Abstract
Objective
To compare the effect of celecoxib with that of a classic non‐steroidal anti‐inflammatory drug (NSAID) on synovial inflammation and on the synovial expression of proinflammatory genes in patients with knee osteoarthritis (OA).
Methods
30 patients with severe knee OA scheduled for total knee replacement surgery were included in a 3 month clinical trial. They were randomised to two groups: patients treated with celecoxib (CBX) (200 mg/24 h) and patients treated with aceclofenac (ACF) (100 mg/12 h). Those patients with OA who did not want to be treated with NSAIDs served as a control group. During knee surgery, synovial fluid (SF) and synovial membrane (SM) were collected. A SM specimen was fixed and embedded in paraffin and another part was frozen for molecular biology studies.
Results
At the end of study both CBX and ACF treated patients showed a significant improvement in pain and knee function compared with controls. Both drugs significantly reduced prostaglandin E2 (PGE2) SF concentration and down regulated COX‐2 mRNA and protein expression at the SM. However, synovial macrophage infiltration (CD68 antigen staining) and expression of proinflammatory mediators, such as interleukin 1β and tumour necrosis factor α, were decreased only by CBX treatment.
Conclusion
Both drugs improved joint pain and function, inhibited SF PGE2 concentration, and induced a decrease in synovial COX‐2 expression and synthesis not related to the tissue inflammatory status. These data suggest that PGE2 blocking agents may decrease PGE2 production not only by direct COX‐2 inhibition but also by down regulating COX‐2 expression and synthesis. However, CBX and ACF appear to have different anti‐inflammatory profiles in controlling OA synovial macrophage infiltration and proinflammatory expression.
Keywords: osteoarthritis, COX‐2, synovial membrane, non‐steroidal anti‐inflammatory drugs, celecoxib
Osteoarthritis (OA) is the most common chronic joint disease. Joint cartilage degeneration is the central feature in OA, but is associated with concomitant changes in synovium and subchondral bone metabolism causing pain and stiffness in the affected joints and impaired physical function.1 Although OA is traditionally defined as a non‐inflammatory joint disease, many reports have highlighted the critical role of synovial inflammation in OA progression.1,2,3,4 Several investigators have pointed out the appearance of prominent inflammatory lesions at the synovial tissue, consisting of increased vascularisation, cellular infiltration and oedema of the sublining tissue, and hyperplasia of intimal synovial cells.3 A number of clinical studies have recently demonstrated an interesting association between synovitis, OA inflammation, and progression of structural changes.5,6,7,8,9 In this regard, synovial inflammation and local concentration of proinflammatory mediators seem to be directly involved in pain generation in OA joints.10,11,12
Few structure modifying drugs have been used to date for OA treatment, and the most commonly used drugs are symptom modifying, such as non‐steroidal anti‐inflammatory drugs (NSAIDs). These treatments have been shown to improve pain and disability effectively. Although many approaches have been used to study the effects of NSAID administration during OA, it is still not known whether these drugs might have any long term benefits in OA by modifying cartilage and/or synovial metabolism. Some in vivo and in vitro studies have suggested that NSAID administration may accelerate joint cartilage damage,10,13,14,15 but others have shown favourable effects.14,16,17,18,19 Gineyts and coworkers have recently reported that ibuprofen partially prevents synovial and cartilage degradation in OA.20 However, there are few in vivo studies on the influence of NSAIDs on OA synovial metabolism.
NSAIDs decrease prostaglandin (PG) synthesis by inhibiting cyclo‐oxygenase (COX), the key enzyme required for conversion of arachidonic acid into PGs. At least two COX isoforms have been identified to date: COX‐1, which is usually constitutively expressed in many tissues and has a homoeostatic role; COX‐2, which is only appreciably expressed during inflammatory conditions in response to the presence of several cytokines, growth factors, oncogenes, etc.21 Overexpression of the COX‐2 protein has a critical role in many pathophysiological states, including some forms of joint disease. A number of experimental and human studies have shown that PGE2 and COX‐2 synthesis are up regulated in OA synovial membranes (SMs), and suggest that selective inhibition of COX‐2 activity may result in an improvement of arthritic symptoms.22,23 However, little is known about the in vivo effect of the different drugs employed on synovial COX‐1 and COX–2 expression.
The purpose of this study was to compare the effects of a dual COX inhibitor, aceclofenac (ACF), and a selective COX‐2 inhibitor, celecoxib (CBX), on synovial inflammation, and SM expression of proinflammatory genes in patients with severe knee OA.
Patients and methods
Patients
This study comprised 30 patients with clinical and radiological evidence of knee OA who met the American Rheumatism Association criteria for functional classes III or IV. At the time of recruitment, patients had to have had a diagnosis of knee OA for at least 6 months based on clinical and radiological criteria. All patients had been scheduled for total knee replacement surgery at the orthopaedic surgery department of Hospital Virgen de la Cinta (Tortosa, Tarragona, Spain) and were offered treatment with NSAIDs until surgery. Those who accepted NSAID treatment were randomised to receive either CBX (200 mg/24 h) or ACF (100 mg/12 h). The duration of the treatment was 3 months±5 days for all the patients included in this study, and no differences were found between groups. At the beginning of the study we included 10 patients who received CBX and 10 who received ACF. Treatment was started after 1 week of washout. Ten patients who agreed to participate in the study but preferred not to be treated with an NSAID were also recruited and used as a control group. For all patients, acetaminophen was used as a rescue analgesic drug for pain control (500 mg three times a day maximum). Corticosteroid and hyaluronate injections were not allowed during the trial or in the 3 months before enrolment.
Patients were evaluated at three times: at enrolment (T0), after the washout period (T1), and 2 or 3 days before surgery (T2). Patient symptoms and physical disability were measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC),24 which evaluates pain, stiffness, and physical performance.
The day before surgery, serum samples were obtained and stored at −20°C until analysis. During knee surgery, synovial fluid (SF) was collected and stored at −70°C until analysis. Three separated fragments from the suprapatellar synovial tissue were excised and immediately frozen in liquid nitrogen for further gene expression studies. Two additional specimens from the same region of 2.5 cm length each were dissected and fixed for 24 hours in 4% paraformaldehyde, dehydrated, and divided into small pieces to be embedded in paraffin.
Synovial histopathological studies
For light microscopy, paraffin embedded SM sections were prepared and stained with haematoxylin and eosin and Masson's trichrome stain. Synovitis was evaluated in each sample according to the Krenn scale25 by two independent, blinded observers. In brief, three synovial compartments were scored as follows: intima layer (0 = low cell density, one to two rows; 1 = mild or focal hyperplasia; 2 = diffuse hyperplasia or focal areas of severe hypercellularity; 3 = diffuse prominent hyperplasia); interstitium (0 = normal interstitium; 1 = focal sublining fibrotic changes; 2 = increased interstitial matrix and vascularity; 3 = widespread fibrosis and vessel congestion); and leucocyte infiltrates (0 = scattered cells or no infiltration; 1 = groups of cells, small aggregates or foci of polymorphonuclear cells; 2 = frequent aggregates or abscess formation; 3 = spread infiltration or large aggregates). At least two paraffin blocks for each patient were evaluated to obtain a synovitis score.
Immunohistochemistry
Immunohistochemistry was performed as previously reported.26 A monoclonal antihuman CD68 antibody was used as primary antibody (KP‐1, DakoCytomation, at 4 μg/ml). Negative controls were performed in parallel by replacing the primary antibody with a non‐immune normal mouse serum. Only synovial sections in which the lining layer was identifiable were included in the analysis. All tissue sections were randomly evaluated by one investigator (OS‐P) who was unaware of the clinical details of the patients. A semiquantitative analysis was carried out using a 0–5 scale to allow discrimination between low percentages of positive cells, as described27,28 (0 = 0–1% positive cells; 1 = 2–10%; 2 = 11–25%; 3 = 26–50%; 4 = 51–75%; 5 = 76–100%). The density of CD68 positive cells was established in six different ×100 fields randomly selected by the examiner from a minimum of three specimens for each patient.
PGE2 concentration in SF
SF was collected in tubes containing EDTA and then treated with hyaluronidase (Boehringer) for 1 hour at 37°C (10 U/ml). Tubes were then centrifuged, and PGE2 concentration was measured in supernatants using a commercial kit (Assay Designs), according to the manufacturer's instructions. All the measurements were done in duplicate for each patient. Results are expressed in ng/ml as the mean (SEM) for each group.
Gene expression studies using real time polymerase chain reaction (PCR)
Total RNA from the SM was obtained by the acid guanidinium–phenol–chloroform method.29 The high‐capacity cDNA archive kit (Applied Biosystems) was used, and 1 μg of total RNA was reverse transcribed with 2.5 U/μl of a reverse transcriptase for 2 hours at 37°C in a total volume of 50 μl to obtain total cDNA. Measurement of specific RNA was performed by single‐reporter real time PCR using the ABI Prism 7500 Sequence Detection system (Applied Biosystems). The specific oligonucleotide primer pairs labelled with a reporter fluorescent dye (FAM) at the 5′ end, and the specific FAM TaqMan probe used for COX‐1 (assay ID: Hs00924811_m1; gene accession No NM_080591), COX‐2 (assay ID: Hs00153133_m1; gene accession No NM_000963), interleukin (IL) 1β (assay ID: Hs00174097_m1; gene accession No NM_000576), and tumour necrosis factor (TNF) α (assay ID: Hs00174128_m1; gene accession No NM_000594), were purchased from Applied Biosystems. A predesigned 18S ribosomal RNA (rRNA) assay (assay ID: Hs99999901_s1; gene accession No X03205) (Applied Biosystems) was also used as an endogenous control. TaqMan reactions were performed in a total volume of 25 μl containing forward primer, reverse primer, FAM TaqMan probe (or VIC dye for internal control), and 12.5 μl of TaqMan Universal PCR Master Mix (Applied Biosystems). The default thermal cycling programme of the ABI Prism 7500 sequence detection system was used for real time PCR (95°C for 10 minutes, followed by 40 cycles of two step PCR, including denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute). After confirmation that the amplification efficiency of COX‐1, COX‐2, IL1β, and TNFα and the endogenous control 18S rRNA were approximately equal, differences were calculated with the threshold cycle (Ct), and the comparative Ct method was used for relative quantification. COX‐1, COX‐2, IL1β, and TNFα mRNA were normalised relative to 18S rRNA in each well, and each patient value for each gene expression was then normalised relative to the calibrator value (one of the CBX patients was chosen as calibrator = 1). Three different RNA extractions from different synovial specimens for each patient were done. Similar results were obtained for each extraction.. The gene expression for each protein in each patient is the mean of these different RNA extractions.
Western blot analysis
For COX‐1, COX‐2, TNFα, and IL1β determinations we isolated SM total proteins, and western blot studies were performed as previously published.30 Again, two different protein extractions from different specimens for each patient were carried out. Primary antibodies against human COX‐1 and COX‐2 were obtained from Santa Cruz Biotech, TNFα antibody from PeproTech, and IL1β antibody from MBL. To ensure that equal amounts of total proteins were charged, each membrane was also hybridised with anti‐α‐tubulin (Sigma). Densitometric analysis of COX‐1, COX‐2, TNFα, and IL1β expression were then corrected by levels of α‐tubulin expression. Data are shown as densitometric arbitrary units as the mean (SEM) for each group.
Statistical analysis
All statistical analyses were performed using SPSS 8.0 for Windows (SPSS Inc). Results are expressed as mean (SEM), unless otherwise stated. Data from the three groups were compared by Kruskal‐Wallis non‐parametric analysis, and a pairwise comparison by Mann‐Whitney test was applied when overall differences were identified. Differences were considered significant for p<0.05.
Results
Baseline information and clinical response
Five of the 30 patients enrolled into the study were lost to follow up. One patient from the ACF group was excluded because of adverse events during treatment (gastric pain). Three patients were excluded because synovial samples could not be obtained during surgery owing to technical problems, and one final patient refused to undergo knee replacement at this time. So, the number of patients that completed the study were: nine for the CBX group, seven for the ACF group, and nine for the control group.
Table 1 shows the demographic and clinical characteristics of the subjects. Patient symptoms and physical disability were evaluated at different times: T0, T1, and T2, as described in “Patients and methods”. The differences in the WOMAC index at T0 were not statistically significant between the different groups. Both ACF and CBX treated patients reported significantly less pain in the T2 than in the T1 evaluation (table 1). However, control patients showed a significant increase in the WOMAC index at T2 as compared with T1, probably because no treatment was given during the study time (table 1).
Table 1 Baseline patient information.
| Baseline patient information | CBX | ACF) | Control |
|---|---|---|---|
| (n = 9) | (n = 7) | (n = 9) | |
| Sex (%) | |||
| Male | 22 | 14 | 22 |
| Female | 78 | 86 | 78 |
| Age (years) | 73 (5) | 71 (8) | 72 (8) |
| WOMAC (VAS) | |||
| After washout T1 | 2240 (918) | 2807 (644) | 1996 (390) |
| Before surgery T2 | 1952 (908)* | 2536 (528)* | 2140 (378)* |
| Difference T2−T1 | −289 (308) | −270 (359) | 144 (85) |
Values are shown as mean (SEM) unless stated otherwise.
* p<0.05 v T1 data from the same group of patients.
Synovial membrane histopathology
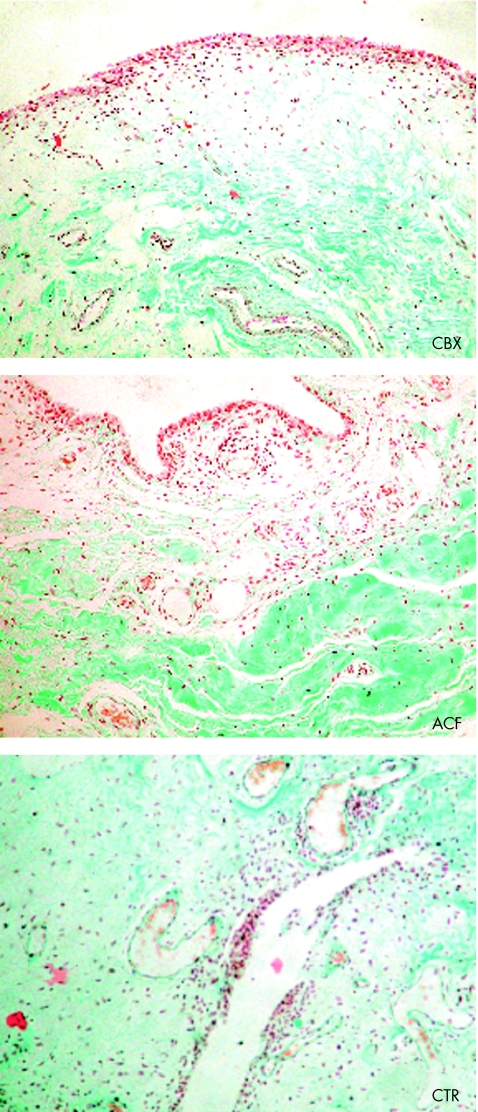
All synovial specimens stained with haematoxylin and eosin and Masson's trichrome stain were evaluated. The histopathological findings which predominated were an increased density of sublining vessels, the appearance of leucocyte infiltrates, and focal to diffuse hyperplasia of the intima layer (fig 1). These changes were scored for each sample as described in the “Patients and methods” section. The mean (SEM) global synovitis scores for each group were 4.0 (0.6) for CBX, 4.1 (0.9) for ACF, and 3.8 (1) for control patients. No significant differences were seen in this score between the three patient groups studied either in the overall synovitis score or in any of the three subscales evaluated (intima layer, interstitium, and leucocyte infiltrate). The global synovitis score obtained for these groups agreed with previous observations reported by Krenn et al in OA tissues.25
Figure 1 Synovial membrane histopathology in CBX, ACF, and control groups of patients from specimens obtained during knee replacement surgery. Photomicrographs are representative for seven to nine patients from each group (haematoxylin and eosin; original magnification ×100).
CD68 immunostaining
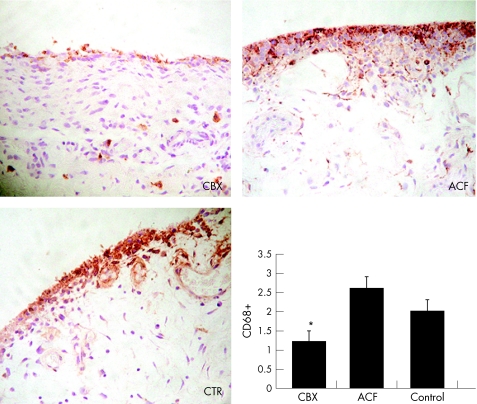
CD68 staining was localised both at the lining and the sublining layer of the synovial samples (fig 2). In control patients, CD68 immunoreactivity was found in a patchy distribution at the sublining area, while it was abundant at the lining layer (fig 2). In both groups of patients treated with COX inhibitors the distribution pattern of CD68 antibody binding was similar to that of control synovial tissues (fig 2), but the amount of positive cells both at the lining layer and at the interstitium was clearly lower in CBX treated patients than in ACF treated and control patients (p<0.05) (fig 2).
Figure 2 Localisation of CD68 positive cells in the SM sections from CBX, ACF, and control patients. Photographs are representative of seven to nine patients from each group (peroxidase technique; original magnification ×200). The bar graph shows the semiquantitative scoring according to the description given in the “Patients and methods” section. *p<0.05 v ACF patients. Data represent the mean (SEM), n = 7–9 per group.
PGE2 concentration in SF
PGE2 was measured in previously digested SF samples. Mean (SEM) SF PGE2 levels were 95 (12) ng/ml for the CBX group, n = 9; 103 (41) ng/ml for the ACF group, n = 7; and 269 (115) for the control group, n = 9, with significant difference between CBX and controls (p = 0.031) and between ACF and controls (p = 0.036). The PGE2 concentration was not significantly different between CBX and ACF treated patients.
COX expression and synthesis
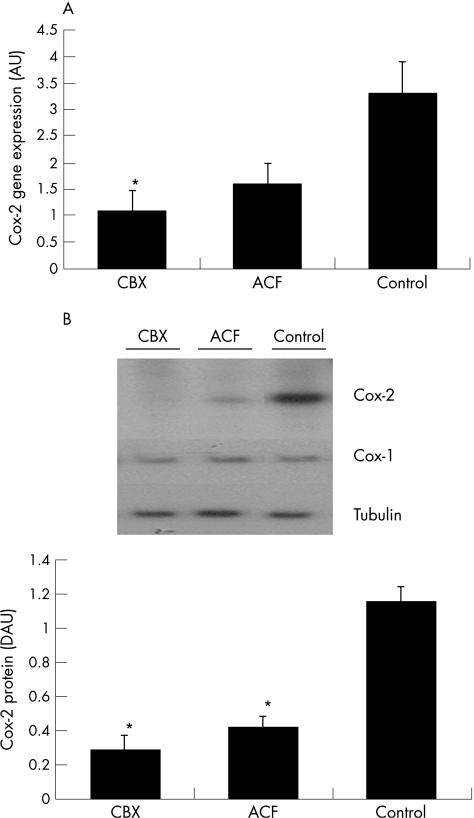
COX‐1 and COX‐2 RNA expression in synovial tissue from patients with OA was assessed by real time PCR studies. CBX, ACF, and control patients showed no statistically significant differences in COX‐1 gene expression. However, COX‐2 gene expression was decreased in CBX treated patients in comparison with the control group (fig 3A). COX‐2 expression in ACF treated patients showed a similar trend, but the difference was not statistically significant when compared with the control group. COX‐2 expression was not significantly different between CBX and ACF treated patients (fig 3A).
Figure 3 COX‐2 gene expression and protein presence at the SM in CBX, ACF, and control groups of patients. (A) COX‐2 expression measured by real time PCR. The comparative Ct method was used for relative quantification. COX‐1 and COX‐2 mRNA expression were normalised relative to 18S rRNA in each well, and each patient value for each gene expression was then normalised relative to the calibrator value (one of the CBX patients was chosen as calibrator = 1). (B) Representative western blots of COX‐2 and α‐tubulin, and a densitometric analysis of COX‐2 levels expressed in arbitrary units (AU) after correction by α‐tubulin are shown. *p<0.05 v control patients. Data represent the mean (SEM), n = 7–9 patients per group.
Induction of COX protein was then examined using western blot studies. As shown in fig 3B, similar results were recorded as obtained for RNA expression. COX‐1 was expressed in similar amounts in the three groups of patients. However, COX‐2 protein was induced to a much greater extent in control patients than in treated patients. Both ACF and CBX treated patients had a statistically significant decrease in COX‐2 protein levels in comparison with control patients (fig 3B). However, no significant differences where shown in the COX‐2 synthesis between CBX and ACF treated patients. Our results also showed a significant correlation between the presence of COX‐2 protein and PGE2 concentration in SF (rs = 0.46; p<0.05) and serum PGE2 concentration (rs = 0.52; p<0.05). The same correlation study for COX‐2 gene expression and PGE2 concentration did not reach statistical significance.
IL1β and TNFα expression and synthesis
To investigate whether NSAID treatment could modify synovial cytokine expression and synthesis during severe OA, we measured the gene expression and protein presence of two proinflammatory cytokines associated with OA development.
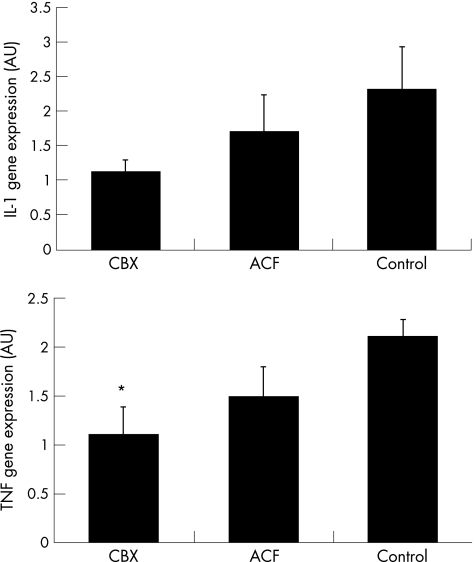
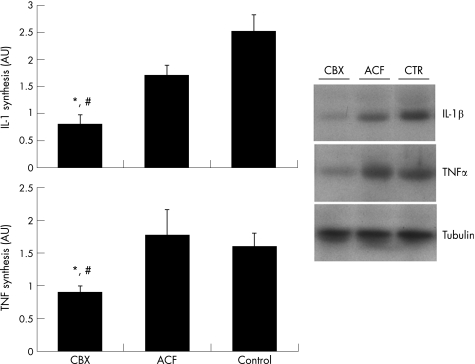
Quantitative PCR experiments showed that IL1β gene expression was not significantly modified by these treatments in the SM of patients with OA, although there was a clear trend in CBX treated patients for a decreased IL1β expression (fig 4). TNFα expression was significantly inhibited in CBX treated patients in comparison with control patients, while no significant effect was noted for the ACF group (fig 4).
Figure 4 Gene expression of IL1β and TNFα at the SM in CBX, ACF, and control groups of patients measured by real time PCR. The comparative Ct method was used for relative quantification. IL1 and TNFα mRNA expression were normalised relative to 18S rRNA in each well, and each patient value for each gene expression was then normalised relative to the calibrator value (one of the CBX patients was chosen as calibrator = 1). All real time PCR samples were processed in triplicate. *p<0.05 v control patients. Data represent the mean (SEM), n = 7–9 per group.
To investigate whether gene expression results correlated with protein presence, a western blot analysis for TNFα and IL1β presence in the SM extracts was performed. Our results showed that both IL1β and TNFα proteins were significantly decreased in the CBX group in comparison with control patients, while no statistically significant differences were found between ACF and control patients (fig 5). TNFα and IL1β synthesis were also significantly different between CBX and ACF treated patients (fig 5).
Figure 5 IL1β and TNFα in the SM of CBX, ACF, and control groups of patients. Right panel: representative western blots of IL1β, TNFα, and α‐tubulin are shown. Left panel: a densitometric analysis of IL1β and TNFα levels expressed in arbitrary units (AU) after correction by α‐tubulin. *p<0.05 v control patients. #p<0.05 v ACF patients. Data represent the mean (SEM), n = 7–9 per group.
Discussion
PGE2, the eicosanoid that has been found in higher concentrations in inflamed joints,31 modulates a number of characteristic features of the progressive tissue destruction occurring in OA, such as proteinase activation, matrix protein synthesis, cell proliferation/apoptosis, and even sensitisation of nociceptors (reviewed by Martel‐Pelletier et al21 and Crofford32). Increased PGE2 levels have been detected in ST and SF in OA.33,34 The SM appears to be the major source of PGE2 production during OA.35 In this study we have shown that two NSAIDs used for OA treatment, ACF and CBX, similarly improved joint pain and function and decreased PGE2 concentration in SF. Moreover, these treatments inhibited COX‐2 gene expression and synthesis in the SM of patients with OA. Our data show that both ACF and CBX decrease PGE2 production not only by enzyme activity inhibition but also by a direct effect on COX‐2 gene expression and synthesis. To our knowledge, this is the first in vivo demonstration of a significant effect of NSAIDs on tissue COX‐2 regulation in humans. These data are in line with published results obtained with indometacin and a COX‐2 selective inhibitor in the SM of arthritic rats.36 Previous in vitro studies have also suggested that COX‐2 expression may be regulated by NSAIDs, although different results have been reported. In cultured synovial fibroblasts, several types of anti‐inflammatory drugs (for example, dexamethasone or nimesulide) inhibited COX‐2 synthesis,37 while others (for example, naproxen, meloxicam, indometacin) had no effects.35,37,38 However, a recent report on a study in human leukaemic cells postulated that such discrepancies may be due both to differences in the cell activator and to the different anti‐inflammatory potency of NSAIDs.39 Authors of this study demonstrated that all NSAIDs tested inhibited COX‐2 expression if their concentration was sufficiently high.39
The mechanism by which COX‐2 might be inhibited in the presence of a decreased PGE2 concentration remains unknown. However, PGE2 may be partially responsible for this situation, as it has been shown that PGE2 stabilises COX‐2 mRNA and promotes its translation in synovial cells.40 In this context, we have recently shown that the presence of PGE2 greatly induced COX‐2 synthesis in cultured synovial fibroblasts stimulated with IL1β.30
In addition to PGE2 concentration inhibition, COX‐2 inhibitors may act through new mechanisms that are currently being discussed. In this regard, in vivo anti‐tumour effects have been shown for a number of NSAIDs.41,42 This effect might be explained, at least in part, by indirect mechanisms such as an anti‐angiogenic action43,44 or changes in cell motility and invasiveness evoked both by COX‐2 selective and non‐selective inhibitors.42 Whether CBX or ACF can modify angiogenesis in the SM of patients with OA remains unknown. In our study, these treatments appeared ineffective for modifying vessel proliferation at the SM along with the global synovitis score. But in this regard, Kreen's method25 probably lacks sensitivity to examine synovial angiogenesis as a relevant feature of synovial injury.
NSAIDs appear to share some degree of similarity in that they all inhibit PGE2 synthesis. However, their other activities are widely different—for example, inhibition of cytokine synthesis, toxicity for normal or OA chondrocytes, activity on cartilage matrix synthesis, and so on. To assess whether a COX‐2 selective inhibitor such as CBX might have a different anti‐inflammatory profile from ACF in these patients, we studied expression and synthesis of two cytokines with an essential role during OA—namely, IL1β and TNFα. These experiments showed that, in addition to decreasing PGE2 concentration and COX‐2 presence, long term CBX administration inhibited the expression and synthesis of IL1β and TNFα in the SM of patients with OA in comparison with untreated patients. However, ACF treatment did not significantly modify these measures. In addition, macrophage presence was significantly decreased by CBX in comparison with ACF treatment. Local levels of cytokines, mainly IL1β and TNFα from inflamed synovial tissue which are locally increased during OA,45 control the mechanisms of structural joint degradation in OA.46 An increased cytokine synthesis occurring in parallel to PGE2 decrease during NSAID treatment has been reported by other authors both in vivo and in vitro.47,48,49,50 Different authors have attributed these results to suppression of the protective effect of PGE2.48,50 Because CBX and ACF treated patients show different cytokine expression and macrophage density, while having similar PGE2 and COX‐2 profiles, our data suggest that an additional anti‐inflammatory mechanism independent of PGE2 and COX‐2 must take place in the SM of CBX treated patients. Cytokines have an essential role in inflammation in chronic synovitis and are mainly produced upon activation of monocytes/macrophages.51 It might be speculated that macrophage depletion is responsible, at least partly, for the decreased cytokine synthesis shown in our study. In this sense, CBX has been shown to be a pro‐apoptotic agent for several cell types, including human macrophages and synovial fibroblasts.52,53 The hypothesis that the pro‐apoptotic effect of CBX might be a COX independent event is supported by recent findings that demonstrate an apoptotic effect of CBX in COX‐2 negative cells. Furthermore, CBX derivatives that were unable to inhibit COX‐2 act as apoptotic agents.54,55,56
The significance of synovitis in the pathophysiology of OA is increasingly recognised.2,57 Recent studies have shown the existence of a strong association between OA progression and inflammation.58,59 Therefore, suppression of inflammation might slow disease progression. In this study we have shown that in patients with knee OA, long term NSAID treatment with both CBX or ACF similarly improved joint pain and function, inhibited PGE2 concentration in SF, and decreased COX‐2 expression and synthesis in the SM. However, CBX and ACF appear to have different anti‐inflammatory profiles in controlling macrophage infiltration and proinflammatory cytokine expression in the SM.
Acknowledgements
This work was partially supported by research grants from the Spanish Ministry of Education (SAF 2003/08379); Comunidad Autonoma de Madrid (GR/SAL/0798/2004), Fondo de Investigaciones Sanitarias (CP03/00011; G03/152; 04/0259; 05/0485), Fundacion Mapfre Medicina and Pfizer Spain.
Abbreviations
ACF - aceclofenac
CBX - celecoxib
COX - cyclo‐oxygenase
IL - interleukin
NSAIDs - non‐steroidal anti‐inflammatory drugs
OA - osteoarthritis
PCR - polymerase chain reaction
PG - prostaglandin
SF - synovial fluid
SM - synovial membrane
TNF - tumour necrosis factor
WOMAC - Western Ontario and McMaster Universities Osteoarthritis Index
Footnotes
Both authors contributed equally to this work
Ethics approval: the local ethics committee approved the study, and informed consent was obtained from all patients.
References
- 1.Pelletier J P. Etiopathogenesis of osteoarthritis. In: Koopman WJ, ed. A textbook of rheumatology. Arthritis and allied conditions. Baltimore: Lippincott Williams & Wilkins, 20011969–1984.
- 2.Pelletier J P, Martel‐Pelletier J, Abramson S B. Osteoarthritis, an inflammatory disease. Potential implication for the selection of new therapeutic targets. Arthritis Rheum 2001441237–1247. [DOI] [PubMed] [Google Scholar]
- 3.Saito I, Koshino T, Nakashima K, Uesugi M, Saito T. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthritis Cartilage 200210156–162. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg W B, van der Kraan P, van Beuningen H M.Synovial mediators of cartilage damage and repair in OA. Oxford, UK: Oxford University Press, 1998157–167.
- 5.Sharif M, Shepstone L, Elson C J, Dieppe P A, Kirwan J R. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis 20005971–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharif M, Kirwan J, Elson C J, Granell R, Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum 2004502479–2488. [DOI] [PubMed] [Google Scholar]
- 7.Petersson I F, Boegard T, Svensson B, Heinegard D, Saxne T. Changes in cartilage and bone metabolism identified by serum markers in early osteoarthritis of knee joint. Br J Rheumatol 19983746–50. [DOI] [PubMed] [Google Scholar]
- 8.Spector T D, Hart D J, Nandra D, Doyle D V, Mackillop N, Gallimore J R.et al Low‐level increases in serum C‐reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum 199740723–727. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg R L, Huff J P, Lenz M E, Glickman P, Katz R, Thonar E J. Elevated plasma levels of hyaluronate in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 199134799–807. [DOI] [PubMed] [Google Scholar]
- 10.Brandt K D. Effects of nonsteroidal anti‐inflammatory drugs on chondrocyte metabolism in vitro and in vivo. Am J Med 19878329–34. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura M, Segami N, Kaneyama K, Suzuki T, Miyamaru M. Relationship between pain‐related mediators and both synovitis and join pain in patients with internal derangements and osteoarthritis of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 200294328–332. [DOI] [PubMed] [Google Scholar]
- 12.Trumble T N, Billinghurst R C, McIlwraith C W. Correlation of prostaglandin E2 concentrations in synovial fluid with ground reaction forces and clinical variables for pain and inflammation in dogs with osteoarthritis induced by transection of the cranial cruciate ligament. Am J Vet Res 2004651269–1275. [DOI] [PubMed] [Google Scholar]
- 13.Rainsfold K D. Mechanisms of NSAIDs on joint destruction in osteoarthritis. Agents Actions Suppl 19934439–43. [PubMed] [Google Scholar]
- 14.Dingle J T. The efffects of NSAIDs in human articular cartilage GAG synthesis. Eur J Rheumatol Inflamm 19961647–52. [Google Scholar]
- 15.Serni U, Mannoni A M, Benucci M. Is there preliminary in vivo evidence for an influence of nonsteroidal antiinflammatory drugs on progression in osteoartritis? Part II: evidence from animal models. Osteoarthritis Cartilage 19997351–352. [DOI] [PubMed] [Google Scholar]
- 16.Dooley M, Spencer C M, Dunn C J. Aceclofenac: a reappraisal of its use in the management of pain and rheumatic disease. Drugs 2001611351–1378. [DOI] [PubMed] [Google Scholar]
- 17.Blot L, Marcelis A, Devogrlaer J P, Manicourt D H. Effect of diclofenac, aceclofenac and meloxicam on the metabolism of proteoglycans and hyaluronan in osteaoarthritic human cartilage. Br J Pharmacol 20001311413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes J C, Caron J P, Martel‐Pelletier J, Jovanovic D, Mineau F, Tardif G.et al Effects of tenidap on the progression of osteoarthritic lesions in a canine experimental model. Suppression of metalloprotease and interleukin‐1 activity. Arthritis Rheum 199740284–294. [DOI] [PubMed] [Google Scholar]
- 19.Mastbergen S C, Bijlsma J W, Lafeber F P. Selective COX‐2 inhibition is favorable to human early and late‐stage osteoarthritic cartilage: a human in vitro study. Osteoarthritis Cartilage 200513519–526. [DOI] [PubMed] [Google Scholar]
- 20.Gineyts E, Mo J A, Ko A, Henriksen D B, Curtis S P, Gertz B J.et al Effects of ibuprofen on molecular markers of cartilage and synovium turnover in patients with knee osteoarthritis. Ann Rheum Dis 200463857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martel‐Pelletier J, Pelletier J P, Fahmi H. Cyclooxygenase‐2 and prostaglandins in articular tissues. Semin Arthritis Rheum 200333155–167. [DOI] [PubMed] [Google Scholar]
- 22.Siegle I, Klein T, Backman J T, Saal J G, Nusing R M, Fritz P. Expression of cyclooxygenase 1 and cyclooxygenase 2 in human synovial tissue: differential elevation of cyclooxygenase 2 in inflammatory joint diseases. Arthritis Rheum 199841122–129. [DOI] [PubMed] [Google Scholar]
- 23.Iñiguez M A, Pablos J L, Carreira P, Cabre F, Gomez‐Reino J J. Detection of COX‐1 and COX‐2 isoforms in synovial fluid cells from inflammatory joint diseases. Rheumatology (Oxford) 199837773–778. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan W W, Goldsmith C H, Campbell J, Stitt L W. Validation study of WOMAC: a health status instrument for measuring clinically important participant relevant outcomes to antirheumatic drug therapy in participants with osteoarthritis of the hip or knee. J Rheumatol 1998151833–1840. [PubMed] [Google Scholar]
- 25.Krenn V, Moravietz L, Häupl T, Neidel J, Petersen I, Köning A. Grading of chronic synovitis ‐ a histopathological grading system for molecular and diagnosis pathology. Pathol Res Pract 2002198317–325. [DOI] [PubMed] [Google Scholar]
- 26.Palacios I, Lopez‐Armada M J, Hernandez P, Sanchez‐Pernaute O, Gutierrez S, Miguelez R.et al Tenidap decreases IL‐8 and monocyte chemotactic peptide‐1 (MCP‐1) mRNA expression in the synovial tissue of rabbits with antigen arthritis and in cultured synovial cells. Clin Exp Immunol 199811588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolhain R J, Tak P P, Dijkmans B A, De Kuiper P, Breedveld F C, Miltenburg A M. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br J Rheumatol 199837502–508. [DOI] [PubMed] [Google Scholar]
- 28.Kane D, Gogarty M, O'Leary J, Silva I, Bermingham N, Bresnihan B.et al Reduction of synovial sublining layer inflammation and proinflammatory cytokine expression in psoriatic arthritis treated with methotrexate. Arthritis Rheum 2004503286–3295. [DOI] [PubMed] [Google Scholar]
- 29.Chomczynski P, Sacchi N. Single‐step method of RNA isolation by acid guanidinium thiocynate‐phenolchloroform extraction. Anal Biochem 1987162155–159. [DOI] [PubMed] [Google Scholar]
- 30.Largo R, Diez‐Ortego I, Sanchez‐Pernaute O, Lopez‐Armada M J, Alvarez‐Soria M A, Egido J.et al EP2/EP4 signalling inhibits monocyte chemoattractant protein‐1 production induced by interleukin 1β in synovial fibroblasts. Ann Rheum Dis 2004631197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetzl E J, An S, Smith W L. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J 199591051–1058. [DOI] [PubMed] [Google Scholar]
- 32.Crofford L J. COX‐2 in synovial tissues. Osteoarthritis Cartilage 19997406–408. [DOI] [PubMed] [Google Scholar]
- 33.Sahap Atik O. Leukotriene B4 and prostaglandin E2‐like activity in synovial fluid in osteoarthritis. Prostaglandins Leukot Essent Fatty Acids 199039253–254. [DOI] [PubMed] [Google Scholar]
- 34.Wittenberg R H, Willburger R E, Kleemeyer K S, Peskar B A. In vitro release of prostaglandins and leukotrienes from synovial tissue, cartilage, and bone in degenerative joint diseases. Arthritis Rheum 1993361444–1450. [DOI] [PubMed] [Google Scholar]
- 35.Hardy M M, Seibert K, Manning P T, Currie M G, Woerner B M, Edwards D.et al Cyclooxygenase 2‐dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum 2002461789–1803. [DOI] [PubMed] [Google Scholar]
- 36.Anderson G D, Hauser S D, McGarity K L, Bremer M E, Isakson P C, Gregory S A. Selective inhibition of cyclooxygenase (COX)‐2 reverses inflammation and expression of COX‐2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest 1996972672–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahmi H, He Y, Zhang M, Martel‐Pelletier J, Pelletier J P, Di Battista J A. Nimesulide reduces interleukin‐1beta‐induced cyclooxygenase‐2 gene expression in human synovial fibroblasts. Osteoarthritis Cartilage 20019332–340. [DOI] [PubMed] [Google Scholar]
- 38.Kojima F, Naraba H, Miyamoto S, Beppu M, Aoki H, Kawai S. Membrane‐associated prostaglandin E synthase‐1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res Ther 20046R355–R365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takada Y, Bhardwaj A, Potdar P, Aggarwal B B. Nonsteroidal anti‐inflammatory agents differ in their ability to suppress NF‐kappaB activation, inhibition of expression of cyclooxygenase‐2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene 2004239247–9258. [DOI] [PubMed] [Google Scholar]
- 40.Faour W H, He Y, He Q W, de Ladurantaye M, Quintero M, Mancini A.et al Prostaglandin E(2) regulates the level and stability of cyclooxygenase‐2 mRNA through activation of p38 mitogen‐activated protein kinase in interleukin‐1 beta‐treated human synovial fibroblasts. J Biol Chem 200127631720–31731. [DOI] [PubMed] [Google Scholar]
- 41.Moore B C, Simmons D L. COX‐2 inhibition, apoptosis and chemoprevention by nonsteroidal antiinflammatory drugs. Curr Med Chem 200071131–1144. [DOI] [PubMed] [Google Scholar]
- 42.Zha S, Yegnasubramanian V, Nelson W G, Isaacs W B, de Marzo A M. Cyclooxygenases in cancer: progress and perspective. Cancer Lett 20042151–20. [DOI] [PubMed] [Google Scholar]
- 43.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois R N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 199893705–716. [DOI] [PubMed] [Google Scholar]
- 44.Masferrer J L, Koki A, Seibert K. COX‐2 inhibitors. A new class of antiangiogenic agents. Ann N Y Acad Sci 199988984–86. [DOI] [PubMed] [Google Scholar]
- 45.Smith M D, Triantafillou S, Parker A, Youssef P P, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 199724365–371. [PubMed] [Google Scholar]
- 46.Fernandes J C, Martel‐Pelletier J, Pelletier J P. The role of cytokines in osteoarthritis physiology. Biorheology 200239237–246. [PubMed] [Google Scholar]
- 47.Ferrari A J, Van Linthoudt D, Morrone L, Branigan P, Schumacher H R, Baker D G. Nonsteroidal anti‐inflammatory drugs and prostaglandins: their interactions and effects on the particulate‐induced inflammatory process implicated in joint implant‐loosening and monosodium urate crystal‐induced inflammation. Am J Ther 19963189–194. [PubMed] [Google Scholar]
- 48.Schumacher H R, Meng Z, Sieck M, Zonay L, Clayburne G, Baker J F.et al Effect of a nonsteriodal antiinflammatory drug on synovial fluid in osteoarthritis. J Rheumatol 1996231774–1777. [PubMed] [Google Scholar]
- 49.He W, Pelletier J P, Martell‐Pelletier J, Laufer S, Di Battista J A. Synthesis of interleukin‐1β, tumor necrosis factor‐α and interstitial collagenase (MMP‐1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with inflammatory cytokines. J Rheumatol 200229546–553. [PubMed] [Google Scholar]
- 50.Lopez‐Armada M J, Sánchez‐Pernaute O, Largo R, Diez‐Ortego I, Palacios I, Egido J.et al Modulation of cell recruitment by anti‐inflammatory agents in antigen‐induced arthritis. Ann Rheum Dis 2002611027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burger D, Dayer J M. The role of human T‐lymphocyte‐monocyte contact in inflammation and tissue destruction. Arthritis Res 20024(suppl 3)S169–S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusunoki N, Yamazaki R, Kawai S. Induction of apoptosis in rheumatiod synovial fibroblast by celecoxib but not by other selective cyclooxigenase 2 inhibitors. Arthritis Rheum 2002463159–3167. [DOI] [PubMed] [Google Scholar]
- 53.Subhashini J, Mahipal S V, Reddanna P. Anti‐proliferative and apoptotic effects of celecoxib on human chronic myeloid leukemia in vitro. Cancer Lett 20051631–43. [DOI] [PubMed] [Google Scholar]
- 54.Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX‐2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX‐2 inhibitor celecoxib. FASEB J 2001152742–2744. [DOI] [PubMed] [Google Scholar]
- 55.Song X, Lin H P, Johnson A J, Tseng P H, Yang Y T, Kulp S K.et al Cyclooxygenase‐2, player or spectator in cyclooxygenase‐2 inhibitor‐induced apoptosis in prostate cancer cells. J Natl Cancer Inst 200294585–591. [DOI] [PubMed] [Google Scholar]
- 56.Waskewich C, Blumenthal R D, Li H, Stein R, Goldenberg D M, Burton J. Celecoxib exhibits the greatest potency amongst cyclooxygenase (COX) inhibitors for growth inhibition of COX‐2‐negative hematopoietic and epithelial cell lines. Cancer Res 2002622029–2033. [PubMed] [Google Scholar]
- 57.Bonnet C S, Walsh D A. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005447–16. [DOI] [PubMed] [Google Scholar]
- 58.Pelletier J P. Rationale for the use of structure‐modifying drugs and agents in the treatment of osteoarthritis. Osteoarthritis Cartilage 200412S63–S68. [DOI] [PubMed] [Google Scholar]
- 59.Ayral X, Pickering E H, Woodworth T G, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis ‐ results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 200513361–371. [DOI] [PubMed] [Google Scholar]