Abstract
Background
Familial Mediterranean fever (FMF) is the most frequent of the recurrent inherited fevers. This autosomal recessive disorder is characterised by periodic episodes of fever and serositis that commonly affect the people of Arab, Armenian, Sephardic Jewish and Turkish origin. Most of the described MEFV gene anomalies responsible for the disease are missense mutations. In the absence of any functional test, epidemiological studies or pedigree analyses are the only means of proving the deleterious character of these sequence variations. Evidence was provided by our recent study using a population‐based approach, that the p.E148Q allele is probably a benign polymorphism and not a disease‐causing mutation. Its implication in FMF remains, however, controversial.
Objective
To evaluate the segregation of the p.E148Q MEFV allele with FMF disease by using pedigree analysis.
Participants
21 patients and 48 unaffected relatives belonging to 18 independent families with FMF.
Results
Segregation analysis of the p.E148Q allele was compatible with a Mendelian autosomal recessive transmission of the disease phenotype in only three families. In 15 of 18 families, segregation was partly or completely defective. The p.E148Q allele was not transmitted to 14 of 19 (74%) affected children.
Conclusions
No evidence of preferential transmission of p.E148Q from heterozygous parents to their affected offspring was observed. MEFV is not associated with the clinical manifestations of several patients carrying this variant. Considering p.E148Q to be a benign polymorphism should reduce the possibility of false‐positive diagnoses, while highlighting genetic heterogeneity in FMF.
Correctly interpreting information gathered from genealogical trees is essential in human genetics. Familial Mediterranean fever (FMF) is an autosomal recessive disorder (MIM #249 100) characterised by recurrent episodes of fever accompanied by abdominal, articular or thoracic pain. The disease is common in people of Arab, Armenian, Sephardic Jewish and Turkish origin. Early diagnosis is crucial to initiate colchicine treatment that prevents the occurrence of episodes of fever and renal amyloidosis, the main complication of the disease. The gene responsible for FMF, MEFV,1,2 encodes a protein called pyrin or marenostrin. This protein is associated with inflammation, and regulates caspase‐1 function, cytokine secretion and apoptosis.
More than 50 missense sequence variations of the MEFV gene have been described in the INFEVERS database, http://fmf.igh.cnrs.fr/. In the absence of any functional test, the deleterious character of these variations can only be proved by familial or epidemiological studies. Thus, the role of the four most frequent mutations located in exon 10 of the MEFV gene (ie, p.M694V, c.2080A>G; p.V726A, c.2177T>G; p.M680I, c.2040G>C; and p.M694I, c.2082G>A) in FMF development is established1,2 and widely accepted. The p.E148Q (c.442G>C) variation situated in exon 2 was initially described as a mutation,3 but its implication in FMF remains controversial.4,5,6,7,8,9 By using a population‐based approach, we recently showed that the p.E148Q allele is a benign polymorphism.7 Here, we use pedigree analysis to assess the association of the p.E148Q allele with FMF.
Participants and methods
Among the 387 families with FMF previously studied in our laboratory, we selected those with at least one carrier of the p.E148Q allele. The 18 families selected had 21 patients and 48 unaffected relatives. Phenotypic features were recorded through a standardised clinical form by physicians from different medical specialties and the diagnosis of FMF was established according to a usual clinical criteria set.10 Patients gave informed consent, according to the recommendations of the French ethics committees.
The analysis of mutations was carried out as described previously.11 Briefly, exons 2, 3, 5 and 10 of the MEFV gene were analysed by denaturing gradient gel electrophoresis. In the samples displaying a mobility shift, the mutations were identified by enzyme restriction analysis or by direct sequencing.
Results
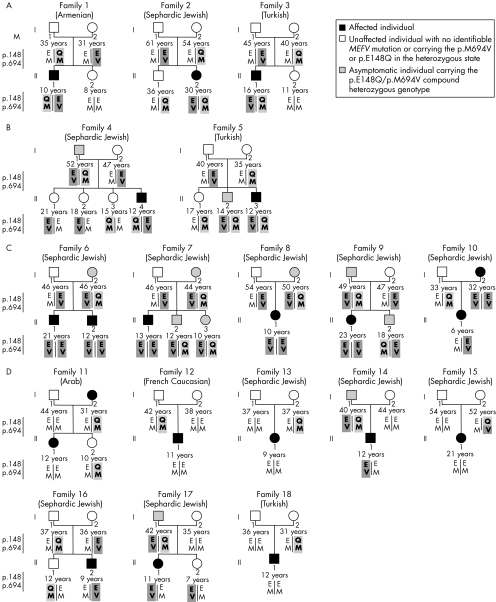
The p.E148Q variation was found in 18 of 387 (4.7%) families with FMF genotyped in our laboratory. Among the 69 people belonging to these families, 31 carried the p.E148Q allele and one carried the p.E148Q–p.M694V complex allele (fig 1D; family 15; I:2). In all, 21 people had clinical manifestations compatible with FMF according to the usual clinical criteria set,10 and 48 were asymptomatic.
Figure 1 Genealogical trees of families with familial Mediterranean fever. Ethnic origin is shown under family number. The p.E148Q and the p.M694V alleles are shown in light and dark shades, respectively. The p.E148Q–p.M694V complex allele is shown in a mixed light and dark shade.
Genealogical trees were classified in four groups according to the likelihood of the implication of the p.E148Q allele in the observed phenotypes. In group A (families 1–3), we observed a classic Mendelian recessive transmission of the disease phenotype compatible with the association of the p.E148Q allele in FMF: all affected people carried the p.E148Q/p.M694V compound heterozygous genotype; healthy heterozygous parents transmitted one mutated allele to affected compound heterozygous children. In group B (families 4 and 5), segregation was partly defective. In family 4, the asymptomatic father (I:1) and his affected child (II:4) had the same p.M694V/p.E148Q genotype. In family 5, one person with the p.E148Q/p.M694V genotype (II:3) showed symptoms, but his brother, who carried the same genotype (II:2), was devoid of any symptoms. In group C, except for family 10, a perfect segregation of the disease phenotype with the p.M694V allele was observed, all affected children being homozygous for this allele. It is, however, striking to note that all those (n = 7) who carried the p.E148Q/p.M694V compound heterozygous genotype were asymptomatic. Family 10 is particular in that the affected child carried only the p.M694V mutation in the heterozygous state (inherited from her mother) and did not receive the p.E148Q allele from her father. In group D, one of the parents who was shown to carry at least one p.E148Q allele never transmitted this allele to his or her affected child. Taken together, in groups C and D (families 6–18), the p.E148Q variation was clearly not causative of FMF, because it was never transmitted to affected people; moreover, all of the nine compound heterozygous p.E148Q/p.M694V people were healthy.
In addition, we evaluated the frequency with which the p.E148Q allele was transmitted from heterozygous parents to affected children. In 17 of the families described above, one of the parents was heterozygous for this allele; an additional person carried the p.E148Q–M694V complex allele (family 15). Only 5 of 19 (26%) affected children inherited the p.E148Q sequence variation.
Discussion
An examination of the 18 families with FMF genotyped in our laboratory, carrying the p.E148Q variation, showed correct segregation in only 3 of them. In most (n = 13) families, the p.E148Q allele was not transmitted to affected people. Among 16 p.E148Q/p.M694V compound heterozygous people, only 5 (31%) displayed a phenotype similar to that of FMF. We suggest that this may be a random association, because the high frequency of p.E148Q (4–12%) in classically affected populations4,5,8,12,13 could represent a confounding factor.
FMF is an autosomal recessive disorder. Most of the deleterious MEFV mutations—for example, p.M694V, pM694I, p.M680I and p.V726A—are located in exon 10 coding for the B‐30.2 pyrin domain that probably plays an important part in protein function. These four mutations generally have a correct segregation with the disease in a classic Mendelian autosomal recessive pattern, and their frequency is much higher in patients than in healthy populations. Since its discovery, the role of the p.E148Q variant remains controversial. p.E148Q/p.E148Q homozygous people are generally asymptomatic.4,5,14,15,16 Several authors have suggested that this variant is a low‐penetrance mutation.3,14 According to this hypothesis, the p.E148Q allele frequency must be higher in patients than in control populations. Many examples of low‐penetrance mutations are described in the literature; the most widely known is p.C282Y HFE1 gene mutation, responsible for hereditary haemochromatosis. The penetrance of the p.C282Y HFE1 allele is low, but its frequency is considerably higher in patients than in asymptomatic controls.17 By contrast, the p.E148Q allele frequency is comparable among patients and controls.4,5,7,12 In addition, p.E148Q is frequent in some populations in which FMF has not been reported, such as Chinese and Punjabi Indians (allele frequencies of 15% and 21%, respectively).6 p.E148Q may be a functional polymorphism that has a phenotypic effect only in trans‐association with a disease‐causing MEFV mutation.4 Nevertheless, our recent study on a Sephardic Jewish population showed that the frequency of p.E148Q/p.M694V compound heterozygotes was similar among patients and among healthy relatives.7
Despite these data, the p.E148Q sequence variation is still considered to be a deleterious mutation. This is, for instance, the case in a recent study on Turkish patients with FMF.8 Among 26 p.E148Q/p.E148Q homozygous individuals, 4 were asymptomatic and only 16 presented with fever. In addition and most importantly, the frequency of the p.E148Q allele was 12% in controls and only 3.5% in patients with FMF.8 Another study on Greek populations from Rhodes and Crete9 showed a higher p.E148Q allele frequency in patients than in controls (18.3% v 1.8%). To explain this unexpected observation, the authors proposed that the deleterious effect of p.E148Q “should be considered in connection with origin data”. In the absence of haplotype data, this result does not rule out the possible existence of a founder effect in the studied population, with a linkage disequilibrium between p.E148Q and a so‐far‐unidentified deleterious MEFV mutation.
Some studies have shown the association of p.E148Q with chronic inflammatory diseases other than FMF or with AA amyloidosis,6,16,18,19,20 but several other studies did not show such association.21,22,23,24,25,26 In our study, none of the people carrying the p.E148Q allele had other inflammatory disorders. Further investigations on larger cohorts of patients are necessary to verify the hypothesis on implication of the p.E148Q sequence variation in chronic inflammatory disorders.
Among the 21 patients presented in our study, in only six (group C) does the MEFV gene seem to be implicated in observed clinical manifestations. Two hypotheses may explain this observation. Firstly, the molecular screening protocol failed to detect the responsible MEFV mutations; however, our recent studies argue against this hypothesis.27,28 Secondly, as established clinical criteria cannot discriminate between MEFV‐related FMF and MEFV‐unrelated FMF‐like syndromes, other hereditary or non‐hereditary fevers may be responsible for the phenotype of patients.
In conclusion, the present study, which shows the defective segregation of the p.E148Q MEFV allele with the FMF phenotype in most of the families studied, strengthens the fact that this sequence variant is not implicated in FMF. Thus, considering this allele to be disease causative would lead to establishing false‐positive diagnoses and to neglecting further necessary diagnostic investigations.
Acknowledgements
We thank Ray Horn for a critical reading of the manuscript.
Abbreviations
FMF - familial Mediterranean fever
Footnotes
Funding: This work was supported by the Assistance Publique/Hôpitaux de Paris grant PHRC AOM97201 and by a grant from the GIS Maladies Rares.
Competing interests: None declared.
References
- 1.The French FMF Consortium A candidate gene for familial Mediterranean fever. Nat Genet 19971725–31. [DOI] [PubMed] [Google Scholar]
- 2.The International FMF Consortium Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 199790797–807. [DOI] [PubMed] [Google Scholar]
- 3.Bernot A, da Silva C, Petit J L, Cruaud C, Caloustian C, Castet V.et al Non‐founder mutations in the MEFV gene establish this gene as the cause of familial Mediterranean fever (FMF). Hum Mol Genet 199871317–1325. [DOI] [PubMed] [Google Scholar]
- 4.Ben‐Chetrit E, Lerer I, Malamud E, Domingo C, Abeliovich D. The E148Q mutation in the MEFV gene: is it a disease‐causing mutation or a sequence variant? Hum Mutat 200015385–386. [DOI] [PubMed] [Google Scholar]
- 5.Stoffman N, Magal N, Shohat T, Lotan R, Koman S, Oron A.et al Higher than expected carrier rates for familial Mediterranean fever in various Jewish ethnic groups. Eur J Hum Genet 20008307–310. [DOI] [PubMed] [Google Scholar]
- 6.Booth D R, Lachmann H J, Gillmore J D, Booth S E, Hawkins P N. Prevalence and significance of the familial Mediterranean fever gene mutation encoding pyrin Q148. Q J Med 200194527–531. [DOI] [PubMed] [Google Scholar]
- 7.Tchernitchko D, Legendre M, Cazeneuve C, Delahaye A, Niel F, Amselem S. The E148Q MEFV allele is not implicated in the development of familial Mediterranean fever. Hum Mutat 200322339–340. [DOI] [PubMed] [Google Scholar]
- 8.Topaloglu R, Ozaltin F, Yilmaz E, Ozen S, Balci B, Besbas N.et al E148Q is a disease causing MEFV mutation: a phenotypic evaluation in patients with familial Mediterranean fever. Ann Rheum Dis 200564750–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantopoulos K, Kanta A, Lilakos K, Papanikolaou G, Meletis I. Familial Mediterranean fever and E148Q pyrin gene mutation in Greece. Int J Hematol 20058126–28. [DOI] [PubMed] [Google Scholar]
- 10.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T.et al Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum 1997401879–1885. [DOI] [PubMed] [Google Scholar]
- 11.Cazeneuve C, Sarkisian T, Pecheux C, Dervichian M, Nedelec B, Reinert P.et al MEFV‐gene analysis in Armenian patients with familial Mediterranean fever: diagnostic value and unfavorable renal prognosis of the M694V homozygous genotype‐genetic and therapeutic implications. Am J Hum Genet 19996588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershoni‐Baruch R, Shinawi M, Leah K, Badarnah K, Brik R. Familial Mediterranean fever: prevalence, penetrance and genetic drift. Eur J Hum Genet 20019634–637. [DOI] [PubMed] [Google Scholar]
- 13.Al‐Alami J R, Tayeh M K, Najib D A, Abu‐Rubaiha Z A, Majeed H A, Al‐Khateeb M S.et al Familial Mediterranean fever mutation frequencies and carrier rates among a mixed Arabic population. Saudi Med J 2003241055–1059. [PubMed] [Google Scholar]
- 14.Aksentijevich I, Torosyan Y, Samuels J, Centola M, Pras E, Chae J J.et al Mutation and haplotype studies of familial Mediterranean fever reveal new ancestral relationships and evidence for a high carrier frequency with reduced penetrance in the Ashkenazi Jewish population. Am J Hum Genet 199964949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kogan A, Shinar Y, Lidar M, Revivo A, Langevitz P, Padeh S.et al Common MEFV mutations among Jewish ethnic groups in Israel: high frequency of carrier and phenotype III states and absence of a perceptible biological advantage for the carrier state. Am J Med Genet 2001102272–276. [DOI] [PubMed] [Google Scholar]
- 16.Gershoni‐Baruch R, Broza Y, Brik R. Prevalence and significance of mutations in the familial Mediterranean fever gene in Henoch‐Schonlein purpura. J Pediatr 2003143658–661. [DOI] [PubMed] [Google Scholar]
- 17.Hanson E H, Imperatore G, Burke W. HFE gene and hereditary hemochromatosis: a HuGE review. Human Genome Epidemiology. Am J Epidemiol 2001154193–206. [DOI] [PubMed] [Google Scholar]
- 18.Aganna E, Hawkins P N, Ozen S, Pettersson T, Bybee A, McKee S A.et al Allelic variants in genes associated with hereditary periodic fever syndromes as susceptibility factors for reactive systemic AA amyloidosis. Genes Immun 20045289–293. [DOI] [PubMed] [Google Scholar]
- 19.Imirzalioglu N, Dursun A, Tastan B, Soysal Y, Yakicier M C. MEFV gene is a probable susceptibility gene for Behcet's disease. Scand J Rheumatol 20053456–58. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovich E, Livneh A, Langevitz P, Brezniak N, Shinar E, Pras M.et al Severe disease in patients with rheumatoid arthritis carrying a mutation in the Mediterranean fever gene. Ann Rheum Dis 2005641009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dode C, Hazenberg B P, Pecheux C, Cattan D, Moulin B, Barthelemy A.et al Mutational spectrum in the MEFV and TNFRSF1A genes in patients suffering from AA amyloidosis and recurrent inflammatory attacks. Nephrol Dial Transplant 2002171212–1217. [DOI] [PubMed] [Google Scholar]
- 22.Olgun A, Akman S, Kurt I, Tuzun A, Kutluay T. MEFV mutations in familial Mediterranean fever: association of M694V homozygosity with arthritis. Rheumatol Int 200525255–259. [DOI] [PubMed] [Google Scholar]
- 23.Fidder H, Chowers Y, Ackerman Z, Pollak R D, Crusius J B, Livneh A.et al The familial Mediterranean fever (MEVF) gene as a modifier of Crohn's disease. Am J Gastroenterol 2005100338–343. [DOI] [PubMed] [Google Scholar]
- 24.Karban A, Dagan E, Eliakim R, Herman A, Nesher S, Weiss B.et al Prevalence and significance of mutations in the familial Mediterranean fever gene in patients with Crohn's disease. Genes Immun 20056134–139. [DOI] [PubMed] [Google Scholar]
- 25.Brucato A, Shinar Y, Brambilla G, Robbiolo L, Ferrioli G, Patrosso M C.et al Idiopathic recurrent acute pericarditis: familial Mediterranean fever mutations and disease evolution in a large cohort of Caucasian patients. Lupus 200514670–674. [DOI] [PubMed] [Google Scholar]
- 26.Espinosa G, Arostegui J I, Plaza S, Rius J, Cervera R, Yague J.et al Behcet's disease and hereditary periodic fever syndromes: casual association or causal relationship? Clin Exp Rheumatol 200523(Suppl 38)S64–S66. [PubMed] [Google Scholar]
- 27.Cazeneuve C, Hovannesyan Z, Genevieve D, Hayrapetyan H, Papin S, Girodon‐Boulandet E.et al Familial Mediterranean fever among patients from Karabakh and the diagnostic value of MEFV gene analysis in all classically affected populations. Arthritis Rheum 2003482324–2331. [DOI] [PubMed] [Google Scholar]
- 28.Tchernitchko D, Moutereau S, Legendre M, Delahaye A, Cazeneuve C, Lacombe C.et al MEFV analysis is of particularly weak diagnostic value for recurrent fevers in western European Caucasian patients. Arthritis Rheum 2005523603–3605. [DOI] [PubMed] [Google Scholar]