Abstract
Background
Anti‐citrullinated protein antibodies (ACPA) are specifically and frequently detected in sera of patients with rheumatoid arthritis (RA). Citrullinated fibrin or fibrinogen is a candidate autoantigen of such antibodies.
Objective
To investigate the presence of citrullinated fibrinogen (cFBG) in the plasma or synovial fluid of patients with RA and control patients, and to determine cFBG levels and their relationship with serum markers for RA if it is present.
Methods
A sandwich enzyme linked immunosorbent assay (ELISA) to measure cFBG was established using monoclonal antibodies cF16.1 and cF252.1, generated by immunising mice with R16Cit and R252Cit, the fibrinogen Aα chain derived sequences with citrulline at position 16 and 252, respectively, and the presence of cFBG was further investigated with immunoprecipitation‐western blotting.
Results
Positive signals were detected in 11/15 RA synovial fluids (RASFs), but not in osteoarthritis synovial fluids or RA plasma with sandwich ELISA for cFBG using cF16.1 and an anti‐modified citrulline (AMC) antibody. The presence of cFBG in RASFs was confirmed by immunoprecipitation‐western blotting. Furthermore, most RA sera strongly reacted against R16Cit. No relationship was seen between RASF cFBG levels and C reactive protein or anti‐cyclic citrullinated peptide antibody levels of the paired sera.
Conclusion
cFBG is detected as a soluble citrullinated autoantigen in RASFs and may therefore be a genuine candidate antigen for ACPA in patients with RA.
Keywords: anti‐citrulline‐containing peptide antibody, rheumatoid arthritis, synovial fluid
Anti‐citrullinated protein antibodies (ACPA), such as antifilaggrin antibodies and anti‐cyclic citrullinated peptide (CCP) antibodies, are useful serological markers for the diagnosis of rheumatoid arthritis (RA).1,2,3,4,5 Citrulline is formed by the post‐translational modification of peptidylarginine by peptidylarginine deiminase (PADI). Five isotypes have been identified6,7,8,9,10,11,12 and PADI1‐4, at least, require calcium ions. PADI2 and PADI4 are thought to be related to RA, both of which are found in the RA synovium.13,14,15,16 Our large scale, genome‐wide case‐control study using single nucleotide polymorphisms found that a PADI4 polymorphism is distinctly associated with RA and also with levels of antibodies to citrullinated filaggrin in the sera of Japanese patients with RA.15
Citrullinated forms of the α and β chains of fibrin have been identified in the RA synovium by Masson‐Bessiere et al,17 and these results were supported by subsequent reports.14,18,19 Fibrinogen, which constitutes a substrate for PADI2 and PADI4,16 is produced in the liver, and infiltrates synovial fluid (SF) through vessels. Then, under the activated coagulating pathway in the RA synovium,20,21,22,23,24,25 it polymerises into insoluble fibrin. The real target of the anti‐citrulline‐containing peptide antibody is still unknown, but because anti‐citrullinated fibrinogen (cFBG) antibody has been identified as a useful marker for RA with excellent sensitivity and specificity,26,27 we assume that citrullinated fibrin(ogen) is a genuine autoantigen for ACPA.
Though citrullinated fibrin is commonly observed, it is not yet known whether it is citrullinated only after fibrinogen polymerisation or whether fibrinogen is also citrullinated in blood or SF. Furthermore, if cFBG is present, its relevance to RA disease characteristics or activities is of exceptional interest. Thus, we were prompted to set up a sandwich enzyme linked immunosorbent assay (ELISA) to measure cFBG levels to investigate the presence of cFBG and its association with RA using monoclonal antibodies (mAbs) that recognise cFBG.
To generate mAbs, we prepared two peptides, R16Cit and R252Cit as immunogens, respectively corresponding to positions 11–21, 247–257 of the secreted Aα chain with the citrulline substituted from arginine at position 16 and 252, both of which are recognised by PADI4.16 Thrombin recognises 16Arg for the cleavage of fibrinopeptide A (FPA), and thus holds a key to fibrinogen polymerisation into fibrin. We also investigated RA sera reactivity against these peptides to determine whether these positions are citrullinated or not, based on the study by Schellekens et al,3 in which amino acid sequences adjoining citrulline were shown to be crucial in the determination of antigenicity.
Materials and methods
Citrullination of human fibrinogen
Human fibrinogen (100 μl; 5 mg/ml; American Diagnostics, Pendleton, IN, USA) was citrullinated with 30 μl of recombinant human PADI4 (prepared as previously described,16 1.3 U/mg fibrinogen, for the standard of sandwich ELISA) or 10 μl of rabbit PADI2 (Sigma, St Louis, MO, USA, 5 U/mg fibrinogen, for the selection of mAbs) in a 1 ml reaction buffer (100 mM Tris, 93 mM NaCl, 20 mM CaCl2, 5 mM dithiothreitol, pH 7.4). The incubation was performed for 2 hours at 37°C and, finally, 200 μl of 0.2 M EDTA was added to stop the reaction. For the control, 0.2 M EDTA was added to native fibrinogen (nFBG) and other reaction mix components before the incubation.
Generation of murine monoclonal antibodies against human citrullinated fibrinogen
BALB/c mice were immunised with synthetic peptides R16Cit and R252Cit (fig 1A) conjugated with keyhole limpet haemocyanin. After immunisation, the spleen cells of the mice were fused with myeloma cells and the hybridoma cell lines were cloned with a limiting dilution method. The heavy chain isotypes of mAbs were identified with a mouse mAb isotyping kit (Amersham, Town, UK), according to the manufacturer's protocol. Selected clones were cultured in RPMI 1640 with 10% fetal bovine serum, and antibodies from culture media were purified by a HiTrap IgM purification column (Amersham) after pretreatment with a HiTrap protein G.
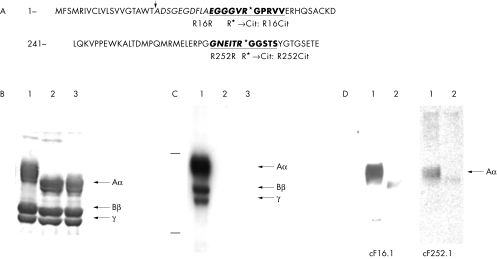
Figure 1 Generation and selection of mAbs against cFBG. (A) The sequences of position 1–49 and 241–284 of the fibrinogen Aα chain are shown, and the sequences of R16R and R252R are underlined. The mRNA encoded fibrinogen is modified post‐translationally and at the point indicated by an arrow the secreted form of fibrinogen starts. R16R and R252R, respectively, correspond to positions 11–21 and 247–257 of the secreted fibrinogen Aα chain. R16Cit and R252Cit, used as antigens for mAbs, have citrulline substituted from arginine at position 16 and 252, respectively. Fibrinopeptide A is indicated by italics. (B–D) Selection of the mAbs against cFBG. The enzymatic modification was confirmed by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) followed by (B) Coomassie brilliant blue staining and western blotting using (C) the anti‐modified citrulline (AMC) antibody and (D) mAbs cF16.1 and cF252.1. Lane 1, cFBG; lane 2, nFBG; lane 3, fibrinogen treated by rabbit PADI2 as in lane 1, with EDTA added before treatment.
The clones obtained more or less recognised the non‐citrulline control R16R or R252R (fig 1A), as well as R16Cit and R252Cit. A further selection of clones that preferentially recognise the cFBG Aα chain was performed by western blotting. cFBG and nFBG were subjected to 10% SDS‐PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Bio‐Rad, Hercules, CA, USA). After blocking by 5% skimmed milk in Tris buffered saline with 0.1% Tween 20 (TBST), the membranes were incubated for 2 hours at room temperature with mAbs. After washes with TBST, 1:2000 goat antimouse immunoglobulin, horseradish peroxidase (HRP) conjugate (Sigma) was added and they were incubated for 2 hours. After washes, the membranes were visualised with electrochemiluminescence western blotting detection reagents (Amersham).
Plasma, serum, and synovial fluid samples
Twenty seven plasma and 36 serum samples were collected from patients who fulfilled the American College of Rheumatology criteria for RA, and normal control sera were collected from eight healthy subjects. Fifteen RA synovial fluids (RASFs) and five osteoarthritis synovial fluids (OASFs) were collected in EDTA‐containing or heparinised tubes at the time of therapeutic arthrocentesis, centrifuged at 450 g for 5 minutes to remove any debris, and the supernatants were collected. Informed consent was obtained for all the samples.
Sandwich ELISA for cFBG using the AMC antibody
cF16.1 and cF252.1, the selected mAbs (described in detail in “Results”), were coated on plate wells (Nunc Maxisorp, Roskilde, Denmark) at 10 μg/ml in 50 mM carbonate buffer, pH 9.5. After an incubation at 4°C overnight the wells were washed with phosphate buffered saline with 0.05% Tween 20 (PBST), then blocked with 3% bovine serum albumin (BSA; Sigma)/PBST for 1 hour. Then, the standard cFBG, control nFBG, plasma, and SF samples diluted in 1% BSA/HEPES buffered saline: 100 mM HEPES, 100 mM NaCl, pH 7.2 with 0.1% Tween 20(HBST) were applied to the wells. After incubation at 4°C overnight, the wells were washed, incubated with 1% glutaraldehyde/PBS for 1 hour, then with 0.2 M Tris‐Cl, pH 7.8 for 30 minutes. After washes, reagent A and B (modified citrulline detection kit; Upstate, Chicago, IL, USA) were applied and the wells were incubated overnight at 37°C.
After washes, the wells were blocked with 5% BSA/PBST for 30 minutes, then incubated with 1:2500 AMC antibody (Upstate) in 2.5% BSA/PBST for 3 hours at 37°C, successively with goat F(ab′)2 antirabbit immunoglobulin HRP conjugate, 1:50 000 (Biosource, Camarillo, CA, USA) for 2 hours at 37°C. After washes, 3,3′,5,5′‐tetramethylbenzidine substrate (KPL, Gaithersburg, MD, USA) was applied and the colour development was stopped with 0.5 M H2SO4. Then, the absorbance at a wavelength of 450 nm (A450) was read.
For all the ELISAs in our study, the samples were tested in duplicate and the A450 of blank wells was subtracted from that of the other wells.
Fibrinogen is very abundant in plasma (1.5–3 g/l) and is normally expected to be present in native forms. Thus, we investigated whether the mAbs could capture cFBG from nFBG‐rich samples such as plasma. Normal plasma (1:50), which contains 100–1000‐fold as much nFBG as the cFBG used for the standard curve, was added to the diluent and the assays were performed as above. For the comparison, goat F(ab′)2 antihuman fibrinogen (Cappel, Aurora, OH, USA) was used as the capture antibody.
Sandwich ELISA for cFBG using an antihuman fibrinogen antibody for the detection and quantification of fibrinogen in plasma and SFs
To detect cFBG, plate wells were coated with cF16.1 and cF252.1, respectively, incubated at 4°C overnight, and blocked with 3% BSA/PBST for 1 hour. Then cFBG, nFBG, plasma, and SFs in 1% BSA/HBST were applied, and the wells were incubated for 3 hours at 37°C. After washing, the wells were incubated with 1:250 antihuman fibrinogen antibody, HRP conjugate (FG‐EIA‐D; Affinity Biologicals, Ontario, Canada) for 2 hours at room temperature. After washes and colour development, A450 was read.
For the quantification of fibrinogen the procedures were the same, except that goat F(ab′)2 antihuman fibrinogen (Cappel) was coated, and normal referential plasma was used as the standard.
Immunoprecipitation and western blotting
cF16.1 (5 μg) was added to 400 μl of 1:20 diluted RASFs and OASFs and incubated for 2 hours at 4°C. Then, Dynabeads coupled with rat antimouse IgM (Dynal, Oslo, Norway) were added to the samples and they were incubated for a further 2 hours at 4°C. Next, the beads were collected and washed as instructed by the manufacturer. Laemmli sample buffer was added to the beads and they were heated for 5 minutes at 95°C. Then, the supernatants were collected after centrifugation and subjected to SDS‐PAGE and western blotting. SF samples diluted 1:15 were prepared for comparison.
For the detection of citrullination, blotted PVDF membranes were disposed of as instructed by the manufacturer (modified citrulline detection kit; Upstate). After chemical modification, the membrane was washed with distilled water and blocked with 5% skimmed milk/TBST for 30 minutes, incubated with 1:1000 AMC antibody in 2.5% BSA/TBST for 1 hour, then successively with 1:5000 antirabbit immunoglobulin antibody (Upstate) for 1 hour after washes with TBST. The membrane was visualised with Supersignal WestDura substrate (Pierce, Rockford, IL, USA). To detect fibrinogen, membranes were blocked with 5% skimmed milk/TBST for 30 minutes, incubated with 1:1500 rabbit antihuman fibrinogen (Hyphen BioMed, Andresy, France) for 1 hour, then with 1:30 000 antirabbit immunoglobulin HRP conjugate (Biosource) for 1 hour. The membranes were visualised with electrochemiluminescence‐plus.
Measurement of reactivity against R16Cit and R252Cit by sera
The peptides R16R, R16Cit, R252R, R252Cit conjugated with BSA were coated on wells at 10 μg/ml and incubated at 4°C overnight. The wells were washed and blocked with 3% BSA/PBST and incubated with 1:200 diluted sera in 1% BSA/PBST at 4°C overnight. Then, the wells were washed and incubated with 1:50 000 goat F(ab′)2 antihuman IgG, HRP conjugate (Biosource) at 37°C for 2 hours. Colour development was performed and A450 of the reaction against R16Cit minus that against R16R (ΔR16Cit−R16R), and A450 of the reaction against R252Cit minus that against R252R (ΔR252Cit−R252R) were assessed.
Measurement of autoantibodies to CCP
CCP antibody levels were measured with a DIASTAT anti‐CCP ELISA kit (Axis‐Shield, Cambridgeshire, UK).
Statistical analyses
The non‐parametric data obtained for the sera reactivity against peptides and the correlations between CCP antibody or C reactive protein and A450 levels measured with cF16.1‐AMC ELISA were analysed with Wilcoxon's rank sum test and Spearman's rank correlation test, respectively, for significance (p<0.05).
Results
Preparation and characterisation of murine anti‐cFBG mAbs
As described in “Materials and methods”, the two clones cF16.1 and cF252.1 were selected by western blotting (fig 1B–D). These clones, both IgM, preferentially recognised the cFBG Aα chain.
Establishment of sandwich ELISA standard curves for citrullinated fibrinogen
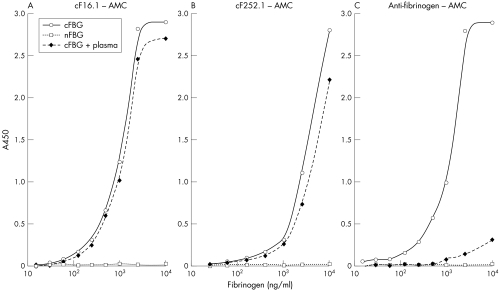
The standard curves were established with cF16.1 and cF252.1 as capture antibodies and AMC and antihuman fibrinogen as detection antibodies. Though not definitely specific to fibrinogen, the ELISA with AMC was specific to citrulline up to 10 μg/ml and the standard curves were almost unaffected by the addition of 1:50 normal plasma (figs 2A and B), which is obvious when compared with the case in which the anti‐fibrinogen antibody was used for capturing (fig 2C), whereas the ELISA with antihuman fibrinogen was preferential for cFBG, but cross reacted with nFBG at a higher concentration (figs 3A and B)
Figure 2 The standard curves of sandwich ELISA for cFBG using the AMC antibody for detection. (A) cF16.1, (B) cF252.1, (C) goat F(ab′)2 antihuman fibrinogen antibodies were used as capture antibodies. cFBG + plasma means cFBG was prepared in a diluent containing 1:50 diluted normal referential plasma. In (C), nFBG in diluted plasma accounted for almost all the binding capacities of capture antibodies, while in (A) and (B) cF16.1 and cF252.1 selectively captured cFBG, even in the presence of abundant nFBG.
Figure 3 Standard curves of sandwich ELISA for cFBG using the antihuman fibrinogen antibody for detection. (A) cF16.1, (B) cF252.1 were used as the capture antibodies. In (A) and (B) cFBG was preferentially recognised, but nFBG was also detected at a higher concentration.
All the samples were first tested with AMC for citrulline specificity and negligible interference by nFBG, which is abundantly present in plasma and SF, and the positive samples with AMC were further tested with anti‐fibrinogen.
Sandwich ELISA using cF16.1 or cF252.1 and the AMC antibody
The sensitivity of the ELISA was determined to be 16 ng/ml and 30 ng/ml for cF16.1 and cF252.1, respectively, and because all the samples were tested at a dilution of 1:25, the cut off lines for positivity were set at 400 and 750 ng/ml, respectively.
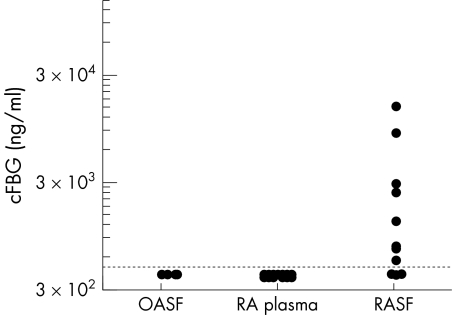
With cF16.1, positive signals were detected in 11/15 RASFs, while the signals of OASFs and plasma samples were below the level of detection (fig 4).
Figure 4 The levels of cFBG measured by cF16.1‐AMC sandwich ELISA. All the samples were tested at 1:25 and the cut off line for sensitivity was 400 ng/ml. Signals for cFBG were detected in 11/15 RASFs, while all of the RA plasma (n = 27) and OASF samples (n = 5) were below the level of detection.
Assuming that the degree of citrullination was the same between samples and the standard cFBG citrullinated in vitro with PADI4, the levels of cFBG were 500 ng/ml to 15.2 μg/ml for positive RASFs.
With cF252.1 as the capture antibody, only two RASFs were positive for cFBG. Both were also positive with cF16.1, although the concentrations were 1.2 μg/ml and 1.7 μg/ml, much lower than with cF16.1 (8.7 and 15.2 μg/ml, respectively).
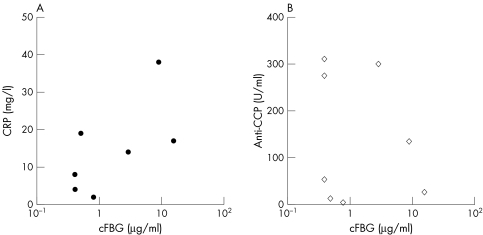
For patients with paired sera available (n = 8), no clear correlation was observed between RASF cFBG levels and serum C reactive protein (fig 5A, Spearman's coefficient (rs) = 0.57, p = 0.13) or anti‐CCP antibody levels (fig 5B, rs = −0.26, p = 0.48).
Figure 5 The correlations between RASF cFBG levels measured by cF16.1‐AMC ELISA and C reactive protein (CRP) (A) or anti‐CCP antibody levels (B) of the paired sera. No significant correlation was observed between them. (rs = 0.57 and p = 0.13 for CRP, rs = −0.26 and p = 0.48 for anti‐CCP antibody).
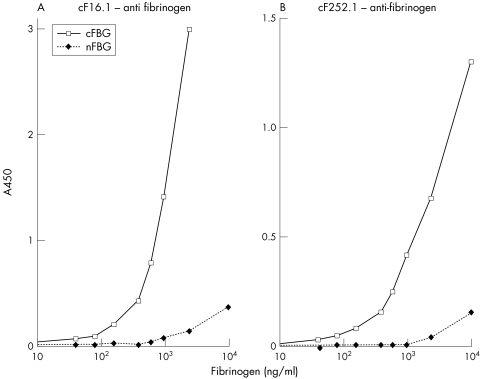
Sandwich ELISA using cF16.1 and cF252.1 and an antihuman fibrinogen antibody for the detection
Samples were serially diluted and positive signals of plasma samples, proved to be citrulline negative in the ELISA with AMC, were observed up to 1:200 owing to the cross reactivity against nFBG. They disappeared at 1:400 completely, at which level nFBG interference in SFs was also negligible because the fibrinogen concentration was below 1000 mg/l for all the SFs (data not shown), and was lower than plasma levels. Eight samples selected from SF and plasma were diluted 1:400 and subjected to the assay. cFBG positive RASFs with AMC were also positive in this assay (table 1). This further demonstrated the presence of cFBG.
Table 1 A450 levels of 1:400 diluted plasma and synovial fluid samples in cF16.1‐anti fibrinogen sandwich ELISA.
| Sample | Fibrinogen | cFBG* | A450 |
|---|---|---|---|
| (mg/l) | (μg/ml) | ||
| RASF1 | 504 | 2.9 | −0.02 |
| RASF3 | 950 | 8.7 | 0.15 |
| RASF4 | 558 | 15.2 | 0.23 |
| RASF6 | 162 | <0.4 | −0.04 |
| OASF4 | 93 | <0.4 | −0.03 |
| Normal plasma | 1800 | <0.4 | −0.04 |
| RA plasma 18 | 3780 | <0.4 | 0.04 |
| RA plasma 19 | 3200 | <0.4 | 0.04 |
*cFBG levels were obtained from the results of the cF16.1‐AMC sandwich ELISA.
Immunoprecipitation and western blot
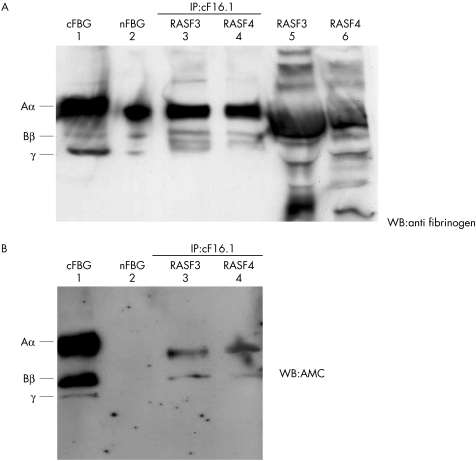
To further confirm the citrullination of fibrinogen in RASFs, RASF3, 4 (cFBG concentrations measured by cF16.1‐AMC ELISA were 8.7, 15.2 μg/ml, respectively) were immunoprecipitated with cF16.1, and the precipitates were subjected to western blotting by anti‐fibrinogen (fig 6A) and AMC (fig 6B).
Figure 6 A demonstration of the presence of cFBG in RA synovial fluids using the immunoprecipitation (IP)‐western blotting (WB) method. Samples diluted 1:15 and immunoprecipitates using cF16.1 were obtained for cFBG positive RASFs (RASF3, RASF4) and subjected to western blotting by anti‐ fibrinogen (A) and AMC (B). Lane 1, cFBG as the positive control; lane 2, nFBG as the positive control for anti‐ fibrinogen and negative control for AMC; lanes 3 and 4, immunoprecipitates of RASF3 and 4 using cF16.1; lanes 5 and 6, RASF3 and 4 diluted at 1:15. Three consecutive chains at the levels of fibrinogen Aα, Bβ, γ were immunoprecipitated (A, lanes 3, 4) from many fibrinogen related products (A, lanes 5, 6), and Aα and Bβ chains in RASF 3, 4 were confirmed to be citrullinated. (B) The signal for AMC was stronger in Aα than in Bβ.
As shown in fig 6A, three bands at the levels of the fibrinogen Aα, Bβ, γ chains were immunoprecipitated (lanes 3, 4) from numerous fibrinogen related bands observed in the same SFs (lanes 5, 6). For the same precipitates Aα and Bβ were positive for AMC, with Aα more citrullinated than Bβ (fig 6B). Thus, the presence of cFBG in RASFs was demonstrated.
ELISA for measurement of the reactivity of RA sera against R16Cit and R252Cit
The mAbs were expected to recognise the immunised peptides R16Cit and R252Cit, but it is difficult to tell whether they exclusively recognised the epitopes. Amino acids adjoining citrulline are critical in the determination of antigenicity, and if RA sera react against the peptides differently or disproportionately, a difference in the degree of citrullination at positions 16 and 252 is likely.
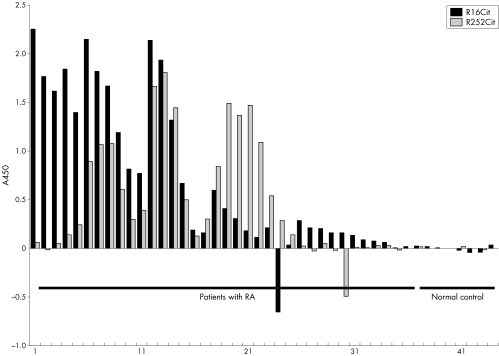
The median (2SD) of ΔR16Cit−R16R was 0.301 (1.55) (range −0.609 to 2.263) and that of ΔR252Cit−R252R was 0.262 (1.202) (range −0.490 to 1.811) for 36 patients with RA, and 0.010 (0.019) (range −0.013 to 0.032) and 0.008 (0.021) (range −0.024 to 0.027), respectively, for eight normal controls. Although the patterns of reactivity differed among samples (fig 7), R16Cit was significantly more strongly antigenic to RA sera (p<0.05), at least in comparison with R252Cit.
Figure 7 The A450 levels of RA sera reactivity against R16Cit and R252Cit. RA sera in general reacted against R16Cit significantly more strongly (p<0.05), some even exclusively, but the patterns showed a wide variety depending on the patient.
Discussion
Fibrin has been shown to be citrullinated in synovial tissues of inflamed joints.14,17,18,19 The presence of a soluble citrullinated antigen, cFBG, was newly demonstrated in our study. cFBG was not detected in RA plasma, whereas it was detected in 11/15 RASFs, and this showed that fibrinogen is citrullinated, not in blood, but exclusively in joints before its polymerisation, suggesting leakage of PADI enzymes in SFs and highly activated citrullination.
cF252.1 did not work effectively in capturing cFBG. This may be due to the lower sensitivity compared with cF16.1 or the interference by citrullinated fibrin degradation products, in which R252Cit is included, but position 252 may be less citrullinated than position 16. In contrast, cF16.1 captured cFBG from RASFs successfully, and RA sera strongly reacted against R16Cit, which implies the citrullination of position 16, the thrombin cleavage site, in cFBG. Dysfibrinogenaemia is often associated with the mutation of position 16 and impaired FPA release.28,29,30,31,32 The replacement of basic arginine with neutral citrulline at this position may hamper the binding of thrombin. Thus protected from polymerisation and made stable as a soluble antigen, cFBG may become antigenic as a modified form of the self antigen, fibrinogen. cFBG was detected only in RASFs and not in OASFs in our study, while the citrullination of synovial fibrin, which shares almost the same structure as fibrinogen, was shown to be a common phenomenon in any synovitis, including OA.18,19 We recently found that PADI4, expressed in neutrophils, is associated with RA in Japanese subjects,15 and is immunohistochemically colocalised with apoptotic cells.14 Therefore, the discrepancy may be explained by the difference in the degree of neutrophil infiltration between RASF and OASF. Whereas almost no neutrophils are present in OASFs, they massively infiltrate RASFs and at the time of their apoptosis PADI4 is assumed to leak out abundantly and recognise and citrullinate fibrinogen at an early stage before its polymerisation. On the other hand, fibrin deposits are very stable and may be citrullinated with as minute an amount of PADI as is detectable in OA synovium. A larger number and a wider variety of SFs should be tested to examine whether cFBG is specifically detected in RA. If cFBG is specifically observed in RA, it may be a real target for ACPA.
Aα chains were more visible than Bβ in the immunoprecipitate of RASFs. This is consistent with our previous report using liquid chromatography with tanden mass spectrometry,16 in which more peptidylarginines proved to be citrullinated in Aα than in Bβ. The γ chain was shown to be less efficiently citrullinated than Aα or Bβ16,17 and unrecognised by sera positive for ACPA,17,19 and we assume that it was undetectable owing to this even lower citrullination.
For the association with RA disease characteristics, cFBG may work not only as a pathogenic antigen but also as a disease exacerbating factor by forming immunocomplexes in RASFs with anti‐cFBG antibodies that are frequently detected in patients with RA.26,27,33
In conclusion, we detected, for the first time, cFBG in RASFs as a soluble citrullinated autoantigen and confirmed that fibrinogen citrullination takes place in joints. The thrombin cleavage site is thought to be citrullinated and this makes cFBG antigenic to patients with RA. Also, cFBG may be a genuine antigen for soluble immunocomplexes. Further studies are required to evaluate the precise role of cFBG and its association with PADI4.
Acknowledgements
This work was supported by a grant from the Japanese Millennium Project. We thank Dr Akihiro Yamaguchi and Dr Yuko Okazaki (Department of Allergy and Rheumatology, The University of Tokyo Hospital) for their technical advice and assistance.
Abbreviations
ACPA - anti‐citrullinated protein antibodies
AMC - anti‐modified citrulline
BSA - bovine serum albumin
cFBG - citrullinated fibrinogen
CCP - cyclic citrullinated peptide
ELISA - enzyme linked immunosorbent assay
FPA - fibrinopeptide A
HBST - HEPES buffered saline with 0.1% Tween 20
HRP - horseradish peroxidase
IP - immunoprecipitation
mAb - monoclonal antibody
nFBG - native fibrinogen
OA - osteoarthritis
PADI - peptidylarginine deiminase
PBST - phosphate buffered saline with 0.05% Tween 20
PVDF - polyvinylidene difluoride
RA - rheumatoid arthritis
SDS‐PAGE - sodium dodecyl sulphate‐polyacrylamide gel electrophoresis
SF - synovial fluid
TBST - Tris buffered saline with 0.1% Tween 20
Footnotes
Competing interests: None.
Ethical approval: This study was approved by the University of Tokyo ethical committee and the ethical committee of The Institute of Physical and Chemical Research, Japan.
References
- 1.Vincent C, Simon M, Sebbag M, Girbal‐Neuhauser E, Durieux J J, Cantagrel A.et al Immunoblotting detection of autoantibodies to human epidermis filaggrin: a new diagnostic test for rheumatoid arthritis. J Rheumatol 199825838–846. [PubMed] [Google Scholar]
- 2.Vincent C, Nogueira L, Sebbag M, Chapuy‐Regaud S, Arnaud M, Letourneur O.et al Detection of antibodies to deiminated recombinant rat filaggrin by enzyme‐linked immunosorbent assay: a highly effective test for the diagnosis of rheumatoid arthritis. Arthritis Rheum 2002462051–2058. [DOI] [PubMed] [Google Scholar]
- 3.Schellekens G A, de Jong B A, van den Hoogen F H, van de Putte L B, van Venrooij W J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis‐specific autoantibodies. J Clin Invest 1998101273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schellekens G A, Visser H, de Jong B A, van den Hoogen F H, Hazes J M, Breedveld F C.et al The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 200043155–163. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Sawada T, Murakami A, Matsui T, Tohma S, Nakazono K.et al High diagnostic performance of ELISA detection of antibodies to citrullinated antigens in rheumatoid arthritis. Scand J Rheumatol 200332197–204. [DOI] [PubMed] [Google Scholar]
- 6.Takahara H, Tsuchida M, Kusubata M, Akutsu K, Tagami S, Sugawara K. Peptidylarginine deiminase of the mouse. Distribution, properties, and immunocytochemical localization. J Biol Chem 198926413361–13368. [PubMed] [Google Scholar]
- 7.Senshu T, Kan S, Ogawa H, Manabe M, Asaga H. Preferential deimination of keratin K1 and filaggrin during the terminal differentiation of human epidermis. Biochem Biophys Res Commun 1996225712–719. [DOI] [PubMed] [Google Scholar]
- 8.Rogers G, Winter B, McLaughlan C, Powell B, Nesci T. Peptidylarginine deiminase of the hair follicle: characterization, localization, and function in keratinizing tissues. J Invest Dermatol 1997108700–707. [DOI] [PubMed] [Google Scholar]
- 9.Ishigami A, Kuramoto M, Yamada M, Watanabe K, Senshu T. Molecular cloning of two novel types of peptidylarginine deiminase cDNAs from retinoic acid‐treated culture of a newborn rat keratinocyte cell line. FEBS Lett 1998433113–118. [DOI] [PubMed] [Google Scholar]
- 10.Asaga H, Nakashima K, Senshu T, Ishigami A, Yamada M. Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. J Leukoc Biol 20017046–51. [PubMed] [Google Scholar]
- 11.Guerrin M, Ishigami A, Mechin M C, Nachat R, Valmary S, Sebbag M.et al cDNA cloning, gene organization and expression analysis of human peptidylarginine deiminase type I. Biochem J 2003370167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vossenaar E R, Zendman A J, van Venrooij W J, Pruijn G J. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 2003251106–1118. [DOI] [PubMed] [Google Scholar]
- 13.Vossenaar E R, Radstake T R D, van der Heijden A, van Mansum M A M, Dieteren C, de Rooij D J.et al Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis 200463373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang X, Yamada R, Suzuki A, Sawada T, Yoshino S, Tokuhiro S.et al Localization of peptidyl argininedeiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford) 20054440–50. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M.et al Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet 200334395–402. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama‐Hamada M, Suzuki A, Kubota K, Takazawa T, Ohsaka M, Kawaida R.et al Comparison of enzymatic properties between hPADI2 and hPADI4. Biochem Biophys Res Commun 2005327192–200. [DOI] [PubMed] [Google Scholar]
- 17.Masson‐Bessiere C, Sebbag M, Girbal‐Neuhauser E, Nogueira L, Vincent C, Senshu T.et al The major synovial targets of the rheumatoid arthritis‐specific antifilaggrin autoantibodies are deiminated forms of the alpha‐ and beta‐chains of fibrin. J Immunol 20011664177–4184. [DOI] [PubMed] [Google Scholar]
- 18.Vossenaar E R, Smeets T J M, Kraan M C, Raats J M, van Venrooij W J, Tak P P. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum 2004503485–3494. [DOI] [PubMed] [Google Scholar]
- 19.Chapuy‐Regaud S, Sebbag M, Baeten D, Clavel C, Foulquier C, De Keyser F.et al Fibrin deimination in synovial tissue is not specific for rheumatoid arthritis but commonly occurs during synovitides. J Immunol 20051745057–5064. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez‐Pernaute O, Largo R, Calvo E, Alvarez‐Soria M A, Egido J, Herrero‐Beaumont G. A fibrin based model for rheumatoid arthritis. Ann Rheum Dis 2003621135–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busso N, Hamilton J A. Extravascular coagulation and the plasminogen activator/plasmin system in rheumatoid arthritis. Arthritis Rheum 2002462268–2279. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg J B, Pippen A M, Greenberg C S. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 199134996–1005. [DOI] [PubMed] [Google Scholar]
- 23.So A K, Varisco P A, Kemkes‐Matthes B, Herkenne‐Morard C, Chobaz‐Peclat V, Gerster J C.et al Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost 200312510–2515. [DOI] [PubMed] [Google Scholar]
- 24.Clemmensen I, Holund B, Andersen R B. Fibrin and fibronectin in rheumatoid synovial membrane and rheumatoid synovial fluid. Arthritis Rheum 198326479–485. [DOI] [PubMed] [Google Scholar]
- 25.Carmassi F, de Negri F, Morale M, Song K Y, Chung S I. Fibrin degradation in the synovial fluid of rheumatoid arthritis patients. A model for extravascular fibrinolysis. Semin Thromb Hemost 199622489–496. [DOI] [PubMed] [Google Scholar]
- 26.Nielen M M, van der Horst A R, van Schaardenburg D, van der Horst‐Bruinsma I E, van de Stadt R J, Aarden L.et al Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann Rheum Dis 2005641199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nugueira L, Chapuy‐Regaud S, Constantin A, Clavel C, Sebbag M, Cantagre A.et al Autoantibodies to deiminated fibrinogen are the most efficient serological criterion for early rheumatoid arthritis diagnosis. Arthritis Res Ther 20035(suppl 1)S6 [Google Scholar]
- 28.Haverkate F, Samama M. Familial dysfibrinogenemia and thrombophilia: report on a study of the SCC subcommittee on fibrinogen. Thromb Haemost 199573151–161. [PubMed] [Google Scholar]
- 29.Stucki B, Zenhausern R, Biedermann B, Baudo F, Redaelli R, Lammle B.et al Fibrinogens Bern IV, Bern V and Milano XI: three dysfunctional variants with amino acid substitutions in the thrombin cleavage site of the Aalpha‐chain. Blood Coagul Fibrinolysis 19991093–99. [PubMed] [Google Scholar]
- 30.Mosesson M W. Dysfibrinogenemia and thrombosis. Semin Thromb Hemost 199925311–319. [DOI] [PubMed] [Google Scholar]
- 31.Martinez J. Congenital dysfibrinogenemia. Curr Opin Hematol 19974357–365. [DOI] [PubMed] [Google Scholar]
- 32.Galanakis D K. Inherited dysfibrinogenemia: emerging abnormal structure associations with pathologic and nonpathologic dysfunctions. Semin Thromb Hemost 199319386–395. [DOI] [PubMed] [Google Scholar]
- 33.Zvaifler N J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol 197316265–336. [DOI] [PubMed] [Google Scholar]