global disease activity of −34 (−17 to −56; p = 0.018), in SJC of −9 (−4 to −13; p = 0.018), and in TJC of −7 (3 to −18; p = 0.028), but ESR levels did not change (1 mm/1st h, from −19 to 5). Despite this consistent clinical improvement over 14 weeks, there were no significant changes in synovial markers in these patients. As was seen in the global cohort, in the DAS28 responders there was a trend towards reduction of lining layer hyperplasia (p = 0.063) and CD68+ lining macrophages (p = 0.059), but there was no decrease in all the other markers and, more specifically, global synovial infiltration, CD3+ T lymphocytes, CD20+ B lymphocytes, and sublining CD68+ and CD163+ macrophages (data not shown).
Absence of synovial changes over time is not due to technical variability
To exclude the possibility that a high background variability due to the technical approach might mask synovial changes by reducing the sensitivity to change, we additionally assessed the reproducibility of our synovial tissue analysis on 29 synovial tissue samples from patients with RA and spondyloarthropathy (SpA). These samples were selected based on a wide range of types and degree of inflammatory infiltration. Interassay analysis of paired samples for all synovial markers used in this study resulted in a high agreement in semiquantitative scores, with 80% of complete concordance and 98.5% of scores with a maximum one step difference between both analyses (table 2). Similarly, interobserver analysis of the same samples by two investigators resulted in 95% of scores with a maximum one step difference (table 2). Taken together, these data indicate a good reproducibility and a low background variability of the synovial tissue analysis.
Table 2 Assessment of the intraobserver and interobserver variability for the evaluation of synovial histopathology.
| Variable | Intraobserver variability | Interobserver variability | ||||
|---|---|---|---|---|---|---|
| Complete concordance | ⩽1 Step difference | >1 Step difference | Complete concordance | ⩽1 Step difference | >1 Step difference | |
| (%) | (%) | (%) | (%) | (%) | (%) | |
| Lining layer thickness | 90 | 10 | 0 | 61 | 26 | 13 |
| Vascularity | 59 | 39 | 2 | 35 | 65 | 0 |
| Lymphoid aggregates | 88 | 12 | 0 | 78 | 21 | 1 |
| Inflammatory infiltration | 76 | 22 | 2 | 30 | 65 | 5 |
| Plasma cells | 76 | 17 | 7 | 48 | 44 | 8 |
| Polymorphonuclear cells | 64 | 34 | 2 | 52 | 35 | 13 |
| CD3+ T lymphocytes | 66 | 33 | 1 | 32 | 66 | 2 |
| CD20+ B lymphocytes | 81 | 15 | 4 | 67 | 28 | 5 |
| CD38+ plasma cells | 86 | 9 | 5 | 83 | 12 | 5 |
| CD1a+ dendritic cells | 85 | 15 | 0 | 67 | 29 | 4 |
| Intracellular citrullinated proteins | 100 | 0 | 0 | 95 | 5 | 0 |
| mAb12A staining | 92 | 8 | 0 | 73 | 27 | 0 |
| Mean of all markers | 80 | 18 | 2 | 60 | 35 | 5 |
Twenty nine synovial samples with a large variability in degree and type of synovial inflammation were blindly scored for 12 different histopathological features on a semiquantitative scale from 0 to 3. The concordance was calculated between two independent scores by the same observer and independent scores by two different observers.
Discussion
This study described the variations of disease activity in the absence of effective treatment over a 14 week period in a cohort of 12 patients with RA. All patients had established disease with joint symptoms for at least 1 year, fulfilled the classification criteria for RA,10 had no concomitant disease that might interfere with the evaluations, and had active disease at baseline. Despite the fact that they did not receive effective treatment during the study period, there was a clear trend towards spontaneous decrease of disease activity, with 7/12 patients reaching the EULAR criteria for moderate or good response.26 Of interest, not only patient related outcomes such as the patient's global assessment of disease activity but also physician related outcomes such as SJC decreased significantly, suggesting a genuine decrease in disease activity. However, this was not paralleled by a decrease of biological inflammatory measures such as the ESR. Taken together, these data illustrate well that placebo effects and natural variations over the disease course complicate conclusive clinical interpretations in small patient cohorts as used in early phase clinical trials in RA.
The main finding of the present study is that in the absence of effective treatment there was no consistent change in synovial histopathology in these patients despite the spontaneous decrease in clinical disease activity. Of the large panel of synovial features analysed in this study, only lining layer thickness and lymphoid aggregates appeared to decrease. Because both lining layer hyperplasia and lymphoid aggregation are not homogeneous features throughout the inflamed synovium and because related markers such as CD68 expression in the lining layer did not decrease, it is possible that these results might have been influenced by technical variability or sampling biases. Moreover, when applying a more conservative analysis with correction for multiple testing, none of these differences reached statistical significance.
In contrast with these features, there was no decrease at all in synovial inflammatory infiltration. On the contrary, there was even a trend towards an increase in the number of synovial sublining macrophages, which were previously shown to correlate with disease activity14,27,28 and to be reduced in treatment‐induced remission.29 Similarly, the other infiltrating inflammatory cell types did not decrease, including the CD3+ T lymphocytes, which also reflect disease activity and response to treatment in RA.4,30 The idiosyncrasy of this finding was emphasised by the fact that changes in DAS28 scores did not correlate with changes in synovial biomarkers during the natural course of the disease, as usually occurs with effective treatment.4,5
One intriguing finding in this analysis was the inverse correlation between changes in disease activity and synovial plasma cells. Although an increase rather than a decrease of synovial plasma cells was also seen upon tumour necrosis factor α blockade in SpA,31,32 the interpretation of the present finding in RA remains speculative until further confirmation by independent studies. Indeed, because we tested a large panel of features this increases the possibility of false positive findings due to multiple comparisons. Therefore, this finding certainly warrants caution and further confirmation. Independently of the synovial plasma cells, however, the striking absence of correlation between changes in disease activity and synovial features was confirmed by the fact that even in clinical responders (according to EULAR response criteria) we observed no changes in synovial inflammatory infiltration. These data also support the pathophysiological concept that not only clinical synovitis but also subclinical joint inflammation may be of particular importance in RA.33
A caveat which should be considered when interpreting the absence of synovial changes as seen in the present study is the sensitivity to change of the synovial tissue analysis methodology. In a separate analysis of 29 synovial tissue samples we demonstrated good reproducibility of the methodology in order to exclude the possibility that a high background variability might mask changes over time. As to sensitivity to change upon effective treatment, the present study did not include such a control group, but numerous previous studies using the same synovial markers and comparable cohort sizes have demonstrated convincingly that effective treatment led to a significant decrease of synovial inflammation in RA as well as in other forms of chronic arthritis.4,5,6,7,8,31,32,34,35,36,37,38,39 Although the semiquantitative scoring methods may be less sensitive to change than manual counting or digital image analysis and may thus miss minor changes,40 these data indicate that the methodology used is sufficiently reproducible and sensitive to detect profound and consistent synovial changes upon effective treatment.31,32,34,35,39 Further standardisation of more sensitive techniques such as digital image analysis will allow future studies to refine and extend these findings.
It should be emphasised that the relevance of these findings is directly related to the global context of the analysis: the search for useful biomarkers in early phase clinical trials in RA. This type of study is generally restricted to cohorts of small to intermediate size, which explains why clinical disease activity scores are particularly prone to random changes due to spontaneous variations of disease activity, placebo effects, and regression to the mean, and are thus not always indicative of the genuine clinical effect seen in larger cohorts. Although relatively small, the size of the cohort included in the present study is representative of such studies and similar to, or larger than, previous studies assessing the effect of placebo on synovial histopathology.2,4,6,7,8 Taken together with extensive previous work on the validation and standardisation of biopsy sampling and analysis in RA studies with effective treatments,41 these data provide further evidence that synovial histopathology can be a useful additional tool to discriminate between effective and ineffective treatment. Although synovial histopathology is certainly not aimed to be a surrogate marker replacing clinical outcomes, it may be useful together with clinical measures and classical biomarkers such as C reactive protein and ESR to create a high density of outcome data in small patient cohorts. The added value of synovial markers of treatment response had been suggested by the high sensitivity to detect changes in RA clinical trials5 and the recent demonstration in SpA that the discriminative power of synovial markers is similar to, or better than, C reactive protein and ESR.42
In conclusion, the absence of consistent and significant synovial changes not only confirmed the previously demonstrated absence of synovial immunomodulation in placebo or ineffective treatment4,6,7,8 but also extended these findings by demonstrating that synovial inflammation is less sensitive than clinical disease assessments to variations in the absence of effective treatment. This study adds to the existing evidence that synovial biomarkers may facilitate conclusive early phase clinical trials by reducing the size and duration of this type of study.
Acknowledgements
Dominique Baeten is a senior clinical investigator of the Fund for Scientific Research‐Vlaanderen (FWO‐Vlaanderen). Bernard Vandooren is a research assistant of the Fund for Scientific Research‐Vlaanderen (FWO‐Vlaanderen).
This study was supported by a research grant from Organon NV, Oss, The Netherlands.
Abbreviations
ACR - American College of Rheumatology
DAS28 - 28 joint count Disease Activity Score
DMARDs - disease modifying antirheumatic drugs
ESR - erythrocyte sedimentation rate
gp - glycoprotein
HC - human cartilage
mAb - monoclonal antibody
RA - rheumatoid arthritis
SJC - swollen joint count
SpA - spondyloarthropathy
TJC - tender joint count
Footnotes
Competing interests: J Houbiers, A M Boots, and A MM Miltenburg are employed by NV Organon, Oss, The Netherlands.
References
- 1.Lipsky P E. Integrating biologic therapy into the comprehensive care of patients with rheumatoid arthritis. J Rheumatol 20057254–57. [PubMed] [Google Scholar]
- 2.Haringman J J, Kraan M C, Smeets T J, Zwinderman K H, Tak P P. Chemokine blockade and chronic inflammatory disease: proof of concept in patients with rheumatoid arthritis. Ann Rheum Dis 200362715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresnihan B, Baeten D, Firestein G, FitzGerald O, Gerlag D M, Haringman J J.et al Synovial tissue analysis in randomized clinical trials. J Rheumatol 2005322481–2484. [PubMed] [Google Scholar]
- 4.Gerlag D M, Haringman J J, Smeets T J, Zwinderman A H, Kraan M C, Laud P J.et al Effects of oral prednisolone on biomarkers in synovial tissue and clinical improvement in rheumatoid arthritis. Arthritis Rheum 2004503783–3791. [DOI] [PubMed] [Google Scholar]
- 5.Haringman J J, Gerlag D M, Zwinderman A H, Smeets T J, Kraan M C, Baeten D.et al Synovial tissue macrophages: highly sensitive biomarkers for response to treatment in rheumatoid arthritis patients. Ann Rheum Dis 200564834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smeets T J M, Kraan M C, Versendaal J, Breedveld F C, Tak P P. Analysis of serial synovial biopsies in patients with rheumatoid arthritis: description of a control group without clinical improvement after treatment with interleukin 10 or placebo. J Rheumatol 1999262089–2093. [PubMed] [Google Scholar]
- 7.Smeets T J, Kraan M C, van Loon M E, Tak P P. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum 2003482155–2162. [DOI] [PubMed] [Google Scholar]
- 8.Van Holten J, Pavelka K, Vencovsky J, Stahl H, Rozman B, Genovese M.et al A multicentre, randomised, double‐blind, placebo controlled phase II study of subcutaneously administered interferon beta 1a in the treatment of patients with active rheumatoid arthritis. Ann Rheum Dis 20056464–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruynesteyn K, Landewe R, van der Linden S, van der Heijde D. Radiography as primary outcome in rheumatoid arthritis: acceptable sample sizes for trials with 3 months' follow‐up. Ann Rheum Dis 2004631413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felson D T, Anderson J J, Boers M, Bombardier C, Chernoff M, Fried B.et al The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 199336729–740. [DOI] [PubMed] [Google Scholar]
- 11.Felson D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C.et al American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. [DOI] [PubMed] [Google Scholar]
- 12.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 13.Baeten D, Van den Bosch F, Elewaut D, Stuer A, Veys E M, De Keyser F. Needle arthroscopy of the knee with synovial biopsy sampling: technical experience in 150 patients. Clin Rheumatol 199918434–441. [DOI] [PubMed] [Google Scholar]
- 14.Baeten D, Demetter P, Cuvelier C, Van den Bosch F, Kruithof E, Van Damme N.et al Comparative study of synovial histology in rheumatoid arthritis, spondyloarthropathy and osteoarthritis: influence of disease duration and activity. Ann Rheum Dis 200059945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten D, Kruithof E, De Rycke L, Vandooren B, Wyns B, Boullart L.et al Diagnostic classification of spondyloarthropathy and rheumatoid arthritis by synovial histopathology: a prospective study in 154 consecutive patients. Arthritis Rheum 2004502931–2941. [DOI] [PubMed] [Google Scholar]
- 16.Baeten D, Peene I, Union A, Meheus L, Sebag M, Serre G.et al Specific presence of intracellular citrullinated proteins in rheumatoid arthritis synovium: relevance to antifilaggrin autoantibodies. Arthritis Rheum 2001442255–2262. [DOI] [PubMed] [Google Scholar]
- 17.Baeten D, Boots A M H, Steenbakkers P G A, Bos E, Verheijden G F M, Elewaut D.et al HC gp‐39 expression by monocytic cells in rheumatoid synovium is correlated with joint destruction. Arthritis Rheum 2000431233–1243. [DOI] [PubMed] [Google Scholar]
- 18.Steenbakkers P G, Baeten D, Rovers E, Veys E M, Rijnders A W, Meijerink J.et al Localization of MHC class II/human cartilage glycoprotein‐39 complexes in synovia of rheumatoid arthritis patients using complex‐specific monoclonal antibodies. J Immunol 20031705719–5727. [DOI] [PubMed] [Google Scholar]
- 19.Baeten D, Steenbakkers P G A, Rijnders A M W, Boots A M, Veys E M, De Keyser F. Detection of MHC/HC gp‐39 complexes in rheumatoid arthritis synovium as a specific and independent histological marker. Arthritis Rheum 200450444–451. [DOI] [PubMed] [Google Scholar]
- 20.Kruithof E, Baeten D, De Rycke L, Vandooren B, Foell D, Roth J.et al Synovial histopathology of psoriatic arthritis, oligo‐ and polyarticular, resembles more spondyloarthropathy than rheumatoid arthritis. Arthritis Res Ther 20057569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tak P P, Thurkow E W, Daha M R, Kluin P M, Smeets T J, Meinders A E.et al Expression of adhesion molecules in early rheumatoid synovial tissue. Clin Immunol Immunopathol 199577236–242. [DOI] [PubMed] [Google Scholar]
- 22.Kraan M C, Haringman J J, Ahern M J, Breedveld F C, Smith M D, Tak P P. Quantification of the cell infiltrate in synovial tissue by digital image analysis. Rheumatology (Oxford) 20003943–49. [DOI] [PubMed] [Google Scholar]
- 23.Kraan M C, Smith M D, Weedon H, Ahern M J, Breedveld F C, Tak P P. Measurement of cytokine and adhesion molecule expression in synovial tissue by digital image analysis. Ann Rheum Dis 200160296–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeten D, Demetter P, Cuvelier C A, Kruithof E, Van Damme N, De Vos M.et al Macrophages expressing the scavenger receptor CD163: a link between immune alterations of the gut and synovial inflammation in spondyloarthropathy. J Pathol 2002196343–350. [DOI] [PubMed] [Google Scholar]
- 25.Baeten D, Moller H J, Delanghe J, Veys E M, Moestrup S K, De Keyser F. Association of CD163+ macrophages and local production of soluble CD163 with lower lymphocyte activation in spondyloarthropathy synovitis. Arthritis Rheum 2004501611–1623. [DOI] [PubMed] [Google Scholar]
- 26.van Gestel A M, Haagsma C J, van Riel P L C M. Validation of RA improvement criteria that include simplified joint counts. Arthritis Rheum 1998411845–1850. [DOI] [PubMed] [Google Scholar]
- 27.Tak P P, Smeets T J, Daha M R, Kluin P M, Meijers K A, Brand R.et al Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum 199740217–225. [DOI] [PubMed] [Google Scholar]
- 28.Smeets T J, Barg E C, Kraan M C, Smith M D, Breedveld F C, Tak P P. Analysis of the cell infiltrate and expression of proinflammatory cytokines and matrix metalloproteinases in arthroscopic synovial biopsies: comparison with synovial samples from patients with end stage, destructive rheumatoid arthritis. Ann Rheum Dis 200362635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith M D, Kraan M C, Stavotinek J, Au V, Weedon H, Parker A.et al Treatment‐induced remission in rheumatoid arthritis patients is characterized by a reduction in macrophage content of synovial biopsies. Rheumatology (Oxford) 200140367–374. [DOI] [PubMed] [Google Scholar]
- 30.Baeten D, Kruithof E, De Rycke L, Boots A M, Mielants H, Veys E M.et al Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res Ther 20057359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baeten D, Kruithof E, Van den Bosch F, Demetter P, Van Damme N, Cuvelier C.et al Immunomodulatory effects of anti‐tumor necrosis factor alpha therapy on synovium in spondylarthropathy: histologic findings in eight patients from an open‐label pilot study. Arthritis Rheum 200144186–195. [DOI] [PubMed] [Google Scholar]
- 32.Kruithof E, Baeten D, Van den Bosch F, Mielants H, Veys E M, De Keyser F. Histological evidence that infliximab leads to downregulation of inflammation and tissue remodelling of the synovial membrane in spondyloarthropathy. Ann Rheum Dis 200564529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraan M C, Versendaal H, Jonker M, Bresnihan B, Post W J, t Hart B A.et al Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum 1998411481–1488. [DOI] [PubMed] [Google Scholar]
- 34.Tak P P, Taylor P C, Breedveld F C, Smeets T J, Daha M R, Kluin P M.et al Decrease in cellularity and expression of adhesion molecules by anti‐tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum 1996391077–1081. [DOI] [PubMed] [Google Scholar]
- 35.Dolhain R J, Tak P P, Dijkmans B A, De Kuiper P, Breedveld F C, Miltenburg A M. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br J Rheumatol 199837502–508. [DOI] [PubMed] [Google Scholar]
- 36.Kraan M C, Reece R J, Barg E C, Smeets T J, Farnell J, Rosenbrug R.et al Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Findings in a prospective, randomized, double‐blind, parallel‐design clinical trial in thirty‐nine patients at two centers. Arthritis Rheum 2000431820–1830. [DOI] [PubMed] [Google Scholar]
- 37.Kraan M C, van Kuijk A W, Dinant H J, Goedkoop A Y, Smeets T J, de Rie M A.et al Alefacept treatment in psoriatic arthritis: reduction of the effector T cell population in peripheral blood and synovial tissue is associated with improvement of clinical signs of arthritis. Arthritis Rheum 2002462776–2784. [DOI] [PubMed] [Google Scholar]
- 38.Kane D, Gogarty M, O'Leary J, Silva I, Bermingham N, Bresnihan B.et al Reduction of synovial sublining layer inflammation and proinflammatory cytokine expression in psoriatic arthritis treated with methotrexate. Arthritis Rheum 2004503286–3295. [DOI] [PubMed] [Google Scholar]
- 39.De Rycke L, Baeten D, Foell D, Kruithof E, Veys E M, Roth J.et al Differential expression and response to anti‐TNFalpha treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol 200520617–27. [DOI] [PubMed] [Google Scholar]
- 40.Youssef P P, Smeets T J, Bresnihan B, Cunnane G, FitzGerald O, Breedveld F.et al Microscopic measurement of cellular infiltration in the rheumatoid arthritis synovial membrane: a comparison of semi‐quantitative and quantitative analysis. Br J Rheumatol 1998371003–1007. [DOI] [PubMed] [Google Scholar]
- 41.Bresnihan B, Baeten D, Firestein G, FitzGerald O, Gerlag D M, Haringman J J.et al Synovial tissue analysis in clinical trials. J Rheumatol 2005322481–2484. [PubMed] [Google Scholar]
- 42.Kruithof E, De Rycke L, Vandooren B, De Keyser F, FitzGerald O, McInnes I.et al Identification of synovial biomarkers of response to experimental treatment in early phase clinical trials in spondylarthropathy. Arthritis Rheum. (in press) [DOI] [PubMed]