Abstract
Objectives
To evaluate the outcome of balloon angioplasty in the arteries of the upper extremities in patients with giant‐cell arteritis (GCA) and stenosing extracranial involvement.
Methods
Percutaneous transluminal angioplasty (PTA) for symptomatic upper limb artery stenoses (n = 29) and occlusions (n = 1) resistant to medical treatment was carried out in 10 patients (all women, mean age 65 years) with GCA. Vascular lesions were located in the subclavian (n = 4), axillary (n = 10) and brachial (n = 16) arteries. Interventional treatment was accompanied by immunosuppressive drugs in all patients. Follow‐up included clinical and serological examination, magnetic resonance angiography and colour duplex ultrasound.
Results
Initial technical success of angioplasty was achieved in the case of all vascular lesions. In five patients, marked recurrent stenoses (vascular territories; n = 10/30) were found during follow‐up (mean 24 months). The cumulative primary patency rate was 65.2%. All recurrent lesions developed in the territories of the initial long‐segment stenoses. Repeated PTA (vascular territories, n = 8; patients, n = 5) provided a cumulative secondary patency rate of 82.6% and a cumulative tertiary patency rate of 89.7%.
Conclusions
Despite a tendency to restenoses, balloon angioplasty of the upper‐extremity artery, in combination with immunosuppressive treatment, is an efficient method for the treatment of extracranial GCA.
Giant‐cell arteritis (GCA) is an immune‐mediated vasculitis of the large and medium‐sized arteries, which commonly occurs among people aged ⩾50 years. Clinical manifestations may be non‐specific and variable, including features attributable to systemic inflammation and local complications of vascular injury. GCA most commonly affects the temporal arteries.1,2 Apart from affecting the carotid branches (temporal, occipital and ophthalmic arteries), GCA may affect the aorta or branches of the aortic arch, particularly the subclavian, axillary and brachial arteries (figs 1–3).3,4,5,6 Characteristic clinical manifestations include diminished or absent pulses and blood pressure (pulseless disease), and arm claudication.4,5,6,7 Because of increasing attention and newer diagnostic procedures, large‐vessel involvement is increasingly recognised in GCA.8 Clinical studies showed a stenotic involvement of the arteries of the upper extremities in 4–15% of cases, but sonography and positron emission tomography studies suggest much greater involvement of the larger arteries in GCA, emphasising that the manifestation is apparently often undetected.9,10,11,12,13
Figure 1 Long‐segment stenosis (white arrows) of the left brachial artery in a 60‐year‐old patient with claudication of the left arm before (A) and immediately after angioplasty (B). Long‐term success is proved by follow‐up. Although magnetic resonance angiography (C) before percutaneous transluminal angioplasty (PTA) shows severe obstruction, only mild, focal restenosis is found 14 months later (D). Similar results are provided using duplex ultrasound: before PTA (E), blood flow is extremely decreased because of extensive hypoechic vessel wall proliferation in the course of giant‐cell arteritis. (F) Fourteen months after interventional treatment, no sign of haemodynamically relevant stenosis is seen.
Figure 2 Angiography of the aortic arch arteries in a 67‐year‐old patient. (A) The left subclavian, axillary and proximal brachial artery are stenotic owing to vasculitis, but the right side is not affected. (B) Percutaneous transluminal angioplasty is carried out successfully. After 15 months, (C) magnetic resonance angiography and (D) duplex ultrasound show absence of recurrent stenosis. (C) An irregularity of the right subclavian artery on magnetic resonance imaging is caused by artefacts due to venous contrast agents given on the right arm.
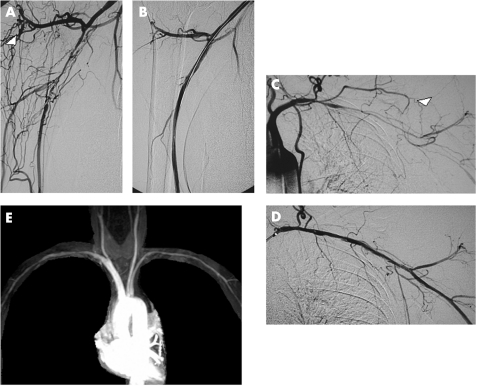
Figure 3 Initially successful interventional treatment of (A,B) severe right brachial artery stenosis, (C,D) severe left subclavian and axillary artery stenosis and brachial artery occlusion, accompanied by disappearance of collateral vessels (white arrowheads). During follow‐up, repeated angioplasties had to be because of recurrent stenoses on both sides. Despite minor vessel wall irregularities, (E) magnetic resonance angiography finally showed sufficient reconstruction of the arteries of the upper extremities.
Standard treatment for GCA consists of glucocorticoids.1,2 Although general signs of inflammatory disease often respond adequately, ischaemic symptoms may persist despite medical treatment.6,14,15
Percutaneous transluminal angioplasty (PTA) is an established interventional treatment for occlusive artherosclerotic diseases, but less evidence exists on the endovascular treatment of inflammatory vascular diseases such as GCA or Takayasu's arteritis.16,17,18,19,20 However, reports about PTA in large‐vessel GCA are restricted to a limited number of cases.14,15,20,21,22
We describe the results of PTA in 10 patients with GCA and symptomatic extracranial manifestations that are refractory to medical treatment.
Patients and methods
Patient population
Ten consecutive patients, diagnosed as having GCA, presented with symptomatic large‐artery involvement to the Rheumaklinik Bad Bramstedt and to the University Hospital Schleswig‐Holstein, Campus Kiel, Germany, between 1995 and 2004. All patients had painful unilateral or bilateral intermittent arm claudication without rest pain. Of the 10 patients, 9 fulfilled the American College of Rheumatology criteria for GCA and 1 (patient 2, tables 1 and 2) had classic bilateral upper‐extremity artery manifestation without cranial complications.4,23 In this patient, large‐artery vasculitis was accompanied with polymyalgia rheumatica, a condition closely related to GCA.1,2 Takayasu's arteritis as a differential diagnosis was ruled out because age at onset of disease was >50 years. The mean age at diagnosis was 65 (range 56–78) years. Unilateral temporal artery biopsy was carried out in all cases and was positive in 4 of 10 patients. Polymyalgia rheumatica was diagnosed in five patients.1,2 The initial manifestation of GCA in three patients consisted of ischaemic symptoms of the upper extremities (table 1). In the other seven patients, median time between diagnosis of GCA and symptomatic extracranial manifestations was 24 months. Prednisolone was introduced to all patients initially (median dose 45 (range 7.5–80) mg). Because of the complicated course of the disease or major side effects of systemic glucocorticoids, methotrexate (median dosage 21 (range 7.5–25) mg/week) was introduced in eight patients. Because of recurrent disease activity, treatment was switched to cyclophosphamide (intravenously 800–1000 mg every 15–21 days) in two patients. Large‐artery involvement was regarded as resistant to medical treatment when there was no release from arm claudication.
Table 1 Clinical characteristics and medical treatment of 10 patients treated with percutaneous transluminal angioplasty for stenosis of upper limbs due to giant‐cell arteritis.
| Patient | Age (years) | Individual symptoms related to upper extremity artery stenosis | ACR criteria PMR | Immunosuppressive drugs after PTA | CRP/ESR/thrombocytes | |
|---|---|---|---|---|---|---|
| At PTA | At control | |||||
| 1 | 71 | Claudication of the left arm | 4 (ta) PMR | Prednisolone | <0.5/5/354 | <0.5/11/ND |
| Methotrexate | ||||||
| 2 | 62 | Claudication of both arms, initial manifestation of vasculitis | 2 PMR | PrednisoloneMethotrexate | 1.2/44/3830.9/70/374 | <0.5/6/216 |
| 3 | 56 | Claudication of both arms, initial manifestation of vasculitis | 3 | PrednisoloneMethotrexate | <0.5/52/288<0.5/12/299 | <0.5/18/272 |
| 4 | 69 | Claudication of both arms, initial manifestation of vasculitis | 3 | PrednisoloneMethotrexate | <0.5/60/362<0.5/50/451 | <0.5/16/ ND |
| 5 | 78 | Claudication of both arms | 3 | Prednisolone | 0.5/6/484 | 0.8/11/375 |
| Cyclophosphamide | ||||||
| 6 | 67 | Claudication of the left arm | 3 (ta) | Prednisolone | 1.8/32/230 | 1.2/14/ ND |
| Methotrexate | ||||||
| 7 | 60 | Claudication of the left arm | 3 PMR | Prednisolone | 1.2/26/312 | 1.5/21/ ND |
| Methotrexate | ||||||
| 8 | 64 | Claudication of the left arm | 3 (ta) PMR | Prednisolone | <0.5/9/285 | <0.5/5/ ND |
| Methotrexate | ||||||
| 9 | 62 | Claudication of both arms | 3 PMR | Prednisolone | <0.5/23/287 | <0.5/10/270 |
| Methotrexate | <0.5/20/293 | |||||
| Cyclophosphamide | <0.5/12/261 | |||||
| 10 | 64 | Claudication of both arms | 3 (ta) PMR | Prednisolone | <0.5/13/300 | <0.5/30/270 |
| Cyclophosphamide | <0.5/44/282 |
ACR criteria, American College of Rheumatology 1990 criteria for the classification of giant‐cell arthritis; CRP, C reactive protein (mg/dl); ESR, erythrocyte sedimentation rate according to the Westergren method (mm at the end of the first hour); ND, not determined; PMR, additional diagnosis of polymyalgia rheumatica; ta: positive biopsy result of the temporal artery; thrombocytes, platelet count (1000/µl).
Table 2 Vascular territories treated with percutaneous transluminal angioplasty (PTA).
| Patient | Treated artery | Follow‐up | |||||
|---|---|---|---|---|---|---|---|
| Months | MRA | US | DSA | Clinical success | Long‐term patency | ||
| 1 | Left brachial | 101 | + | + | + | + | |
| 2* | Left axillary | 8 | – | – | |||
| 2 | Second PTA | 42 | + | + | + | + | |
| 2 | Left brachial | 50 | + | + | + | + | |
| 2* | Right axillary | 8 | – | – | |||
| 2 | Second PTA | 42 | + | + | + | + | |
| 2 | Right brachial | 50 | + | + | + | + | |
| 3* | Left brachial | 3 | – | – | |||
| 3* | Second PTA | 5 | – | – | – | – | |
| 3* | Right axillary | 5 | – | – | – | – | |
| 3* | Right brachial | 5 | – | – | – | – | |
| 4 | Left axillary | 35 | + | + | + | + | |
| 4* | Left brachial | 9 | – | – | |||
| 4 | Second PTA | 26 | + | + | + | + | |
| 4 | Right axillary | 35 | + | + | + | + | |
| 4* | Right brachial | 9 | – | – | |||
| 4 | Second PTA | 26 | + | + | + | + | |
| 5 | Left brachial | 5 | + | + | + | ||
| 5 | Right brachial | 5 | + | + | + | ||
| 6 | Left subclavian | 15 | + | + | + | + | |
| 6 | Left axillary | 15 | + | + | + | + | |
| 6 | Left brachial | 15 | + | + | + | + | |
| 7 | Left brachial | 14 | + | + | + | + | |
| 8 | Left axillary | 14 | + | + | + | + | |
| 8 | Left brachial | 14 | + | + | + | + | |
| 9 | Left subclavian | 16 | + | + | + | + | |
| 9 | Left axillary | 16 | + | + | + | + | |
| 9* | Left brachial | 4 | – | – | – | – | |
| 9* | Second PTA | 4 | – | – | – | – | |
| 9 | Third PTA | 8 | + | + | + | + | |
| 9* | Right brachial | 4 | – | – | – | – | |
| 9* | Second PTA | 4 | – | – | – | – | |
| 9 | Third PTA | 8 | + | + | + | + | |
| 10 | Left subclavian | 15 | + | + | + | + | |
| 10 | Left axillary | 15 | + | + | + | + | |
| 10* | Left brachial | 8 | – | – | – | – | |
| 10 | Second PTA | 7 | + | + | + | + | |
| 10 | Right subclavian | 15 | + | + | + | + | |
| 10 | Right axillary | 15 | + | + | + | + | |
| 10 | Right brachial | 15 | + | + | + | + | |
DSA, digital subtraction angiography; MRA, magnetic resonance angiography; US, colour duplex ultrasound.
Long‐term patency: comprehensive assessment.
+, openness; –, considerable restenosis of a vascular territory.
*Recurrent stenosis. Follow‐up (observation time in months) using imaging methods includes MRA, US and DSA in different combinations. Clinical success was evaluated by means of anamnesis of recurrent claudication, palpation of peripheral arm pulses and measurement of blood pressure.
Diagnosis of extracranial GCA was based on typical angiographic images with smooth, tapered stenoses or occlusions of the subclavian, axillary and proximal brachial arteries (figs 1–3), associated with clinical findings such as intermittent arm claudication and reduction or absence of blood pressure and arm pulses.3,4 An additional hallmark of giant‐cell vasculitis was the typical circular, hypoechic wall thickening delineated by ultrasound (figs 1 and 2).12 Vascular lesions were without signs of atherosclerotic vessel disease‐like eccentric or calcified plaques.
PTA was indicated if ischaemic symptoms persisted despite immunosuppressive treatment. At the time of primary PTA, 5 of 10 patients still had mild laboratory signs of active disease (level of C reactive protein (CRP) >0.8 (range 1.2–1.8) mg/dl; patients, n = 3; erythrocyte sedimentation rate (ESR) according to the Westergren method >30 (range 32–60) mm at the end of the first hour; patients, n = 4). Four of 10 patients had significant thrombocytosis (platelet count >350 000 (range 354 000–484 000)/µl) at the time of PTA, even if acute phase proteins were normal (n = 2) (table 1).
Before intervention, all patients gave informed consent. In all cases, PTA was carried out for clinical indications. Patients were followed up according to routine protocols to optimise their assessment and to adapt their therapeutic regimens to the stage and activity of disease, so that the requirements of the local ethics committee were fulfilled.
Percutaneous transluminal angioplasty
PTA was carried out in severe stenoses (n = 29) and occlusions (n = 1) located in the subclavian (n = 4), axillary (n = 10) and brachial (n = 16; table 2) arteries. Angioplasties carried out in lesions outside the arteries of the upper extremities less typical for GCA4 (vertebral artery, n = 1; renal artery, n = 1) were excluded from the study. The extent of stenosis was graduated in four categories: mild (<50%), moderate (50–69%), severe (70–99%) and occlusion (100%). On the basis of angiographic morphology, the lesions were characterised as focal (<3 cm) or long segment (⩾3 cm). After anticoagulation (5000 IU heparin intra‐arterially and acetylsalicylic acid 1000 mg intravenously), PTA was carried out in the course of catheter angiography (contrast media, Ultravist 300, Schering, Berlin, Germany) with digital subtraction angiography (DSA; Multistar TOP, Siemens, Erlangen, Germany). Including repeated PTA (n = 10) in 8 of the 10 recurrent stenoses, altogether 40 lesions were treated. The usual over‐the‐wire technique was used with the transfemoral (n = 39) or combined transfemorobrachial approach (n = 1). The size of the balloon (4 or 5 mm) was chosen based on the normal diameter of the lumen either proximal or distal to the lesion. Balloons were inflated for 30–60 s. PTA was considered to be technically successful in cases where the residual stenosis was <30% or the arterial lumen was at least 50% larger than it was before treatment. After intervention, about 24 000 IU heparin was given intravenously for 24 h to increase partial thromboplastine time (60–80 s). In 8 of 10 patients, acetylsalicylic acid 100 mg/day was started, and two patients received clopidogrel 75 mg/day. In all patients, immunosuppressive treatment was continued according to the systemic signs of inflammatory process.
Follow‐up
The follow‐up (mean 24 (range 5–101) months) included anamnesis of recurrent arm claudication, physical examination (ie, measurement of blood pressure and palpation of radial or ulnar artery pulse), acquisition of laboratory parameters (CRP, ESR and platelet count) and imaging methods in each patient (figs 1–3). DSA was used only to assess vascular lesions in the course of interventional procedure. Colour duplex ultrasound and magnetic resonance angiography (MRA) were used in combination in 9 of 10 patients.
All MRA studies (n = 10) were conducted on a 1.5‐T system (Magnetom Vision, Siemens) with a 4‐element phased‐array body coil. The field of view comprised the aortic arch and supra‐aortic vessels including the brachial arteries. After a test bolus, a standard of 20 ml (0.1–0.2 mmol/kg of body weight) Gadolinium‐DTPA (Magnevist, Schering AG, Berlin, Germany) in 20 ml saline was injected intravenously by a power injector at 3 ml/s. Images were acquired with a T1‐weighted three‐dimensional FLASH sequence (repetition time 4.6 ms, echo time 1.8 ms, flip angle 30°, matrix 200×512, effective slice thickness 1.75 mm, acquisition time 23 s). Vascular territories were regarded as patent if the degree of stenosis was below 70%. Colour duplex ultrasound in 9 of 10 patients was carried out with a linear transducer (L 12‐5, 12‐5 MHz, HDI 5000, Philips, Hamburg, Germany). Significant arterial stenosis was defined as a 100% increase in the peak systolic velocity or severe deformation of the spectral waveform in the brachial artery, such as post‐stenotic loss of diastolic flow reversal or broadening and filling of the spectral window.
Interventional treatment was considered to be successful over the long‐term if neither clinical examination nor imaging methods showed signs of haemodynamically relevant restenosis. Patency was patterned on the criteria proposed by the Society for Vascular Surgery and the International Society for Cardiovascular Surgery.24 Primary patency was uninterrupted patency of the treated lesion, with no repeated procedure during follow‐up. Secondary and tertiary patency referred to recurrent severe stenoses or occlusions revascularised by PTA.
The cumulative patency rates were calculated using Kaplan–Meier life‐table analysis. Data were statistically evaluated using the software JMP (V.4.0.2; SAS Institute).
Results
Immediate success of angioplasty
PTA provided an immediate technical success rate of 100% in all primary lesions (n = 30; long‐segment, n = 23; focal, n = 7; range 0.5–8 cm), as well as in cases of repeated PTA (n = 10) in recurrent stenoses (secondary PTA, n = 8; tertiary PTA, n = 2). Angiography after angioplasty showed mild residual stenosis in 20 of the 40 lesions.
As a minor complication, moderate dissection of the vessel wall was found after interventional treatment in 16 of the 40 lesions. Stent implantation was not required in any of the cases. In one patient, a haematoma located at the puncture site (right external iliac artery) had to be surgically resected. In another patient, iatrogenic femoral artery pseudoaneurysm was treated successfully by percutaneous thrombin injection. No further complications were noted.
Long‐term success of angioplasty
During a mean follow‐up of 24 months, 5 of 10 patients showed no clinical signs of a relapsing stenosis. In the other five patients, recurrent claudication of the upper limbs was observed in combination with absent or diminished peripheral arm pulses. The reduction in blood pressure indicated restenosis (one arm, n = 2; both arms, n = 3) after a median period of 5 (range 3–9) months. MRA and duplex ultrasound showed severe recurrent vascular obstructions (total lesions, n = 10; stenoses, n = 8; occlusions, n = 2) only in clinically symptomatic patients (brachial artery, n = 7; axillary artery, n = 3). All recurrent lesions developed in the territory of initially long‐segment vascular processes (⩾3 cm, n = 23), but no relevant restenosis was found in focal lesions (<3 cm, n = 7). Imaging ruled out the involvement of vascular territories that had not been affected before. In all patients examined with ultrasound (9/10), residual hypoechic mural thickening was detected as a characteristic sign of vasculitis.
In three of five patients with recurrent stenosis later in the course of the disease, ESR was raised (44–60 mm at the end of the first hour) at the time of the primary PTA. In contrast, in two other patients presenting with serological disease activity during primary PTA, follow‐up on vascular patency was unremarkable. Two of four patients with initially raised platelet count developed restenoses. At the time of the relapse, three of five patients presented with laboratory signs of active disease (ESR, range 44–70 mm at the end of the first hour, CRP, range <0.5–0.9 mg/dl) and two patients were serologically in remission when recurrent stenoses occurred (table 1).
In five patients (8/10 recurrent lesions), a secondary PTA was carried out. After repeated intervention, only two patients had a second relapse of the same initial stenosis. One of these patients underwent a successful third PTA (two lesions). The overall cumulative primary patency rate during follow‐up was 65.2% (standard error 8.9%). Repetition of angioplasty led to a cumulative secondary patency rate of 82.6% (7.1%) and a cumulative tertiary patency rate of 89.7% (5.6%).
Discussion
Formerly considered to be an infrequent pattern of temporal arteritis, involvement of the extracranial arteries is increasingly being recognised.8 Recently, a prospective study12 used colour Doppler sonography to show peripheral artery manifestation (arteries other than the temporal artery) in 30% of the patients with GCA. Characteristically, patients with extracranial artery involvement were more often female, tended to be almost 10 years younger, had experienced temporal headaches less often and had undergone a negative temporal artery biopsy more often.4 Apart from aortitis, prime location of the extracranial manifestation is the subclavian and axillary arteries as well as the proximal segments of the brachial artery. Conforming to the findings from our study (table 1), stenoses are often found to be in bilateral distribution.3,4,5,6,7,8,9,10
Medical treatment for extracranial involvement does not differ from standard treatment of GCA. The preferred treatment remains immunosuppressive because symptomatic arterial obstruction of the upper extremity often subsides after initiating glucocorticoids.7,25 In chronic cases with occlusions or severe stenoses, arterial perfusion is typically maintained by bridging collateral vessels.3 However, some patients have persistent ischaemic symptoms despite initial treatment.6,14,15 Upper limb artery obstruction resulting in arm amputation has also been reported in a patient with GCA.26
Despite the successful use of endovascular treatment in atherosclerotic disease, only a few studies have reported on the interventional treatment of large‐vessel arteritis, most reporting on PTA or stenting in patients with Takayasu's arteritis.17,18,19,27 Experiences of endovascular treatment in patients with GCA are limited and mainly restricted to case reports.14,15,21,22 To our knowledge, this is the largest reported series of patients with PTA of extracranial GCA.
In a smaller series, Amann‐Vesti et al14 described their experience of balloon angioplasty of the upper extremities in four patients with GCA in a cohort similar to ours (mean age 65 years). In contrast with our protocol, they used duplex ultrasound as the only criterion of relapsing stenosis.14 In a previous study, we evaluated the clinical outcome after PTA in patients having different kinds of vasculitis including GCA (n = 8), but there was no systematic proof of patency by imaging.20 Clinical examination is fundamental in assessing stenotic arterial disease in patients with inflammatory aortic arch syndrome. Nevertheless, imaging is indispensable for providing reliable data with regard to early and long‐term results.24 In the present study, which included three of eight patients with GCA from our previous study,20 patients were followed up using a combination of MRA and colour duplex ultrasound besides clinical and laboratory investigation.
MRA and duplex ultrasound have proved to be reliable imaging methods for detecting arterial stenoses of the upper extremities in patients with atherosclerotic vessel disease, as well as in those with vasculitis.28,29,30 Arterial DSA is increasingly restricted to interventional procedures owing to the risks of iodinated contrast media and arterial puncture.31 Thus, evaluation of long‐term outcome of our patients was based on DSA only in the course of repeated PTA.
The diagnosis of extracranial GCA was established using clinical and serological criteria as well as typical angiographic and sonographic findings. As in previous studies, we observed a high rate of negative findings in the temporal artery biopsy specimens of our patients (table 1), emphasising compartmentalisation of the vasculitis, as some authors have suggested.4,32
Conforming to the limited number of reports on the interventional treatment of peripheral involvement in GCA, the initial technical success of angioplasty was 100% in our cohort. Consequently, it was not necessary to implant a stent. Drug‐eluting stents to avoid restenosis may be considered, but to our knowledge, there is no experience with this method in interventional treatment for GCA. Although Amann‐Vesti et al14 did not find major recurrent stenoses during follow‐up (range 24–120 months) in a series of four patients, restenoses occurred in half of our patients (cumulative primary patency rate 65.2%) after a median of 5 months. In four of the relapsing patients, successful long‐term results were achieved by repeated PTA, providing superior cumulative secondary and tertiary patency rates of 82.6% and 89.7%, respectively. Obviously, recurrence of vessel narrowing was influenced by the extent of the initial inflammatory process. All restenoses detected were found in vascular territories, initially at least 3 cm in length. None of the restenoses occurred in areas that were previously not affected.
The observation of a tendency to recurrent stenoses in our patients is supported by similar results from studies on PTA of subclavian arteries in Takayasu's arteritis,17,18,19 whereas superior primary long‐term patency rates from 72% to 90% have been described in the interventional treatment of atherosclerotic vessel disease in supra‐aortic arteries.19,33,34 Although there are single case reports on successful surgical treatment by bypass in patients with extracranial GCA,35 the cost effectiveness and the low rate of complications observed during interventional treatment in this and other studies emphasises the role of PTA as a minimally invasive method for revascularisation of the upper extremities. Nevertheless, the indication should be based on the severity of clinical symptoms to avoid unnecessary morbidity in the course of interventional treatment.
As medical treatment has the potency to improve symptoms of claudication, interventional treatment should be avoided in case of raised serological signs of active inflammation. Nevertheless, the persistence of arm claudication despite a prolonged period of immunosuppressive treatment induced us to carry out PTA in five patients, although serological markers displayed mild disease activity. Follow‐up showed long‐term patency in two of these patients and recurrent stenoses in the other three, but we found no obvious differences in patients without serological disease activity at the time of PTA (table 1). Also, thrombocytosis as an indirect marker of disease activity, as suggested by other reports,36 was not associated with a tendency to relapse. As serological disease activity was low or absent in all of our patients at the time of restenosis, whether restenoses are a result of fibroblastic proliferation rather than of inflammation will need discussion.
In recent studies, low‐dose acetylsalicylic acid has been suggested as a long‐term treatment to decrease the incidence of cranial ischaemic complications in patients with GCA, probably related to its antiplatelet effects.37 An anti‐inflammatory effect of the usual dosage of acetylsalicylic acid given to most of our patients (100 mg) cannot be suspected unless markedly higher doses are used, as reported in a GCA mouse model.38 Although the antiplatelet effect can be discussed, the indication for aspirin has to be considered carefully in the light of comorbidity.
In conclusion, PTA of symptomatic vascular lesions of the upper extremities caused by GCA is an effective method in patients refractory to immunosuppressive treatment only. A tendency to restenoses, especially in extensive lesions, may be overcome by repeated interventions.
Abbreviations
CRP - C reactive protein
DSA - digital subtraction angiography
ERS - erythrocyte sedimentation rate
GCA - giant‐cell arteritis
MRA - magnetic resonance angiography
PTA - percutaneous transluminal angioplasty
Footnotes
Competing interests: None.
References
- 1.Levine S M, Hellmann D B. Giant cell arteritis. Curr Opin Rheumatol 2002143–10. [DOI] [PubMed] [Google Scholar]
- 2.Salvarani C, Cantini F, Boiardi L, Hunder G G. Polymyalgia rheumatica and giant‐cell arteritis. N Engl J Med 2002347261–271. [DOI] [PubMed] [Google Scholar]
- 3.Stanson A W. Imaging findings in extracranial (giant cell) temporal arteritis. Clin Exp Rheumatol 200018S43–S48. [PubMed] [Google Scholar]
- 4.Brack A, Martinez‐Taboada V, Stanson A, Goronzy J J, Weyand C M. Disease pattern in cranial and large‐vessel giant cell arteritis. Arthritis Rheum 199942311–317. [DOI] [PubMed] [Google Scholar]
- 5.Lambert M, Weber A, Boland B, De Plaen J F, Donckier J. Large vessel vasculitis without temporal artery involvement: isolated form of giant cell arteritis? Clin Rheumatol 199615174–180. [DOI] [PubMed] [Google Scholar]
- 6.Walz‐Leblanc B A, Ameli F M, Keystone E C. Giant cell arteritis presenting as limb claudication. Report and review of the literature. J Rheumatol 199118470–472. [PubMed] [Google Scholar]
- 7.Ninet J P, Bachet P, Dumontet C M, Du Colombier P B, Stewart M D, Pasquier J H. Subclavian and axillary involvement in temporal arteritis and polymyalgia rheumatica. Am J Med 19908813–20. [DOI] [PubMed] [Google Scholar]
- 8.Weyand C M, Goronzy J J. Giant cell arteritis and polymyalgia rheumatica. Ann Intern Med 2003139505–515. [DOI] [PubMed] [Google Scholar]
- 9.Klein R G, Hunder G G, Stanson A W, Sheps S G. Large artery involvement in giant cell (temporal) arteritis. Ann Intern Med 197583806–812. [DOI] [PubMed] [Google Scholar]
- 10.Michel B A, Arend W P, Hunder G G. Clinical differentiation between giant cell (temporal) arteritis and Takayasu's arteritis. J Rheumatol 199623106–111. [PubMed] [Google Scholar]
- 11.Nuenninghoff D M, Hunder G G, Christianson T J, McClelland R L, Matteson E L. Incidence and predictors of large‐artery complication (aortic aneurysm, aortic dissection, and/or large‐artery stenosis) in patients with giant cell arteritis: a population‐based study over 50 years. Arthritis Rheum 2003483522–3531. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt W A, Natusch A, Moller D E, Vorpahl K, Gromnica‐Ihle E. Involvement of peripheral arteries in giant cell arteritis: a color Doppler sonography study. Clin Exp Rheumatol 200220309–318. [PubMed] [Google Scholar]
- 13.Blockmans D, Stroobants S, Maes A, Mortelmans L. Positron emission tomography in giant cell arteritis and polymyalgia rheumatica: evidence for inflammation of the aortic arch. Am J Med 2000108246–249. [DOI] [PubMed] [Google Scholar]
- 14.Amann‐Vesti B R, Koppensteiner R, Rainoni L, Pfamatter T, Schneider E. Immediate and long‐term outcome of upper extremity balloon angioplasty in giant cell arteritis. J Endovasc Ther 200310371–375. [DOI] [PubMed] [Google Scholar]
- 15.Dellaripa P F, Eisenhauer A C. Bilateral percutaneous ballon angioplasty of the axillary arteries in a patient with giant cell arteritis and upper extremity ischemic symptoms not responsive to corticosteroids. J Rheumatol 1998251429–1433. [PubMed] [Google Scholar]
- 16.Motarjeme A. Percutaneous transluminal angioplasty of supra‐aortic vessels. J Endovasc Surg 19963171–181. [DOI] [PubMed] [Google Scholar]
- 17.Liang P, Tan‐Ong M, Hoffman G S. Takayasu's arteritis: vascular interventions and outcomes. J Rheumatol 200431102–106. [PubMed] [Google Scholar]
- 18.Joseph S, Mandalem K R, Rao V R, Gupta A K, Unni N M, Rao A S.et al Percutaneous transluminal angioplasty of the subclavian artery in nonspecific aortoarteritis: results of long‐term follow‐up. J Vasc Intervent Radiol 19945573–580. [DOI] [PubMed] [Google Scholar]
- 19.Tyagi S, Verma P K, Gambhir D S, Kaul U A, Saha R, Arora R. Early and long‐term results of subclavian angioplasty in aortoarteritis (Takayasu disease): comparison with atherosclerosis. Cardiovasc Intervent Radiol 199821219–224. [DOI] [PubMed] [Google Scholar]
- 20.Both M, Jahnke T, Reinhold‐Keller E, Reuter M, Grimm J, Biederer J.et al Percutaneous management of occlusive arterial disease associated with vasculitis: a single center experience. Cardiovasc Intervent Radiol 20032619–26. [DOI] [PubMed] [Google Scholar]
- 21.Monte R, Gonźales‐Gay M A, Garcia‐Porrúa C, López‐Alvarez M J, Pulpeiro J R. Successful response to angioplasty in a patient with upper limb ischaemia secondary to giant cell arteritis. Br J Rheumatol 199737344. [DOI] [PubMed] [Google Scholar]
- 22.Do D D, Jeanneret C, Mahler F. Images in vascular medicine. Giant cell arteritis of axillary artery. Vasc Med 19961293–294. [DOI] [PubMed] [Google Scholar]
- 23.Hunder G G, Bloch D A, Michel B A, Stevens M B, Arend W P, Calabrese L H.et al The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990331122–1128. [DOI] [PubMed] [Google Scholar]
- 24.Rutherford R B, Baker J D, Ernst C, Johnston K W, Porter J M, Ahn S.et al Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 199726517–538. [DOI] [PubMed] [Google Scholar]
- 25.Garcia Vazquez J M, Carreira J M, Seoane C, Vidal J J. Superior and inferior limb ischaemia in giant cell arteritis: angiography follow‐up. Clin Rheumatol 19991861–65. [DOI] [PubMed] [Google Scholar]
- 26.Cohen H E, Shankar P J, Martin J C, Lewis M H. Atypical giant cell arteritis resulting in arm amputation. Ann R Coll Surg Engl 200385260–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mašković J, Janković S, Lušić I, Cambj‐Sapunar L, Mimica Z̆, Bačić A. Subclavian artery stenosis caused by non‐specific arteritis (Takayasu disease): treatment with Palmaz stent. Eur J Radiol 199731193–196. [DOI] [PubMed] [Google Scholar]
- 28.Both M, Reinhold‐Keller E, Muller‐Hulsbeck S, Biederer J, Gross W L, Heller M.et al Inflammatory aortic arch syndrome: contrast‐enhanced, three‐dimensional MR angiography in stenotic lesions. RoFo 200417648–55. [DOI] [PubMed] [Google Scholar]
- 29.Loewe C, Schillinger M, Haumer M, Loewe‐Grgurin M, Lammer J, Thurnher S.et al MRA versus DSA in the assessment of occlusive disease in the aortic arch vessels: accuracy in detecting the severity, number, and length of stenoses. J Endovasc Ther 200411152–160. [DOI] [PubMed] [Google Scholar]
- 30.Taneja K, Jain R, Sawhney S, Rajani M. Occlusive arterial disease of the upper extremity: colour Doppler as a screening technique and for assessment of distal circulation. Australas Radiol 199640226–229. [DOI] [PubMed] [Google Scholar]
- 31.Ruehm S G, Goyen M, Debatin J F. MR Angiography: first choice for diagnosis of the arterial vascular system. RoFo 2002174551–561. [DOI] [PubMed] [Google Scholar]
- 32.Duhaut P, Pinede L, Bornet H, Demolombe‐Rague S, Dumontet C, Ninet J.et al Biopsy proven and biopsy negative temporal arteritis: differences in clinical spectrum at the onset of disease. Ann Rheum Dis 199958335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korner M, Baumgartner I, Do D D, Mahler F, Schroth G. PTA of the subclavian and innominate arteries: long‐term results. Vasa 199928117–122. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan T M, Gray B H, Bacharach J M, Perl J 2nd, Childs M B, Modzelewski L. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg 1998281059–1065. [DOI] [PubMed] [Google Scholar]
- 35.Van Damme H, Fourny J, Zicot M, Limet R. Giant cell arteritis (Horton's disease) of the axillary artery‐case reports. Angiology 198940593–601. [DOI] [PubMed] [Google Scholar]
- 36.Costello F, Zimmerman M B, Podhajsky P A, Hayreh S S. Role of thrombocytosis in diagnosis of giant cell arteritis and differentiation of arteritic from non‐arteritic anterior ischemic optic neuropathy. Eur J Ophthalmol 200414245–257. [DOI] [PubMed] [Google Scholar]
- 37.Nesher G, Berkun Y, Mates M, Baras M, Rubinow A, Sonnenblick M. Low‐dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum 2004501332–1377. [DOI] [PubMed] [Google Scholar]
- 38.Weyand C M, Kaiser M, Yang H, Younge B, Goronzy J J. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum 200246457–466. [DOI] [PubMed] [Google Scholar]