Abstract
Objectives
To evaluate the impact of anti‐tumour necrosis factor (TNF) treatment on growth and to identify the predictors for the change in growth in severe juvenile idiopathic arthritis (JIA).
Methods
Data from 71 JIA patients (43 on etanercept, 28 on infliximab) were reviewed two years before and two years on the anti‐TNF treatment. The patients had polyarticular disease course (48 polyarthritis, 19 extended oligoarthritis, two systemic arthritis, and two enthesitis related arthritis). At the initiation of the anti‐TNF treatment, their mean age was 9.6 years and the mean duration of JIA, 5.7 years.
Results
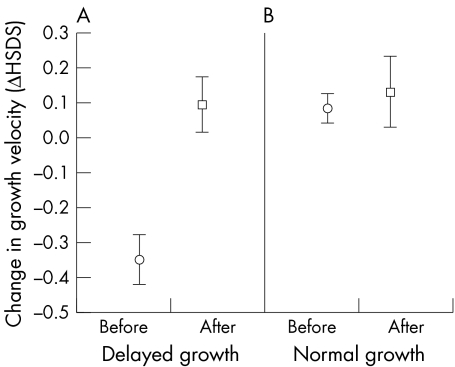
In the patients with delayed growth before anti‐TNF treatment (n = 53), the growth velocity, measured as the change in height standard deviation score, accelerated +0.45 (95% confidence interval, 0.33 to 0.56) (p<0.001) during the anti‐TNF treatment. In the patients with normal or accelerated growth before anti‐TNF treatment (n = 18), the change in growth velocity was +0.05 (0.07 to 0.16) (p = 0.39). At two years on anti‐TNF treatment, the growth velocity between these two groups was similar. No difference was found between the patients treated with etanercept or infliximab. A decelerating growth rate before the anti‐TNF treatment was the strongest predictor for the observed increase in the growth velocity. The change in the inflammatory activity remained a significant predictor of the growth velocity even after the decrease in glucocorticoid dose was taken into account.
Conclusions
In the treatment of polyarticular JIA, the anti‐TNF treatment not only suppresses inflammation but also restores growth velocity.
Keywords: juvenile idiopathic arthritis, anti‐TNF treatment, growth
In juvenile idiopathic arthritis (JIA), one of the well known complications in the long term outcome is growth impairment, which is seen especially in polyarthritis and systemic JIA.1,2 The long term use of glucocorticoids in JIA has been associated with growth retardation,2,3 but growth can also slow down without steroid treatment.4 Advances in drug treatment, especially anti‐tumour necrosis factor (TNF) treatment,5,6,7 have provided a new means of controlling the most aggressive forms of polyarticular JIA. To our knowledge only one study8 has been published on the impact of etanercept, a soluble TNF receptor, on the growth velocity of JIA patients. In that study, linear growth improved on etanercept in seven JIA patients with previous growth delay. In children with Crohn's disease, infliximab—a monoclonal anti‐TNFα antibody—has been shown to restore growth,9 but there are no published data on the effect of infliximab on growth of patients with JIA. Our clinical experience since 1999 has been that growth has been restored in several JIA patients using TNF blockade. This observation led us to initiate a study on a cohort of 71 consecutive JIA patients and their growth during anti‐TNF treatment.
Methods
Assessment of growth
The growth in the JIA patients was recorded for a four year period, two years before and two years after the initiation of anti‐TNF treatment. The growth data were collected retrospectively before 2002 and prospectively thereafter. The child's height and weight measurements were recorded on the Finnish growth chart.10,11 A paediatrician or a registered nurse made the measurements. The height standard deviation score (HSDS; z score), was defined as the observed height minus mean height for age divided by SD, where SD was the standard deviation for the normal population of the same chronological age and sex.11 Growth velocity was defined as the change in HSDS (ΔHSDS) during follow up. A positive value indicated catch up growth and a negative value impaired growth. The height adjusted relative weight (expressed as a percentage) was determined from the ratio of weight for height (W/H) in (kg/cm) to the mean W/H in the normal population of the same calendar age and sex. Body mass index (BMI) was calculated as weight (kg) for height2 (m) (W/H2). An experienced paediatric radiologist used the Greulich‐Pyle method to determine skeletal maturation in relation to the measurements in normal Finnish children.12 A normal bone age was between –2 SD and +2 SD, according to a patient's calendar age and sex.
Patients
The data were analysed on 71 JIA patients, classified according to the ILAR criteria,13 receiving anti‐TNF treatment (43 on etanercept and 28 on infliximab) in three Finnish tertiary care centres of paediatric rheumatology (table 1). All the patients had a severe polyarticular disease course, with a mean disease duration of 5.8 years and refractory to previous treatment regimens with conventional DMARDs (tables 1 and 2). The etanercept dose was 0.4 mg/kg twice weekly subcutaneously. Infliximab was given at a dose of 80 to 200 mg intravenously every six to eight weeks (3–5 mg/kg body weight). All glucocorticoids (oral, intra‐articular, and intravenous) during the four year follow up were summed. When assessing the total corticosteroid exposure, all types of glucocorticoids were converted to prednisolone equivalents, using the following estimate: 4 mg methylprednisolone ≡ 4 mg triamcinolone ≡ 5 mg prednisolone ≡ 0.75 mg betamethasone ≡ 6 mg deflazacort.14
Table 1 Demographic data and clinical characteristics of 71 JIA patients with delayed* or normal† growth before the initiation of anti‐TNF treatment.
| Variable | Growth velocity during the two years before anti‐TNF treatment | ||
|---|---|---|---|
| Delayed* (n = 53) | Normal† (n = 18) | p Value | |
| Sex: female/male | 38/15 (72/28%) | 15/3 (83/17%) | NS |
| Type of JIA | |||
| Seronegative polyarthritis | 34 (64%) | 11 (61%) | NS |
| Extended oligoarthritis | 13 (24%) | 6 (33%) | NS |
| Systemic arthritis | 2 (4%) | 0 | NS |
| Enthesitis related arthritis | 2 (4%) | 0 | NS |
| Seropositive polyarthritis | 2 (4%) | 1 (6%) | NS |
| Psoriatic arthritis | 0 | 0 | NS |
| ANA‐Ab elevated | 23 (43%) | 8 (44%) | NS |
| HLA‐B27 present | 22 (42%) | 5 (28%) | NS |
| Age at the onset of JIA (years) | 4.0 (0.8 to 13.3) | 3.4 (1.1 to 9.7) | NS |
| Duration of JIA (years) | 5.8 (0.3 to 13.7) | 5.5 (1.3 to 12.6) | 0.096 |
| Age at initiation of TNF inhibitor (years) | 9.9 (3.0 to 14.7) | 8.9 (4.7 to 14.5) | NS |
| Measures of disease activity at initiation of TNF inhibitor | |||
| ESR (mm/h) | 33 (3 to 98) | 30 (7 to 65) | 0.051 |
| C reactive protein (mg/l) | 23 (5 to 92) | 23 (5 to 105) | NS |
| Number of active joints | 9 (0 to 31) | 12 (2 to 47) | 0.110 |
Values are n (%) or mean (range).
*Delayed growth: ΔHSDS <0.
†Normal growth: ΔHSDS ⩾0.
ANA‐ab, antinuclear antigen antibody; ESR, erythrocyte sedimentation rate; HSDS, height standard deviation score; JIA, juvenile idiopathic arthritis; TNF, tumour necrosis factor.
Table 2 Drug treatment in 71 JIA patients 24 months before and 24 months after the initiation of the anti‐TNF treatment.
| Treatment | 24 Months before | Initiation of anti‐TNF treatment | 24 Months after |
|---|---|---|---|
| DMARDs and other drug treatments | |||
| Hydroxychloroquine | 23 (32%) | 30 (42%) | 17 (24%) |
| Methotrexate | 46 (65%) | 62 (87%) | 58 (82%) |
| Ciclosporine | 17 (24%) | 32 (45%) | 8 (11%) |
| Sulfasalazine | 15 (21%) | 18 (25%) | 4 (6%) |
| Azathioprine | 6 (8%) | 10 (14%) | 7 (10%) |
| Gold sodium thiomalate | 5 (7%) | 6 (8%) | 1 (1%) |
| Auranofin | 1 (1%) | 0 | 0 |
| Podophyllotoxin | 1 (1%) | 3 (4%) | 0 |
| Leflunomide | 0 | 0 | 5 (7%) |
| Intravenous immunoglobulin | 1 (1%) | 0 | 1 (1%) |
| Prednisolone | 46 (65%) | 55 (77%) | 33 (46%) |
| Treatment strategy | |||
| No DMARDs | 16 (23%) | 2 (3%) | 4 (6%) |
| Prednisolone alone | 0 | 1 (1%) | 0 |
| Single treatment (1 DMARD) | 1 (1%) | 2 (3%) | 25 (35%) |
| Single DMARD with prednisolone | 10 (14%) | 10 (14%) | 17 (24%) |
| Combination treatment | 9 (13%) | 13 (18%) | 9 (13%) |
| Combination treatment with prednisolone | 35 (49%) | 44 (62%) | 16 (23%) |
Values are n (%).
DMARD, disease modifying anti‐rheumatic drug; JIA, juvenile idiopathic arthritis; TNF, tumour necrosis factor.
Inclusion criteria
The JIA patients who had received biological agents for more than one year (mean 23.1 months, median 24, range 13 to 24) were included. The first patient was started on etanercept in April 1999 and on infliximab in September 1999.
Exclusion criteria
From 118 consecutive JIA patients started on biological drugs, 13 were excluded because they were more than 15 years of age at the initiation of anti‐TNF treatment and 13 because of short duration (less than 12 months) of TNFα inhibitor treatment. Twenty one patients were excluded because of the ongoing or previous human growth hormone treatment.
Definition of the patients with delayed and normal growth
The 71 patients were divided into two categories according to the growth velocity (ΔHSDS) during the two years before the initiation of the anti‐TNF treatment: delayed growth (ΔHSDS <0, n = 53) and normal or accelerated growth (ΔHSDS ⩾0, n = 18) (table 1).
Criteria for disease activity
Inactive disease was defined as in Wallace et al.15
Statistical analysis
The growth velocity in the patients with delayed or normal growth was compared using the paired samples t test. Normality of the distribution was assessed using the Kolmogorov–Smirnov test. In the case of non‐normality—for example, in comparing the growth velocity between etanercept and infliximab treatment groups—the non‐parametric Mann–Whitney U test was used. Correlations between the growth velocity and disease related variables (age, duration of disease, disease activity, glucocorticoid dose, and so on) were tested using Spearman's correlation test. The effect of glucocorticoids, age, duration of disease, erythrocyte sedimentation rate (ESR), C reactive protein, and the number of active joints on the growth velocity were calculated using multivariate linear regression analysis. The differences between the category variables were tested with χ2, Fisher's exact, or Kruskall–Wallis tests.
Results
Growth velocity
In the whole group of 71 patients, there was a significant increase in the mean growth velocity (ΔHSDS) between the two‐year follow up periods before and after the initiation of anti‐TNF treatment. This mainly reflected the increase in the growth velocity of the 53 patients with previously delayed growth. Their mean growth velocity increased by +0.45 ΔHSDS (95% CI, 0.33 to 0.56) (p<0.001) during the two years on anti‐TNF treatment. In 18 JIA patients with normal growth before the anti‐TNF treatment, the mean change in the growth velocity of +0.05 ΔHSDS (95% CI, 0.07 to 0.16) was not significant (p = 0.39). At two years on anti‐TNF treatment, neither HSDS nor ΔHSDS differed significantly between these patient groups (fig 1). When growth was calculated as cm/year, the growth rate increased by +1.8 cm/year (95% CI, 1.2 to 2.3) (p<0.001) in the patients with previously delayed growth. There was no change in the growth rate of patients with previously normal growth. There were no differences between the patients receiving etanercept or infliximab treatment in the change of growth velocity, height adjusted relative weight, or BMI.
Figure 1 The mean change with 95% confidence intervals in growth velocity (ΔHSDS) before (circle) and after (square) anti‐TNF treatment in JIA patients with (A) previously delayed growth, and (B) previously normal growth. HSDS, height standard deviation score; JIA, juvenile idiopathic arthritis.
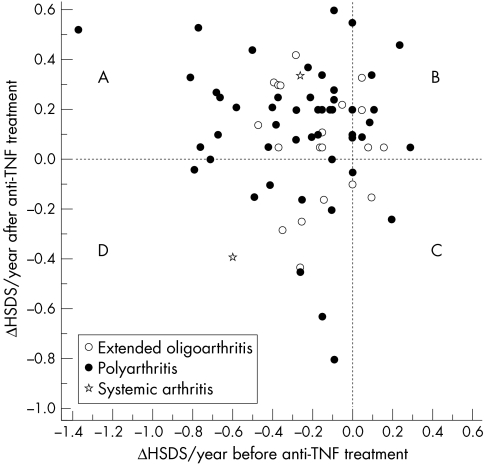
Fifty four patients (76%) improved their growth velocity on anti‐TNF treatment. Of these, 40 had delayed growth before and catch up growth after the initiation of anti‐TNF treatment (fig 2, quadrant A). Their mean accelerated growth velocity was +0.59 ΔHSDS/year (95% CI, 0.47 to 0.69) (p<0.001), range +0.1 to +1.89. Fourteen patients (20%) had a normal or accelerated growth velocity both before and after the initiation of anti‐TNF treatment (fig 2, quadrant B). Four patients (6%) had normal growth velocity before, but a decrease after the initiation of the biological drugs (fig 2, quadrant C). Two of these four patients had received both etanercept and infliximab, and two patients only infliximab for the arthritis, with less than 30% improvement in disease activity. Three of these had also a refractory uveitis, with no response to the biological agents for this symptom either. All four had received an increased dose of glucocorticoids during the past two years of follow up (mean oral cumulative prednisolone increased from 2.7 g to 3.5 g). Thirteen patients (18%) with decelerated growth velocity both before and after the initiation of anti‐TNF treatment (fig 2, quadrant D) were older (mean 11.0 years). Their clinical response to anti‐TNF treatment was less favourable: none could be considered to have inactive disease, and all had a larger number of active joints after the two year treatment period than the rest of the patients (p = 0.015). Their mean use of glucocorticoids was greater (p = 0.005) than in the other patients.
Figure 2 The relations between annual height velocity (ΔHSDS/year) 24 months before (x axis) and 24 months after (y axis) the initiation of anti‐TNF treatment in 71 patients with juvenile idiopathic arthritis. The patients in the left upper quadrant (A) had delayed growth before and catch up growth during the anti‐TNF treatment. The patients in the right upper quadrant (B) had accelerated growth both before and after the anti‐TNF treatment. The patients in the right lower quadrant (C) had catch up growth before, but decreased growth velocity after the anti‐TNF treatment. The patients in left lower quadrant (D) had delayed growth velocity both before and after the anti‐TNF treatment. TNF, tumour necrosis factor.
Bone age
Bone age was assessed before and two years after the initiation of anti‐TNF treatment in 24 (34%) and 63 (89%) patients, respectively. There was no difference in the skeletal maturation between the patients with delayed and normal growth (table 3). In the 24 in whom bone age was determined both before and two years after the initiation of anti‐TNF treatment, the rate of the skeletal maturation remained the same in 17 patients (71%), turned from delayed to normal in six (25%), and from normal to advanced in one, a girl with relatively early puberty. There was no abnormal progression in the skeletal maturation in any of these 24 patients.
Table 3 Comparison of relative weight, body mass index, steroids, disease activity and bone age between the JIA patients with delayed* and normal† growth during the anti‐TNF treatment.
| Growth velocity during the two years before anti‐TNF treatment | |||
|---|---|---|---|
| Delayed* | Normal† | p Value | |
| Height adjusted relative weight (%) at 0 | +11.2 (4.9 to 17.5) | +11.1 (2.9 to 19.4) | NS |
| Height adjusted relative weight (%) at 2 years | +15.4 (7.7 to 23.0) | +13.6 (3.1 to 24.0) | NS |
| BMI‡ at 0 (kg/m2) | +18.5 (17.3 to 19.6) | +18.0 (16.4 to 19.7) | NS |
| BMI‡ at two years (kg/m2) | +19.9 (18.5 to 21.3) | +19.2 (17.2 to 21.2) | NS |
| Oral steroids§ before BA (mg/kg/day) (mean (range)) | 0.16 (0 to 0.60) | 0.16 (0 to 0.42) | NS |
| Oral steroids§ after BA (mg/kg/day) (mean (range)) | 0.12 (0 to 0.83) | 0.08 (0 to 0.31) | NS |
| All steroids§ before BA (mg/kg/day) (mean (range)) | 0.21 (0 to 0.67) | 0.19 (0 to 0.45) | NS |
| All steroids§ after BA (mg/kg/day) (mean (range)) | 0.14 (0 to 0.96) | 0.09 (0 to 0.33) | NS |
| Disease activity at 2 years | |||
| Inactive disease | 27 (51%) | 10 (56%) | NS |
| Improvement of 70–99%¶ | 15 (28%) | 2 (11%) | NS |
| Improvement of 30–69%¶ | 7 (13%) | 5 (28%) | NS |
| Improvement of less than 30%¶ | 4 (8%) | 1 (6%) | NS |
| Bone age at two years | 47 (89%) | 16 (89%) | NS |
| Normal | 32 (68%) | 12 (75%) | NS |
| Delayed | 13 (27%) | 4 (25%) | NS |
| Advanced | 2 (4%) | 0 | NS |
Values are mean (95% confidence intervals) or n (%) unless stated otherwise.
*Delayed growth: ΔHSDS ⩽0 before the initiation of anti‐TNF treatment.
†Normal growth: ΔHSDS ⩾0 before the initiation of anti‐TNF treatment.
‡Body mass index, weight/height2.
§Prednisolone equivalents either two years before or two years after the initiation of anti‐TNF treatment.
¶According to the decrease in the number of active joints, ESR, and C reactive protein from the time of initiation of anti‐TNF treatment.
BA, biological agent; BMI, body mass index; ESR, erythrocyte sedimentation rate; HSDS, height standard deviation score; JIA, juvenile idiopathic arthritis; TNF, tumour necrosis factor.
Growth velocity in prepubertal children
To assess the possibility that the accelerated growth was related to the pubertal growth spurt, the change in the growth velocity was also analysed in JIA patients less than nine years old (n = 27). The change in the growth velocity remained significant (data not shown). If only the girls less than seven years old and the boys less than nine years old at the initiation of anti‐TNF treatment were included in the analyses, the increase in the growth velocity was even greater; the mean change being +0.85 ΔHSDS (95% CI, 0.62 to 1.07) (p<0.001) in 13 patients with delayed growth and non‐significant in four patients with normal growth. Further, based on the observation that a delayed bone age is associated with delayed sexual development,16 we could estimate that in all 37 patients were prepubertal. In this analysis, we included boys with a bone age less than 13 years and girls with a bone age less than 11 years after two years on anti‐TNF treatment. Twenty six of the 37 prepubertal patients with delayed growth (70%) increased their growth velocity by +0.54 ΔHSDS (95% CI, 0.35 to 0.73) (p<0.001) or by +1.8 cm/year (95% CI, 1.1 to 2.5) (p<0.001). In 11 of the prepubertal patients (30%) with normal growth, there were no significant changes in the growth velocity.
Weight and BMI
The height adjusted relative weight and BMI were measured at the initiation of anti‐TNF treatment and after two years of treatment (table 3). The relative weight increased significantly in the patients with delayed growth, from +11.2% to +15.4% (95% CI, 0.7 to 7.6) (p = 0.018), but non‐significantly (p = 0.467) in the patients with normal growth. BMI increased slightly in all patients, from 18.3 to 19.7 kg/m2, the mean increase being +1.5 kg/m2 (95% CI, 0.9 to 2.0) (p<0.001) in the patients with delayed growth, and +1.2 kg/m2 (0.1 to 2.3) (p = 0.033) in the patients with normal growth. During two years on anti‐TNF treatment, seven patients (four with delayed growth) gained weight excessively. Their mean BMI increased by +5.3 kg/m2 (range 2.7 to 8.1) and mean relative weight by +26% (range 12 to 39). Six of the seven were already obese (BMI >25 kg/m2 and relative weight >40%) before anti‐TNF treatment was initiated. In comparison with the others, these seven patients were younger at the onset of JIA (mean age, 1.7 years; p = 0.007) and their disease had lasted longer (mean duration, 9.2 years; p = 0.009).
Glucocorticoids
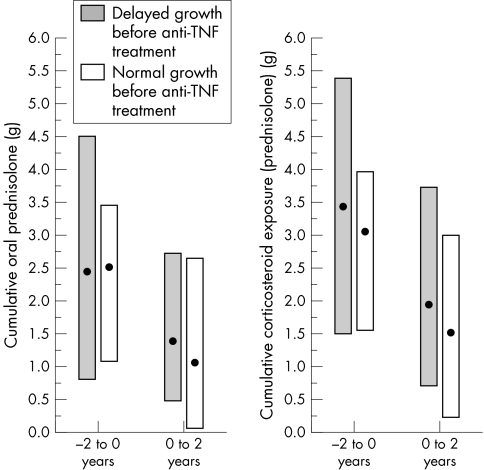
The cumulative dose of oral prednisolone as well as total cumulative glucocorticoid exposure (oral, intravenous, and intra‐articular) before and after the anti‐TNF treatment was carefully reviewed and changed into prednisolone equivalents in all the 71 patients. During the four year follow up, no significant difference was detected in steroid doses between the patients with delayed growth and normal growth (table 3). In both groups, the decrease in glucocorticoid dose was significant (p<0.001) during anti‐TNF treatment (fig 3).
Figure 3 Medians and interquartile ranges (IQR) of cumulative corticosteroid doses of 71 JIA patients with delayed growth (n = 53) or normal growth (n = 18): cumulative oral prednisolone dose (g) and cumulative corticosteroid exposure (oral, intra‐articular, and intravenous prednisolone, g) on a two year time scale before and after the initiation of anti‐TNF treatment. JIA, juvenile idiopathic arthritis; TNF, tumour necrosis factor.
The correlation coefficient (r) between the change in the growth velocity (ΔHSDS) and the change in corticosteroid dose in patients with delayed growth was −0.051 (little or no relation) and in the patients with normal growth, r = −0.670 (moderate). In linear regression analyses, the change of glucocorticoid doses in all 71 patients had a mild age adjusted relation with the change in the growth velocity (r = −0.27 (95% CI, −0.47 to −0.04)). In the patients with delayed growth this relation was not significant, but in the patients with normal growth it was good (r = −0.76, p<0.001).
Next, the growth velocity before the anti‐TNF treatment and ESR at two years on the anti‐TNF treatment were included in a stepwise multivariate linear regression analysis (table 4). In this statistical model, cumulative four year oral prednisolone dose (mean 4.9 g, range 0 to 18.0 g) and cumulative four year intra‐articular steroid dose (mean 1.4 g, range 0.1 to 3.9 g) had a significant predictive value (r = −0.33 and r = −0.26, respectively) for the change in the growth velocity.
Table 4 Predictors of the change in growth velocity.
| Predictor | Standardised coefficients | Partial correlations† | p Value | Colinearity* |
|---|---|---|---|---|
| Growth velocity before the initiation of BA | −0.874 | −0.826 | <0.001 | 0.846 |
| All oral prednisolone during four years | −0.196 | −0.332 | 0.006 | 0.978 |
| ESR at 24 months after the initiation of BA | −0.174 | −0.296 | 0.014 | 0.957 |
| All intra‐articular steroids during four years | −0.157 | −0.258 | 0.034 | 0.865 |
Stepwise multivariate linear regression analysis: the change of growth velocity is the dependent variable, adjusted R square is 0.680 and colinearity is >0.85.
*Measures the interdependence between variables.
†Measures the independent effect of a variable in this model.
BA, biological agent; ESR, erythrocyte sedimentation rate.
Disease activity
At 24 months, 37 of the JIA patients (52%) had inactive disease.15 In 17 of the patients (24%) the number of active joints, ESR, and C reactive protein had decreased by at least 70% but less than 100%; in 12 patients (17%) by at least 30% but less than 70%; and in five patients (7%) by less than 30% (table 3). In all the 71 patients, mean ESR at the initiation of anti‐TNF treatment was 32 mm/h (range 3 to 98) and at 24 months on treatment it was 14 mm/h (range 2 to 60). Mean difference between these two ESR values was 18 mm/h (95% CI, 13 to 23) (p<0.001). Mean C reactive protein decreased from 23 mg/l (range 5 to 105) at baseline to 8 mg/l (range 5 to 65) at 24 months (95% CI, 9 to 22) (p<0.001). The mean number of active joints declined from 10 (range 0 to 47) at baseline to 2 (range 0 to 21) at 24 months (95% CI, 6 to 10) (p<0.001). No serious or life threatening adverse events or side effects were seen during the study period in any of these patients on anti‐TNF treatment.
Discussion
We analysed the effect of etanercept and infliximab on growth in 71 patients with JIA. Those with delayed growth improved their growth velocity significantly during biological drug treatment. In multivariate linear regression analyses, the improvement in the growth velocity was the best in patients with the greatest growth retardation. The growth rate before the anti‐TNF treatment was the strongest predictor for the observed change in the growth velocity. There was no difference in catch up growth between the patients treated with etanercept or infliximab.
In our clinical study, the change in inflammatory activity remained a significant predictor of the growth velocity, even after glucocorticoids were taken into account. This suggests that the improvement in the growth velocity may be accounted for the decrease in inflammation, and not by a direct effect of biological agents on growth or on skeletal maturation. In clinical work, a common hypothesis is that the major reason for growth retardation in JIA is the high inflammatory activity, mediated by cytokines, especially TNFα and interleukin 1β. In a recent in vitro study, these cytokines suppressed longitudinal bone growth locally. This suppression was only partially reversed by insulin‐like growth factor 1 (IGF1).17 In clinical practise, some beneficial effects on growth have been observed with growth hormone treatment in JIA patients with severely retarded growth.18 However, our study suggests that in the treatment of active polyarticular JIA, the highly effective anti‐TNF treatment not only suppresses inflammation but also restores growth velocity.
In our analyses, there was a statistically significant relation between the change in glucocorticoid doses and the change in the growth velocity (ΔHSDS) in 18 patients with normal growth. The small sample size and non‐significant ΔHSDS limits the further implication of the finding. The cumulative oral glucocorticoids and also the cumulative intra‐articular glucocorticoids were significant independent variables predicting the change in the growth velocity during the four year follow up, although in the multivariate regression model their effect was only moderate. The higher the intake of cumulative steroids, the more growth was disturbed. These findings are in accordance with previous studies showing growth impairment with prolonged use of glucocorticoids.19,20 Glucocorticoids disturb longitudinal growth, possibly through direct effects on receptors on the growth plate21 and by interference with other growth modulating pathways—for example, the growth hormone/IGF1 axis.22 In addition, a transient suppression of the pituitary‐adrenal axis after the intra‐articular steroid injections has been described23 and suggested to be dose dependent.24 Based on the present results it can be speculated that with anti‐cytokine treatment resulting in a decreased need for glucocorticoids, steroid related side effects can be minimised.
Anti‐TNF treatment acts by blocking an upstream cytokine, TNFα. Theoretically, this may cause variable effects in several downstream pathways. The effect of anti‐TNF treatment on skeletal maturation is so far not fully understood. The present results indirectly suggest that a pathological acceleration is unlikely. In JIA patients with bone age measured at the baseline and at the end of follow up, the progression of the skeletal maturation did not accelerate but in most patients remained the same or normalised.
During the use of biological agents, the mean height‐adjusted relative weight and BMI increased. The slightly increased weight may reflect a reduction in the catabolic state induced by the inflammation. To avoid obesity, JIA patients with inactive disease should be encouraged to exercise like their healthy peers.
Because of the partly retrospective setting, our study has some limitations. The pubertal staging or the target height was not measured in all our patients, but instead we used the bone age to determine the estimated pubertal growth spurt. The strength in this setting was, however, that none of the patients was lost during follow up. In addition, there was no selection bias, because all patients fulfilling the inclusion criteria and starting anti‐TNF treatment in three centres were included. In this way we were able to collect a substantial amount of data on growth in a rare patient group.
Growth retardation resulting from the extended inflammatory process is one of the most important and permanent complications of polyarticular JIA, affecting the long term quality of life.25,26 Normal growth is an elementary target in the treatment of every chronic condition during childhood. Biological drug treatment is powerful in controlling the inflammation in JIA patients. On the basis of our results, biological agents seem to be effective in restoring normal growth in these children.
Abbreviations
HSDS - height standard deviation score
ILAR - International League of Associations for Rheumatology
JIA - juvenile idiopathic arthritis
TNF - tumour necrosis factor
References
- 1.Zak M, Muller J, Karup Pedersen F. Final height, armspan, subischial leg length and body proportions in juvenile chronic arthritis. A long‐term follow‐up study. Horm Res 19995280–85. [DOI] [PubMed] [Google Scholar]
- 2.Simon D, Fernando C, Czernichow P, Prieur A M. Linear growth and final height in patients with systemic juvenile idiopathic arthritis treated with longterm glucocorticoids. J Rheumatol 2002291296–1300. [PubMed] [Google Scholar]
- 3.Wang S J, Yang Y H, Lin Y T, Yang C M, Chiang B L. Attained adult height in juvenile rheumatoid arthritis with or without corticosteroid treatment. Clin Rheumatol 200221363–368. [DOI] [PubMed] [Google Scholar]
- 4.Polito C, Strano C G, Olivieri A N, Alessio M, Iammarrone C S, Todisco N.et al Growth retardation in non‐steroid treated juvenile rheumatoid arthritis. Scand J Rheumatol 19972699–103. [DOI] [PubMed] [Google Scholar]
- 5.Lovell D J, Giannini E H, Reiff A, Cawkwell G D, Silverman E D, Nocton J J.et al Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med 2000342763–769. [DOI] [PubMed] [Google Scholar]
- 6.Lahdenne P, Vahasalo P, Honkanen V. Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis 200362245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerloni V, Pontikaki I, Gattinara M, Desiati F, Lupi E, Lurati A.et al Efficacy of repeated intravenous infusions of an anti‐tumor necrosis factor alpha monoclonal antibody, infliximab, in persistently active, refractory juvenile idiopathic arthritis: results of an open‐label prospective study. Arthritis Rheum 200552548–553. [DOI] [PubMed] [Google Scholar]
- 8.Schmeling H, Seliger E, Horneff G. Growth reconstitution in juvenile idiopathic arthritis treated with etanercept. Clin Exp Rheumatol 200321779–784. [PubMed] [Google Scholar]
- 9.Borrelli O, Bascietto C, Viola F, Bueno de Mesquita M, Barbato M, Mancini V.et al Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn's disease. Dig Liver Dis 200436342–347. [DOI] [PubMed] [Google Scholar]
- 10.Sorva R, Perheentupa J, Tolppanen E M. A novel format for a growth chart. Acta Paediatr Scand 198473527–529. [DOI] [PubMed] [Google Scholar]
- 11.Pere A. Comparison of two methods for transforming height and weight to normality. Ann Hum Biol 20002735–45. [DOI] [PubMed] [Google Scholar]
- 12.Marttinen E.The growth of hand bones in relation to some body dimensions in normal children and in certain forms of short stature. Helsinki: University of Helsinki, 1983, 23–4. [Thesis. ]
- 13.Petty R E, Southwood T R, Manners P, Baum J, Glass D N, Goldenberg J.et al International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 200431390–392. [PubMed] [Google Scholar]
- 14.Gums J G. Disorders of the adrenal gland. In: DiPiro JT, Talbert RL, Yee GC, eds. Pharmacotherapy – a pathophysiologic approach, 3rd edition. Stamford, Connecticut: Appelton and Lange, 1997
- 15.Wallace C A, Ruperto N, Giannini E, Childhood Arthritis and Rheumatology Research Alliance, PRINTO Pediatric Rheumatology Collaborative Study Group Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004312290–2294. [PubMed] [Google Scholar]
- 16.Wilkins The diagnosis and treatment of endocrine disorders in childhood and adolescence. In: Kappy MS, Blizzard RM, Migeon CJ, editors. Adolescent sexual development. Patterns and timing of physical maturation , 4th edition. Springfield, Illinois: Thomas, 1994
- 17.Martensson K, Chrysis D, Savendahl L. Interleukin‐1beta and TNF‐alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J Bone Miner Res 2004191805–1812. [DOI] [PubMed] [Google Scholar]
- 18.Saha M T, Haapasaari J, Hannula S, Sarna S, Lenko H L. Growth hormone is effective in the treatment of severe growth retardation in children with juvenile chronic arthritis. Double blind placebo‐controlled followup study. J Rheumatol 2004311413–1417. [PubMed] [Google Scholar]
- 19.Blodgett F M, Burgin L, Iezzoni D, Gribetz D, Talbot N B. Effects of prolonged cortisone therapy on the statural growth, skeletal maturation and metabolic status of children. N Engl J Med 1956254636–641. [DOI] [PubMed] [Google Scholar]
- 20.Allen D B. Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin North Am 199625699–717. [DOI] [PubMed] [Google Scholar]
- 21.Abu E O, Horner A, Kusec V, Triffitt J T, Compston J E. The localization of the functional glucocorticoid receptor alpha in human bone. J Clin Endocrinol Metab 200085883–889. [DOI] [PubMed] [Google Scholar]
- 22.Jux C, Leiber K, Hugel U, Blum W, Ohlsson C, Klaus G.et al Dexamethasone impairs growth hormone (GH)‐stimulated growth by suppression of local insulin‐like growth factor (IGF)‐I production and expression of GH‐ and IGF‐I‐receptor in cultured rat chondrocytes. Endocrinology 19981393296–3305. [DOI] [PubMed] [Google Scholar]
- 23.Huppertz H I, Pfuller H. Transient suppression of endogenous cortisol production after intraarticular steroid therapy for chronic arthritis in children. J Rheumatol 1997241833–1837. [PubMed] [Google Scholar]
- 24.Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary‐adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum 200552924–928. [DOI] [PubMed] [Google Scholar]
- 25.David J, Cooper C, Hickey L, Lloyd J, Dore C, McCullough C.et al The functional and psychological outcomes of juvenile chronic arthritis in young adulthood. Br J Rheumatol 199433876–881. [DOI] [PubMed] [Google Scholar]
- 26.Packham J C, Hall M A. Long‐term follow‐up of 246 adults with juvenile idiopathic arthritis: functional outcome. Rheumatology (Oxford) 2002411428–1435. [DOI] [PubMed] [Google Scholar]