Abstract
Background
Severity of rheumatoid arthritis and progression of radiographic joint damage have decreased over the last decades.
Aim
To examine whether this trend is attributable to an underlying trend towards milder disease or to improved treatment.
Methods
The study used an inception cohort of patients with early rheumatoid arthritis seen at the Wichita Arthritis Center, Wichita, Kansas, USA, since 1973 and monitored prospectively since their first clinic visit through clinical, radiographic, laboratory, demographic and self‐reported data. The radiographic disease progression in patients with disease onset in the 1970s, 1980s and 1990s was compared using a multivariate regression model for longitudinal data. The analysis was adjusted for differences in baseline predictors, type of disease‐modifying antirheumatic drugs (DMARDs) and steroid use.
Results
418 patients with rheumatoid arthritis with radiographic follow‐up were included. Patients in earlier decades used fewer DMARDs, had longer disease durations and higher tender joint counts at their first visit. Other important predictors of disease progression did not differ significantly between decades of disease onset. The unadjusted rates of radiographic progression differed between decades (analysis of variance, p = 0.01), with a significant trend towards less radiographic progression in more recent times (trend, p<0.001). However, after adjusting for DMARD use, steroid use and baseline predictors, differences between decades vanished (analysis of variance, p = 0.40) and the trend towards less radiographic progression disappeared (trend, p = 0.45).
Conclusion
These results suggest that the observed trend towards milder disease in rheumatoid arthritis is attributable to more effective antirheumatic treatment and not to a secular trend.
Incidence and severity of rheumatoid arthritis have decreased over the last decades and a secular trend towards a milder disease has been postulated.1,2,3,4 The incidence of rheumatoid arthritis in the USA has dropped by half over the last 50 years,5 and similar developments have been described elsewhere.6,7 The disease has become less frequent, but clinicians have also described a change in the presentation of rheumatoid arthritis, with less extra‐articular manifestations and improved long‐term outcomes.8,9,10,11,12,13,14 Progression of structural joint damage and long‐term functional disability have decreased over time in several large cohorts of rheumatoid arthritis.8,10,15,16,17 Classic complications of rheumatoid arthritis, such as the Felty syndrome or rheumatoid vasculitis, seem to have become less common,13,18 although this trend was not confirmed in other studies.19,20 It remains unclear whether the disease itself has become less severe or whether antirheumatic treatments have become more effective.9
The purpose of this study was to analyse a trend towards milder disease in rheumatoid arthritis, as estimated by radiographic damage progression and functional disability over time. We hypothesised that the observed time trends in disease progression may be due to changes in treatment and aimed at examining the association of time of diagnosis with disease progression, independently of the effect of treatment, which changed over time.
Methods
Study population
Our study cohort included an inception cohort of patients with rheumatoid arthritis seen at the Wichita Arthritis Center, Kansas, USA, between 1 January 1973 and August 1999. The data bank and the characteristics of this cohort have been described.21 Briefly, patients were self‐referred or referred by the doctor and monitored prospectively since their first clinic visit through clinical, radiographic, laboratory, demographic and self‐reported data, including pain, global and Health Assessment Questionnaire (HAQ) measurements.
Inclusion criteria for this analysis consisted of a diagnosis of rheumatoid arthritis by using either the 1958 or the 1987 ARC criteria for rheumatoid arthritis,22,23 early disease at enrolment (<3 years since onset of disease) and available radiographic follow‐up with at least two consecutive sets of radiographs during the first 10 years of disease. The follow‐up period was truncated at 10 years to increase the homogeneity of observation periods and to avoid ceiling effects with radiographic damage scores. Exclusion criteria were the absence of a baseline radiograph taken within the first 3 years of disease.
Study design
This is a longitudinal observational study of a clinic‐based cohort of patients with rheumatoid arthritis. The overall effect of time on the severity of disease encompasses the causal effects of different antirheumatic treatments, different patient selection and potential secular trends. To examine the direct causal effect of time on severity of disease progression, the analysis needs to be controlled for differences in disease‐modifying antirheumatic drug (DMARD) use and in baseline predictors over time.
Outcome variable
The primary end point was the radiographic rate of disease progression as measured by the difference in x ray damage scores divided by the time of follow‐up. For each patient, the progression rate was computed from a linear regression line of all available radiographic time points (see Analysis section). Radiographic damage was assessed using Larsen scores on hand x rays according to the published method24 (range 0–250). All radiographs were taken under the same conditions (postero‐anterior incidence) at the Wichita Arthritis Center. In September 1998, Dr Arvi Larsen scored 3538 radiographs from patients with rheumatoid arthritis enrolled in the Wichita Arthritis cohort.25 Radiographs were read in random order within sets.
A secondary end point of this study was the progression of functional disability, as measured by the change from baseline in the Stanford HAQ,26 which tends to increase slowly over time in rheumatoid arthritis (average of 0.03 units per year27). The HAQ is a 20‐item self‐report questionnaire with scores ranging from 0 to 3, and is the most widely used functional status questionnaire in rheumatology that has been shown to predict work disability,28 joint replacement,29 medical costs30 and mortality31 in rheumatoid arthritis. Radiographic damage and HAQ scores are correlated in advanced disease.32
Exposure variable and predictors
The exposure of interest for this study was the calendar year of disease onset. All patients were classified according to the decade of diagnosis of rheumatoid arthritis. Important predictors of progression of rheumatoid arthritis, such as measures of disease activity, self‐assessed symptom questionnaires, various disease characteristics, demographic characteristics and information on treatment, were extracted from the database to be used in the analysis. Another predictor was the estimated yearly rates of radiographic progression at baseline, which were computed by dividing the radiographic scores at baseline by the disease duration. Estimated yearly progression rates at baseline seem to best predict future radiographic damage progression in randomised controlled trials.33 We determined the time span in years in which each individual DMARD regimen had been used during follow‐up and used this variable to control the analysis for DMARD use. All combination treatments (two or more concomitant DMARDs) were considered together as one additional DMARD regimen, to improve the effectiveness of the analysis.
Analysis
On the basis of previous studies,8,10,15,16,17 we calculated that a sample size of 100 patients per decade with an α error <0.05 would provide more than 90% power to detect differences in radiographic rates of progression of 1 Larsen score unit per year.
We categorised patients into decades according to the diagnosis of rheumatoid arthritis: patients with disease onset in the 1970s, 1980s and the 1990s. Baseline disease characteristics were compared across the three groups. We assessed the significance of differences in means of normally distributed variables with one‐way analysis of variance (ANOVA) and with the Kruskal‐Wallis test for non‐normally distributed variables. For dichotomous variables, Pearson's χ2 test was used to evaluate the significance of differences in proportions.
As the standard of practice has changed over time, patients in different decades are characterised by significant differences in baseline covariates (table 1), hence confounding was a concern in this study. Because such differences may substantially influence disease progression, we used propensity score adjustment to overcome the effects of confounding.34,35 We used a generalised propensity score approach to make the groups comparable regarding covariates believed to be associated with disease progression and minimise the possibility of confounding by indication.36,37,38 The generalised propensity score was computed using a multinomial logistic‐regression model that “predicted” the decade of disease onset, as a function of 13 baseline variables potentially associated with disease progression, such as measures of disease activity (erythrocyte sedimentation rate (ESR), tender joint counts), disease characteristics (rheumatoid factor positive (RF+), nodules), demographic characteristics (age, disease duration at first visit), self‐assessed symptom questionnaires (HAQ, pain levels, global severity), socioeconomic characteristics at enrolment (average income, education level) and radiographic damage (Larsen scores, estimated prior rate of radiographic progression). The propensity score was then used to adjust for differences in baseline variables.
Table 1 Demographic and treatment characteristics.
| 1970s (n = 98) | 1980s (n = 222) | 1990s (n = 98) | p Value* | |
|---|---|---|---|---|
| Female (%) | 71 | 74 | 76 | 0.79 |
| Ethnic origin | ||||
| Caucasian (%) | 94 | 92 | 89 | |
| Hispanic (%) | 1 | 2 | 4 | |
| African‐American (%) | 5 | 5 | 1 | |
| Other (%) | 0 | 1 | 3 | 0.20 |
| Comorbidity score 0–11, Med (IQR) | 1 (1) | 0 (1) | 1 (1) | 0.48 |
| DMARD, % of person‐time in follow‐up | <0.01 | |||
| None | 63 | 45 | 14 | |
| Penicillamine | 10 | 3 | 0 | |
| Hydroxychloroquine | 7 | 15 | 23 | |
| Gold | 13 | 13 | 5 | |
| Sulfasalzine | 0 | 1 | 2 | |
| Methotrexate | 6 | 22 | 45 | |
| Combination therapy | 1 | 1 | 11 | |
| Glucocorticoids, % of person‐time | 13 | 13 | 29 | <0.01 |
| Length of follow‐up in years, Med (IQR) | 7.2 (3.2) | 6.6 (4.0) | 4.6 (3.8) | <0.01 |
DMARD, disease‐modifying antirheumatic drug.
Continuous variables are in medians (Med) and interquartile ranges (IQRs) of variables are reported.
*One‐way analysis of variance of means of normally distributed continuous variables; Kruskal–Wallis test for non‐normally distributed variables; χ2 test for dichotomous variables. Comorbidity scale, number of comorbid conditions (0–11); DMARD, % of person‐time, proportion of follow‐up time on various DMARD regimens; Combination therapy, any combination of two or more DMARDs; Glucocorticoids, low‐dose glucocorticoids.
Progression of radiographic and functional disability was analysed using generalised mixed models for longitudinal data.39 We verified that the multivariate normal assumption for longitudinal models was satisfied and examined whether time as a linear trend or as a polynomial function best fitted the data. Radiographic damage progressed linearly over time, whereas functional disability (HAQ) evolved non‐linearly over time. We selected the best‐fitting model without controlling for potential confounders (crude model). We then adjusted for DMARD use, low‐dose glucocorticoid use and differences in baseline predictors by using the propensity score (adjusted model). Because the indication of DMARD treatment has changed over time, we considered effect modification of antirheumatic treatments by decades, with an interaction term between decades and DMARDs. These interaction terms were included in the model only if found to be substantial confounders, using the 10% change in estimate criteria.40 For the final estimates, a robust estimator of the variance was used. All statistical tests were two sided and were evaluated at the 0.05 significance level. The statistical analysis was carried out using Stata V.8.2 for Windows.
Results
Of the 1240 patients with rheumatoid arthritis seen at the Wichita Arthritis Center, a total of 418 patients with rheumatoid arthritis met the study inclusion criteria (34%). Most of the patients who were excluded (n = 642, 52%) did not have early disease at their first visit; of the remaining, 180 patients (15%) were excluded because they did not have follow‐up hand radiographs. Patients with early rheumatoid arthritis with and without radiographic follow‐up were similar for all baseline socioeconomic and disease characteristics, except for the number of swollen joints (median of 8 v 10 swollen joints). Of the 418 patients with rheumatoid arthritis who met the inclusion criteria, 98 were diagnosed in the 1970s, 222 in the 1980s and 98 in the 1990s. These patients had a median of 2 (interquartile range 2–3) sequential radiographs and a median follow‐up of 3.4 (interquartile range 2.4–5.0) years. As expected, because of changes in prescription patterns, patients in earlier decades were treated mostly without DMARDs, whereas in the 1990s methotrexate (MTX) was the most common antirheumatic treatment regimen (table 1). Patients in later decades also tended to have slightly shorter disease durations at the first visit, were older, reported higher pain levels but fewer tender joints (table 2). Other important predictors of disease progression at baseline, such as rheumatoid factor positivity, levels of ESR or prior rates of radiographic progression, did not differ significantly between decades of disease onset. After adjustment of the propensity score, baseline predictors were balanced, without significant differences between groups (data not shown).
Table 2 Disease characteristics at first visit.
| Disease characteristics at baseline | 1970s (n = 98) | 1980s (n = 222) | 1990s (n = 98) | p Value* |
|---|---|---|---|---|
| Age (years) | 50 (13) | 54 (14) | 57 (15) | <0.01 |
| Education level in years, Med (IQR) | 12 (2) | 12 (2) | 12 (1) | 0.74 |
| Duration of disease in years, Med (IQR) | 1.1 (1.3) | 1.0 (1.1) | 0.7 (0.9) | 0.01 |
| Patient's global assessment (VAS) | 4.8 (1.7) | 5.0 (2.5) | 5.4 (2.4) | 0.22 |
| Pain level (0–10) | 4.5 (2.2) | 4.5 (2.6) | 5.7 (2.7) | < 0.01 |
| RF+ (%) | 83 | 86 | 87 | 0.67 |
| Rheumatoid nodules (%) | 6 | 11 | 10 | 0.31 |
| ESR (mm/h), Med (IQR) | 36 ( 34) | 34 (33) | 36 (30) | 0.84 |
| Tender joint count (0–24) | 12.9 (5.9) | 9.0 (5.4) | 8.5 (5.5) | <0.01 |
| Functional disability (HAQ; 0–3) | 1.1 (1) | 1.2 (0.7) | 1.3 (0.7) | 0.06 |
| Larsen score (0–250), Med (IQR) | 3 (8) | 2 (9) | 1 (7) | 0.41 |
| Estimated prior rate Larsen score progression, Med (IQR) | 2.4 (6.5) | 1.9 (9.5) | 1.7 (10.5) | 0.11 |
ESR, erythrocyte sedimentation rate; HAQ, Stanford Health Assessment Questionnaire25; IQR, interquartile range; VAS, Visual Analogue Scale.
Values are given in mean (SD) if not indicated otherwise. When not normally distributed, medians and IQRs of variables are reported.
*Significance of differences between decades in covariates: one‐way analysis of variance of means of normally distributed continuous variables; Kruskal–Wallis test for non‐normally distributed variables; χ2 test for dichotomous variables.
Education level = total number of years of school and college; disease duration = duration of disease at first visit; patient's global assessment = patient's self‐assessed disease severity based on a Visual Analogue Scale (VAS, range 0–10), higher scores indicate more severe symptoms; pain = patients' pain levels self‐assessed by using a VAS (range 0–10)], higher scores indicate more severe pain. Tender joint count = patients' self‐assessed number of tender joints, Hart‐modified Ritchie index count 41; estimated prior rate of Larsen score progression = Larsen score divided by the disease duration at baseline.33
Radiographic damage progression
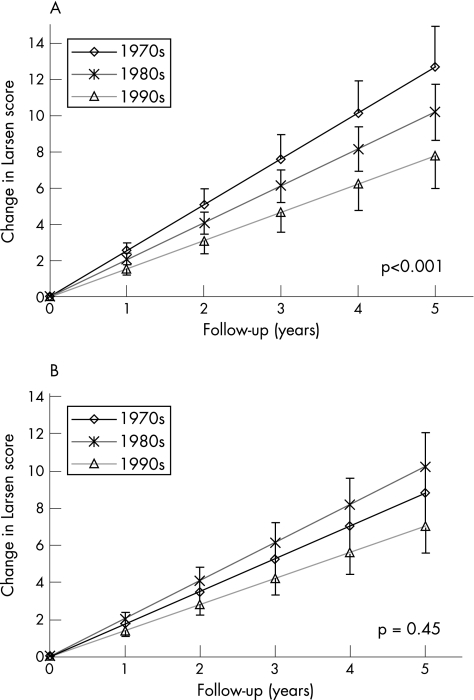
A significant trend towards less radiographic disease progression was evident in more recent decades in the crude analysis (trend test, p<0.001). The mean annual rates of radiographic damage score progression were 2.54 (95% confidence intervals (CI) 2.10 to 2.99), 2.04 (95% CI 1.73 to 2.35) and 1.56 (95% CI 1.19 to 1.93) Larsen score units in the 1970s, 1980s and 1990s, respectively (ANOVA, p = 0.01; fig 1). Adjusting the analysis for differences in baseline predictor profiles did not appreciably change the observed trend towards milder disease progression in later decades (trend test, p = 0.003). However, after adjusting for DMARD use, the differences in radiographic disease progression between decades vanished (ANOVA, p = 0.16) and the trend towards milder disease was no longer apparent (trend test, p = 0.45). The adjusted mean annual rates of radiographic damage score progression were 1.76 (95% CI 1.11 to 2.41), 2.05 (95% CI 1.74 to 2.36) and 1.41 (95% CI 0.98 to 1.84) Larsen score units in the 1970s, 1980s and 1990s, respectively.
Figure 1 The mean radiographic progression in Larsen scores is represented in the different decades. The vertical lines represent the 95% confidence interval of the mean. A secular trend towards milder disease was tested using a trend test (p values). (A) Unadjusted radiographic disease progression. (B) Radiographic progression after adjustment for differences in disease‐modifying antirheumatic drug (DMARD) use and in baseline predictors. A significant trend towards less radiograph disease progression in more recent decades was apparent in the crude analysis (trend test, p<0.001). Adjusting the analysis for differences in baseline predictor profiles did not appreciably change the observed trend towards milder disease progression in later decades. However, after adjusting for DMARD use, the differences in radiographic disease progression between decades vanished and the trend towards milder disease was no longer apparent (trend test, p = 0.45).
Subgroups of patients with RF+ disease and female patients yielded qualitatively similar results. The crude trend towards less radiographic progression over time was even clearer among RF+ patients (trend, p<0.001) or among female patients (trend, p<0.001), but the differences among decades consistently vanished after adjusting for DMARD treatment and baseline characteristics (data not shown). The strongest predictors of radiographic progression were the types and duration of DMARD regimen. Significant interactions existed between the decade of diagnosis and the use of DMARD combination therapy (p = 0.02), MTX treatment (p = 0.005) or the absence of DMARD treatment (p = 0.001), which reflects changing indications of these DMARD regimens over time. In earlier decades, DMARDs such as MTX were indicated only for very severe forms of rheumatoid arthritis with bad prognosis, whereas more recently it became a preferred treatment. Consequently, MTX was associated with considerably more radiographic progression in the 1970s than in the 1980s or the 1990s. On the contrary, “no DMARD” treatment was a common therapeutic option in early rheumatoid arthritis 30 years ago, whereas more recently all patients with rheumatoid arthritis but those with the mildest form of the disease have DMARDs. Consequently, absence of DMARD treatment was associated with markedly less radiographic progression in the 1990s than in the 1970s.
Progression of functional disability
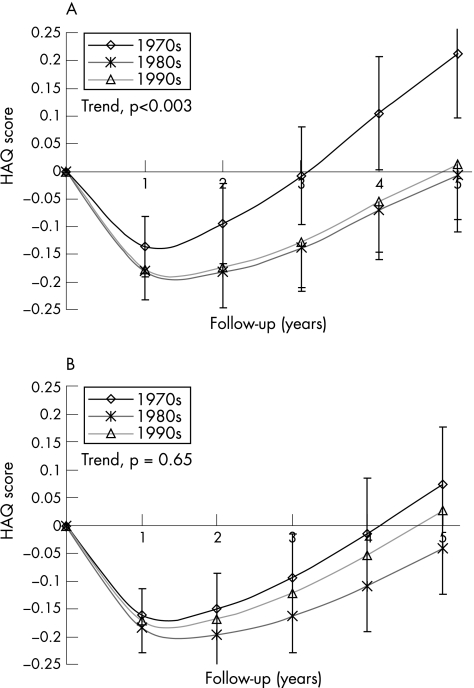
To examine the consistency of the radiographic data, we repeated the analysis with the HAQ score as outcome. Because the progression of functional disability in early rheumatoid arthritis is U shaped, we report mean changes in HAQ scores over time, instead of annual rates of progression (fig 2). The crude HAQ scores decreased in all decades after the first year: in the 1970s by −0.14 (95% CI −0.08 to −0.19), in the 1980s by −0.18 (95% CI −0.13 to −0.23) and in the 1990s by −0.18 (95% CI −0.12 to −0.23). After 5 years, the HAQ scores have generally returned or exceeded baseline levels: in the 1970s by +0.21 (95% CI +0.10 to +0.33), in the 1980s by −0.01 (95% CI −0.09 to +0.07) and in the 1990s by +0.01 (95% CI −0.11 to +0.13).
Figure 2 The mean progression of functional disability (Health Assessment Questionnaire (HAQ) score) is represented in the different decades. The vertical lines represent the 95% confidence interval of the mean. A secular trend towards milder disease was tested using a trend test (p values). (A) The crude analysis of the evolution of functional disability. (B) The evolution of functional disability after adjustment for differences in disease‐modifying antirheumatic drug use and in baseline predictors.
As with the radiographic data, the crude analysis of HAQ scores showed less progression in functional disability in more recent decades (fig 2A). On average, the yearly evolution of HAQ scores decreased by −0.023 units per decade (95% CI −0.01 to 0.04; trend test, p = 0.003). After adjusting for DMARD use and baseline predictors, the differences in the evolution of functional disability between decades vanished (ANOVA, p = 0.58) and the trend towards milder disease was no longer apparent (fig 2A). The adjusted yearly evolution of HAQ scores changed by −0.008 units per decade (95% CI −0.04 to +0.02; trend test, p = 0.60).
Discussion
Several studies have suggested that severity of rheumatoid arthritis has decreased over the past decades. In particular, progression of radiographic joint damage seems to have declined over time, but it is not clear whether the disease itself has become milder or whether antirheumatic treatments have become more effective. This study attempted to analyse the direct causal effect of time of diagnosis on disease progression, to examine the hypothesis of a secular trend towards a milder disease. The crude analysis of both radiographic joint damage and functional disability confirms a significant trend towards milder disease in recent decades. However, after controlling for differences in antirheumatic treatment regimens, the trend for milder disease over time disappears, both for radiographic joint damage and for functional disability. This suggests that the observed trend towards milder disease in rheumatoid arthritis is largely attributable to more effective antirheumatic treatment and not to a secular trend towards milder disease.
Others have examined various outcomes related to severity of rheumatoid arthritis, such as extra‐articular manifestations and mortality with conflicting results.8,9,10,11,12,13,14,15,16,17,18,19,20 Sokka et al15 have described decreasing rates of radiographic damage progression over time in three historic cohorts of rheumatoid arthritis and suggested that this may result from a trend towards milder disease, a different patient selection or improved treatment. In our analysis, crude annual radiographic progression rates showed a similar trend towards milder disease in recent times; however, this effect vanished once differences in treatment were controlled for.
The difference in crude radiographic damage progression between the 1970s and the 1990s is about 1 (95% CI 0.64 to 1.61) Larsen score unit per year. The crude difference in functional disability between the 1970s and the 1990s is about 0.2 HAQ score units (95% CI 0.08 to 0.32) at 5 years. If we assume that this difference is largely explained by differences in antirheumatic treatment, then the more aggressive DMARD regimen used in the 1990s would prevent about 5 Larsen score units and 0.2 HAQ score units in 5 years, which is proportionally a relatively small effect compared with typical randomised controlled trials.42 However, it is important to remember that these are unselected patients, who typically have less severe forms of rheumatoid arthritis than patients enrolled in trials.43
Some limitations inherent to the analysis of historical data need discussion. Firstly, the indication for DMARD treatment has changed greatly over time. As expected, because of changes in prescription patterns, patients with early rheumatoid arthritis in previous decades were initially treated only with non‐steroidal anti‐inflammatory drugs or with mild DMARDs, whereas in the most recent decades early aggressive treatment with DMARDs became the norm. Whenever the exposure variable is linked so closely with an important covariate, analysing the direct effect of the exposure can be difficult because the variables are collinear and tend to obscure each other. Therefore, we could not adjust for the delay of DMARD initiation, but there was enough overlap in the use of DMARD between decades to permit a reliable statistical analysis. We tried alternative ways to control for changes in DMARDs, such as combining DMARD treatments into broader treatment categories or restricting the analysis to patients treated with methotrexate monotherapy only, and found qualitatively similar results (data not shown). Furthermore, this analysis assumes that the choice of a particular DMARD by the rheumatologist is entirely dependent on characteristics of rheumatoid arthritis and on the decade, which may not capture completely the complexity of such therapeutic decisions and therefore not account for unmeasured confounding factors. Secondly, patient selection for practising rheumatologists may have changed over time. Most notably, the disease duration at first visit has decreased over the decades, which explains the variation in the number of eligible cases between the three decades. Patients in the 1970s also reported more tender joints at first visit, but less pain than patients in the 1990s. Other strong predictors of radiographic progression, such as rheumatoid factor, baseline measures of inflammation (ESR) and estimated prior rate of radiographic progression, did not differ markedly between decades. We adjusted for these differences using a propensity score approach, which successfully removed observed imbalances. To assess the stability of our propensity score adjustment, we used an alternative conventional multivariate longitudinal regression model instead of a propensity score, which yielded similar results. Equivalent results were also found in clinical subgroups of RF+ patients and female patients, suggesting that the results are internally consistent. Although we were able to control successfully the analysis for potential confounding by these covariates, we cannot exclude the possibility of residual confounding. Patients with early rheumatoid arthritis not included in the analysis were similar for all baseline socioeconomic and disease characteristics but not for the number of swollen joints (median of 8 v 10 swollen joints), suggesting that the patients may have a slightly more severe disease. Thirdly, comparing annual rates of radiographic progression assumes a linear progression of joint damage over the first few years of disease. Although this is a reasonable assumption for the average progression of radiographic damage of a population, large interindividual differences exist.33,44,45 The power of this study was sufficient to detect relatively small differences in rates of radiographic progression (fig 1A) and was notably larger than other studies that have examined time trends in radiographic progression.15 Strengths of this analysis include a long‐term prospective cohort of rheumatoid arthritis and an analysis accounting for changes in DMARD use.
In conclusion, decreasing progression of radiographic joint damage and functional disability over time seem to be largely explained by more effective DMARD use. This suggests that observed trends to milder disease in rheumatoid arthritis are attributable to more effective antirheumatic treatment and not to a secular trend.
Acknowledgements
We thank Dr Arvi Larsen for performing the radiographic damage scoring.
Abbreviations
ANOVA - analysis of variance
DMARD - disease‐modifying antirheumatic drug
ESR - erythrocyte sedimentation rate
HAQ - Health Assessment Questionnaire
MTX - methotrexate
Footnotes
Funding: AF is supported by the Centocor Health Outcomes in Rheumatic Diseases (CHORD) fellowship and the Kirkland Scholars Fellowship.
Competing interests: None declared.
References
- 1.Silman A J. Trends in the incidence and severity of rheumatoid arthritis. J Rheumatol Suppl 19923271–73. [PubMed] [Google Scholar]
- 2.Silman A J. Are there secular trends in the occurrence and severity of rheumatoid arthritis? Scand J Rheumatol Suppl 19897925–30. [DOI] [PubMed] [Google Scholar]
- 3.Aho K, Tuomi T, Palosuo T, Kaarela K, Von Essen R, Isomaki H. Is seropositive rheumatoid arthritis becoming less severe? Clin Exp Rheumatol 19897287–290. [PubMed] [Google Scholar]
- 4.Pincus T, Sokka T, Chung C, Cawkwell G. Declines of tender and swollen joint counts between 1985 and 2001 in patients with rheumatoid arthritis seen in standard care: possible considerations for revision of inclusion criteria for clinical trials. Ann Rheum Dis 200665878–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doran M F, Pond G R, Crowson C S, O'Fallon W M, Gabriel S E. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty‐year period. Arthritis Rheum 200246625–631. [DOI] [PubMed] [Google Scholar]
- 6.Shichikawa K, Inoue K, Hirota S, Maeda A, Ota H, Kimura M.et al Changes in the incidence and prevalence of rheumatoid arthritis in Kamitonda, Wakayama, Japan, 1965–1996. Ann Rheum Dis 199958751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silman A J. The changing face of rheumatoid arthritis: why the decline in incidence? Arthritis Rheum 200246579–581. [DOI] [PubMed] [Google Scholar]
- 8.Bergstrom U, Book C, Lindroth Y, Marsal L, Saxne T, Jacobsson L. Lower disease activity and disability in Swedish patients with rheumatoid arthritis in 1995 compared with 1978. Scand J Rheumatol 199928160–165. [DOI] [PubMed] [Google Scholar]
- 9.Walji S, Bykerk V P. Rheumatoid arthritis: is the disease becoming milder or is treatment improving? J Rheumatol 2004311023–1025. [PubMed] [Google Scholar]
- 10.Silman A, Davies P, Currey H L, Evans S J. Is rheumatoid arthritis becoming less severe? J Chronic Dis 198336891–897. [DOI] [PubMed] [Google Scholar]
- 11.Wiles N, Symmons D P, Harrison B, Barrett E, Barrett J H, Scott D G.et al Estimating the incidence of rheumatoid arthritis: trying to hit a moving target? Arthritis Rheum 1999421339–1346. [DOI] [PubMed] [Google Scholar]
- 12.Gordon P, West J, Jones H, Gibson T. A 10 year prospective followup of patients with rheumatoid arthritis 1986–96. J Rheumatol 2001282409–2415. [PubMed] [Google Scholar]
- 13.Ward M M. Decreases in rates of hospitalizations for manifestations of severe rheumatoid arthritis, 1983–2001. Arthritis Rheum 2004501122–1131. [DOI] [PubMed] [Google Scholar]
- 14.Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum 2005521009–1019. [DOI] [PubMed] [Google Scholar]
- 15.Sokka T, Kautiainen H, Hakkinen A, Hannonen P. Radiographic progression is getting milder in patients with early rheumatoid arthritis. Results of 3 cohorts over 5 years. J Rheumatol 2004311073–1082. [PubMed] [Google Scholar]
- 16.Heikkila S, Isomaki H. Long‐term outcome of rheumatoid arthritis has improved. Scand J Rheumatol 19942313–15. [DOI] [PubMed] [Google Scholar]
- 17.Aho K, Kaipiainen‐Seppanen O, Heliovaara M, Klaukka T. Epidemiology of rheumatoid arthritis in Finland. Semin Arthritis Rheum 199827325–334. [DOI] [PubMed] [Google Scholar]
- 18.Watts R A, Mooney J, Lane S E, Scott D G. Rheumatoid vasculitis: becoming extinct? Rheumatology (Oxford) 200443920–923. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel S E, Crowson C S, Kremers H M, Doran M F, Turesson C, O'Fallon W M.et al Survival in rheumatoid arthritis: a population‐based analysis of trends over 40 years. Arthritis Rheum 20034854–58. [DOI] [PubMed] [Google Scholar]
- 20.Turesson C, O'Fallon W M, Crowson C S, Gabriel S E, Matteson E L. Extra‐articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis 200362722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi H K, Hernan M A, Seeger J D, Robins J M, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 20023591173–1177. [DOI] [PubMed] [Google Scholar]
- 22.Ropes M W, Bennett G A, Cobb S, Jacox R, Jessar R A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis 19589175–176. [PubMed] [Google Scholar]
- 23.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 24.Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockholm) 197718481–491. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, van der Heijde D M, Larsen A. Assessing radiographic status of rheumatoid arthritis: introduction of a short erosion scale. J Rheumatol 2000272090–2099. [PubMed] [Google Scholar]
- 26.Fries J F, Spitz P, Kraines R G, Holman H R. Measurement of patient outcome in arthritis. Arthritis Rheum 198023137–145. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe F. A reappraisal of HAQ disability in rheumatoid arthritis. Arthritis Rheum 2000432751–2761. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe F, Hawley D J. The longterm outcomes of rheumatoid arthritis: work disability: a prospective 18 year study of 823 patients. J Rheumatol 1998252108–2117. [PubMed] [Google Scholar]
- 29.Wolfe F, Zwillich S H. The long‐term outcomes of rheumatoid arthritis: a 23‐year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum 1998411072–1082. [DOI] [PubMed] [Google Scholar]
- 30.Michaud K, Messer J, Choi H K, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three‐year study of 7,527 patients. Arthritis Rheum 2003482750–2762. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe F, Michaud K, Gefeller O, Choi H K. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum 2003481530–1542. [DOI] [PubMed] [Google Scholar]
- 32.Welsing P M, van Gestel A M, Swinkels H L, Kiemeney L A, van Riel P L. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum 2001442009–2017. [DOI] [PubMed] [Google Scholar]
- 33.Wick M C, Lindblad S, Weiss R J, Klareskog L, Van Vollenhoven R F. Estimated pre‐diagnosis radiological progression: an important tool for studying the effects of early DMARD‐therapy in rheumatoid arthritis. Ann Rheum Dis 200564134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joffe M M, Rosenbaum P R. Invited commentary: propensity scores. Am J Epidemiol 1999150327–333. [DOI] [PubMed] [Google Scholar]
- 35.D'Agostino R B., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med 1998172265–2281. [DOI] [PubMed] [Google Scholar]
- 36.Imbodens G W. The role of the propensity score in estimating dose‐response functions. Biometrika 200087706–710. [Google Scholar]
- 37.Imai K, Van Dyk D A. Causal inference with general treatment regimes: generalizing the propensity score. JASA 200499854–866. [Google Scholar]
- 38.Wang J, Donnan P T, Steinke D, MacDonald T M. The multiple propensity score for analysis of dose‐response relationships in drug safety studies. Pharmacoepidemiol Drug Saf 200110105–111. [DOI] [PubMed] [Google Scholar]
- 39.Skrondal A, Rabe‐Hesketh S. Generalized latent variable modeling: multilevel, longitudinal and structural equation models. Boca Raton, FL: Chapman & Hall, 2004
- 40.Mickey R M, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989129125–137. [DOI] [PubMed] [Google Scholar]
- 41.Thompson P W, Hart L E, Goldsmith C H, Spector T D, Bell M J, Ramsden M F. Comparison of four articular indices for use in clinical trials in rheumatoid arthritis: patient, order and observer variation. J Rheumatol 199118661–665. [PubMed] [Google Scholar]
- 42.Jones G, Halbert J, Crotty M, Shanahan E M, Batterham M, Ahern M. The effect of treatment on radiological progression in rheumatoid arthritis: a systematic review of randomized placebo‐controlled trials. Rheumatology (Oxford) 2003426–13. [DOI] [PubMed] [Google Scholar]
- 43.Sokka T, Pincus T. Most patients receiving routine care for rheumatoid arthritis in 2001 did not meet inclusion criteria for most recent clinical trials or American College of Rheumatology criteria for remission. J Rheumatol 2003301138–1146. [PubMed] [Google Scholar]
- 44.Wolfe F, Sharp J T. Radiographic outcome of recent‐onset rheumatoid arthritis: a 19‐year study of radiographic progression. Arthritis Rheum 1998411571–1582. [DOI] [PubMed] [Google Scholar]
- 45.Hulsmans H M, Jacobs J W, van der Heijde D M, van Albada‐Kuipers G A, Schenk Y, Bijlsma J W. The course of radiologic damage during the first six years of rheumatoid arthritis. Arthritis Rheum 2000431927–1940. [DOI] [PubMed] [Google Scholar]