Abstract
Objectives
To determine the prevalence of anti‐cyclic citrullinated proteins (anti‐CCP) and IgM rheumatoid factor (RF) in sera of patients with TB compared with healthy controls.
Patients and methods
47 consecutive patients with recently diagnosed active pulmonary TB and 39 healthy controls were studied. Data were collected by questionnaire on clinical features of the disease, duration of symptoms, fever, cough, arthralgia, myalgia, sicca symptoms. Serum samples were collected from patients before starting treatment for TB and frozen at −20°C. Anti‐CCP and IgM RF were evaluated by ELISA.
Results
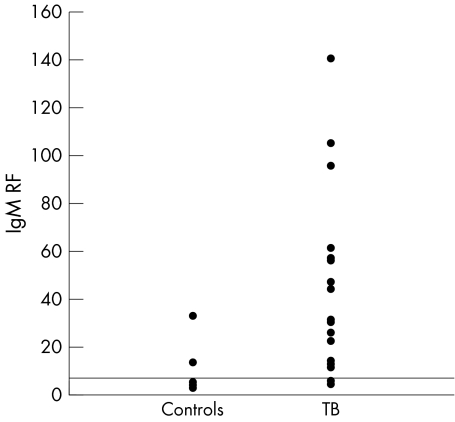
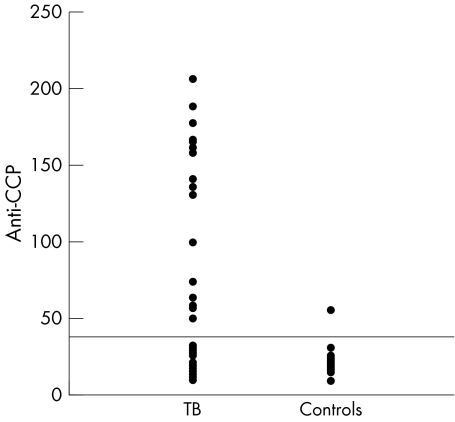
The mean (SD) duration of TB related symptoms was 4.4 (1.7) months, 73% had fever, 94% a cough. Rheumatic symptoms were relatively rare: arthralgia (4%), myalgias (4%), eye and mouth dryness (2% and 9%, respectively). Mean (SD) levels of anti‐CCP were significantly increased in patients with TB compared with controls: 44.9 (51) IU v 20 (7.3) IU (p = 0.002). Serum levels >40 U were found in 15/47 (32%) patients compared with 1/39 (2.6%) controls (p = 0.002). Mean (SD) serum levels of IgM RF were significantly increased in patients with TB: 17.8 (19) v 4.3 (5) (p<0.0001). IgM RF was positive (>6 IU) in 29/47 (62%) patients v 1/39 (2.6%) controls (p<0.0001).
Conclusions
A significant proportion of patients with active TB have an increased titre of anti‐CCP and IgM RF.
Keywords: anti‐CCP IgM RF tuberculosis
The past two decades have been marked by a worldwide resurgence of tuberculosis (TB). About two billion people have latent TB and about eight million will develop active TB.1 It has been shown that both pulmonary TB and also cases of extrapulmonary disease are increasing.2 About 10% of extrapulmonary TB affects the bones and joints.2 Patients with TB, even those without evidence of a direct musculoskeletal/local involvement, may present with a variety of rheumatic symptoms and signs. Although TB arthritis most commonly manifests as a monarthritis of the weightbearing joints,3 oligoarticular or polyarticular presentation is not rare, mimicking inflammatory diseases such as the spondyloarthropathies4 or rheumatoid arthritis (RA), or both.5 Poncet's disease is the classic example of TB‐induced polyarthritis.6 Furthermore, the serum of patients with TB may contain rheumatoid factors (RFs) in up to 40% of cases.7
Antibodies recognising cyclic citrullinated peptides are highly specific for RA.8 The specificity for RA has been shown to be up to 98% in comparison with 0–1% of healthy controls and 2–5% of disease controls.8 Anti‐cyclic citrullinated proteins (anti‐CCP) are present early in the disease process and may even pre‐date the onset of RA by many years.9
This study aimed at determining the prevalence and associations of anti‐CCP in patients with TB.
Patients and methods
Patients
Forty seven patients with recently diagnosed active pulmonary TB were included in the study. All were admitted to the hospital department of tuberculosis, with clinical symptoms and radiological signs of TB as well as positive cultures for Mycobacterium tuberculosis. A questionnaire was used to determine data on the clinical features of the disease, such as duration of symptoms, the presence of fever, cough, as well as rheumatological manifestations such as arthralgia/arthritis, myalgia, rash, mucocutaneous symptoms, sicca symptoms, spontaneous abortion, history of thrombosis, and familial history of autoimmune diseases. Serum samples were collected before starting treatment for TB and frozen at −20°C. Controls were 39 aged matched healthy personnel.
Anti‐CCP
Serum IgG anti‐CCP2 was studied by enzyme linked immunosorbent assay (ELISA) using a commercial kit (Quanta lite; Inova Diagnostics, San Diego CA, USA), according to the manufacturer's instructions. Samples were considered weakly positive if the antibody titre was 20–39 IU, moderately positive between 40 and 59, and strongly positive if >60 IU.
IgM RF
An ELISA commercial kit (Quanta lite; Inova Diagnostics) was used to determine serum IgM RF, according to the manufacturer's instructions. Samples were considered positive if the antibody titre was >6 IU.
Statistical analysis
Statistical analysis was performed by the SPSS software, using Student's t test and a χ2 test to compare antibody titres or positivity rate, respectively, between patients with TB and controls. Pearson correlation coefficients were used to study the relationship between clinical measures and the levels of anti‐CCP and IgM RF. A value of p<0.05 was considered significant.
Results
Patients
Table 1 summarises the demographic and clinical characteristics of patients with TB and healthy controls. The patients had a mean (SD) duration of symptoms of 4.4 (1.7) months; 73% had fever, 94% presented with cough. Only a small minority had symptoms such as arthralgia (4%), myalgias (4%), or eye and mouth dryness (2% and 9%, respectively). None of the patients had signs typical of rheumatoid arthritis such as arthritis, morning stiffness or rheumatoid nodules. None had mucocutaneous aphthae or skin manifestations or a history of spontaneous abortion, thrombosis or known first degree familial autoimmune disease.
Table 1 Demographic and clinical characteristics of patients with TB and healthy controls.
| Characteristics | Patients with TB | Controls |
|---|---|---|
| (n = 47) | (n = 39) | |
| Age (years), mean (SD) | 52.3 (17) | 47 (21) |
| Sex (M/F) | 26/21 | 13/26 |
| Duration of symptoms (months), mean (SD) | 4.4 (1.7) | |
| No (%) of patients with: | ||
| Fever | 34 (72) | |
| Cough | 42 (89) | None |
| Arthralgia | 2 (4) | |
| Myalgia | 2 (4) | |
| Eye dryness | 1 (2) | |
| Mouth dryness | 4 (9) |
Serum levels of anti‐CCP and IgM RF
The mean (SD) levels of anti‐CCP were significantly increased in patients with TB in comparison with controls: 44.9 (51) IU v 20 (7.3) IU (p = 0.002). Serum levels above the upper normal limits (>40 IU) were found in 15/47 (32%) patients in comparison with 1/39 (2.6%) controls (p = 0.002) (fig 1).
Figure 1 Serum IgM RF in patients with TB and controls.
The mean (SD) serum levels of IgM RF were significantly increased in patients with TB: 17.8 (19) v 4.3 (5) (p<0.0001). IgM RF was found positive (>6 IU) in 29/47 (62%) patients in comparison with 1/39 (2.6%) controls (p<0.0001) (fig 2).
Figure 2 Serum anti‐CCP in patients with TB and controls.
In patients with increased levels of anti‐CCP and IgM RF, the mean (SD) levels were of 126.3 (52) IU (range 49.7–205) and 32.8 (31.4) IU (range 6.1–105), respectively.
Associations between clinical manifestations and serological studies
The presence of anti‐CCP significantly correlated with a history of prolonged fever (p = 0.005). No correlation was found between the presence of anti‐CCP or IgM RF and any rheumatic symptom. A significant correlation was found between symptoms of fever and cough (p = 0.003), and between arthralgia and sicca (p<0.0001).
No association was found between anti‐CCP and RF.
Discussion
In this study we found that 15/47 (32%) patients with TB have positive levels of anti‐CCP. Although the presence of anti‐CCP correlated with fever, it was not associated with symptoms and signs of arthritis.
Anti‐CCP are a family of antibodies with specificities directed against a variety of citrullinated peptides.8 They are present in most patients with RA and have been found to have a specificity of >90%.8 However, recent investigators have shown that anti‐CCP are present in the serum of 8% of patients with psoriatic arthritis and have questioned its specificity.10 It is not clear whether the false positive anti‐CCP reactivity seen in patients with TB is directed against citrullinated or non‐citrullinated epitopes in the substrate for the CCP test.
Except for hepatitis C virus (HCV), the presence of anti‐CCP in infectious diseases has not been well studied. It seems that in contrast with RF, which is present in the great majority of patients with hepatitis C arthritis, as well as in a proportion of patients with other subacute infections, particularly bacterial endocarditis, anti‐CCP is negative in patients with HCV and may help in discriminating between HCV related arthritis and RA.11,12
TB is a multifaceted disease, which may present with a variety of symptoms, sometimes mimicking autoimmune diseases. Involvement of the bones and joints is the most common extrapulmonary manifestation of TB.2 There are many pitfalls in the diagnosis of tuberculous arthritis. Clinically, it may imitate other inflammatory rheumatic diseases, such as RA.5 The triad of radiological abnormalities observed in tuberculous arthritis may simulate RA and includes periarticular osteoporosis, peripherally located osseous erosion, and gradual diminution of the joint space.13 Furthermore, it has been reported that RF is present in up to 40% of patients with TB.7 Djavad et al have analysed the RFs produced by Epstein‐Barr virus transformed monoclonal B cells obtained from patients with RA and TB, and shown that they have a similar avidity and affinity to different antigens.14 In addition to RF, patients with TB may present a variety of autoantibodies15 such as antinuclear antibodies, anticardiolipin,15 and antineutrophil cytoplasmic antibodies.16 Thus patients with TB may present both clinical and laboratory parameters indicative of rheumatic conditions, in general, and RA, in particular.
In conclusion, a significant proportion of our present series of patients with active TB had higher titres of anti‐CCP and IgM RF. These findings should be kept in mind in the interpretation of serological studies in the differential diagnosis of patients with systemic manifestations.
Abbreviations
CCP - cyclic citrullinated protein
HCV - hepatitis C virus
RA - rheumatoid arthritis
RF - rheumatoid factor
TB - tuberculosis
References
- 1.Mandalakas A M, Starke J R. Current concepts of childhood tuberculosis. Semin Pediatr Infect Dis 20051693–104. [DOI] [PubMed] [Google Scholar]
- 2.Malaviya A N, Kotwal P P. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol 200317319–343. [DOI] [PubMed] [Google Scholar]
- 3.Watts H G, Lifeso R M. Tuberculosis of bones and joints. J Bone Joint Surg Am 199678288–298. [DOI] [PubMed] [Google Scholar]
- 4.Hammoudeh M, Khanjar I. Skeletal tuberculosis mimicking seronegative spondyloarthropathy. Rheumatol Int. 2004;24: 50–2, Epub 2003 May 29. [DOI] [PubMed]
- 5.Bush D C, Schneider L H. Tuberculosis of the hand and wrist. J Hand Surg [Am] 19849391–398. [DOI] [PubMed] [Google Scholar]
- 6.Sood R, Wali J P, Handa R. Poncet's disease in a north Indian hospital. Trop Doct 19992933–36. [DOI] [PubMed] [Google Scholar]
- 7.Isenberg D A, Maddison P, Swana G, Skinner R P, Swana M, Jones M.et al Profile of autoantibodies in the serum of patients with tuberculosis, klebsiella and other gram‐negative infections. Clin Exp Immunol 198776516–523. [PMC free article] [PubMed] [Google Scholar]
- 8.Van Venrooj W J, Hazes J M W, Visser H. Anti‐citrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med 200260383–388. [PubMed] [Google Scholar]
- 9.van Gaalen F A, Linn‐Rasker S P, van Venrooij W J, de Jong B A, Breedveld F C, Verweij C L.et al Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum 200450709–715. [DOI] [PubMed] [Google Scholar]
- 10.Vander Cruyssen B, Hoffman I E, Zmierczak H, Van den Berghe M, Kruithof E, De Rycke L.et al Anti‐citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis 2005641145–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wener M H, Hutchinson K, Morishima C, Gretch D R. Absence of antibodies to cyclic citrullinated peptide in sera of patients with hepatitis C infection and cryoglobulinemia. Arthritis Rheum 2004502305–2308. [DOI] [PubMed] [Google Scholar]
- 12.Bombardieri M, Alessandri C, Labbadia G, Iannuccelli C, Carlucci F, Riccieri V.et al Role of anti‐cyclic citrullinated peptide antibodies in discriminating patients with rheumatoid arthritis from patients with chronic hepatitis C infection‐associated polyarticular involvement. Arthritis Res Ther 20046R137–R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugosson C, Nyman R S, Brismar J, Larsson S G, Lindahl S, Lundstedt C. Imaging of tuberculosis. Peripheral osteoarticular and soft tissue tuberculosis. Acta Radiol 199637512–516. [DOI] [PubMed] [Google Scholar]
- 14.Djavad N, Bas S, Shi X, Scwager J, Jeannet M, Vischer T.et al Comparison of rheumatoid factors of rheumatoid arthritis patients, of individuals with mycobacterial infections and of normal controls: evidence for maturation in the absence of an autoimmune response. Eur J Immunol 1996262480–2486. [DOI] [PubMed] [Google Scholar]
- 15.Adebajo A O, Charles P, Maini R N, Hazleman B L. Autoantibodies in malaria, tuberculosis and hepatitis B in a west African population. Clin Exp Immunol 19939273–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores‐Suarez L F, Cabiedes J, Villa A R, van der Woude F J, Alcocer‐Varela J. Prevalence of antineutrophilic cytoplasmatic autoantibodies in patients with Tuberculosis. Rheumatology (Oxford) 200342711. [DOI] [PubMed] [Google Scholar]