Abstract
Objective
To identify susceptibility loci for nodal osteoarthritis.
Methods
A genome screen at an average marker spacing of 9.29 cM was carried out on 558 people from 202 families, of whom 491 had nodal osteoarthritis. All genotyped people were graded for the incidence and severity of distal interphalangeal (DIP) nodes, and radiographs from 354 people were graded for joint‐space narrowing (JSN) and osteophytes (OSTs). Age‐regressed indices for DIP nodes, JSN and OSTs were calculated using these phenotypic data. Affected sibling pair (ASP) and quantitative trait analyses were carried out using MERLIN.
Results
The data analysis identified suggestive linkage to loci on chromosomes 3 (for JSN and OST), 4 (for JSN), 8 (for DIP), 11 (for radiographic osteoarthritis) and 16 (for JSN). Both the ASP and quantitative analyses identified the loci on chromosomes 4 and 11. The loci on chromosomes 3 and 16 overlap with those previously identified for large‐joint osteoarthritis. Of the loci identified by the quantitative analyses with the logarithm of the odds of linkage >1.5, two were linked to more than one trait, whereas nine were linked to single traits: one for DIP, six for JSN and two for OST.
Conclusion
The ASP and quantitative analyses of the cohort with nodal osteoarthritis suggest that multiple susceptibility loci for osteoarthritis influence the traits, which combine to form the osteoarthritis phenotype, and that these loci may not act exclusively on the joints of the hand.
Osteoarthritis is the most common form of human joint disease and causes considerable pain and disability.1 It is a heterogeneous condition, both in terms of its aetiology and pathogenesis, which leads to the failure of the diarthrodial joint.2 Of the common osteoarthritis subsets, nodal generalised osteoarthritis shows strong familial predisposition, implying that heritable factors contribute towards predisposition to the condition. Although there is no single definition for this disorder, it can be characterised clinically by polyarticular involvement of the distal and proximal interphalangeal (DIP and PIP) joints of the finger, Heberden's (DIP) and Bouchard's (PIP) nodes, preponderance in women, peak onset in middle age and predisposition to osteoarthritis of the knee, hip, cervical and lumbar apophysial joints.3,4
The familial clustering of multiple DIP nodes, particularly among women, has long been recognised.5 Data from twin studies have supported a strong genetic component for the occurrence of DIP nodes and for radiographic hand osteoarthritis.6 Studies on the relative risk of osteoarthritis of both the interphalangeal joints and the first carpometacarpal joint conducted in Iceland provide further support for a genetic component to radiographic hand osteoarthritis that increases with severity,7 as do segregation analyses in families from the Framingham study8 and in the isolated Chuvashan population.9 Although multiple DIP nodes may manifest earlier than radiographic hand osteoarthritis, there is some evidence of a correlation between nodes and radiographic interphalangeal joint osteoarthritis, particularly in elderly people.10
Genetic linkage and association studies have been conducted using cohorts of patients with osteoarthritis primarily of the hip, knee or hand, and several loci have been suggested as harbouring susceptibility genes.11,12 Several studies seem to be concordant for loci on chromosome 2q.13,14,15,16,17,18 We, however, found no evidence of a major linkage between markers on chromosome 2q in our cohorts of affected sibling pairs (ASPs) with either nodal or knee osteoarthritis,19 in common with two other studies on hand osteoarthritis.20,21 We thus extended our search for susceptibility loci in our family members with nodal osteoarthritis by carrying out a genomewide linkage scan. To analyse the genotype data, we used both qualitative (ASP) and quantitative methods. ASP analysis is a popular method for the analysis of complex diseases of late onset, as this method considers only those patients who are classified as affected according to a predetermined set of diagnostic criteria. This method has the disadvantage, however, of ignoring phenotypic variability that may exist between affected people and excludes information from those who are marginally affected, thereby reducing the power to detect linkage. By contrast, quantitative analyses use this variability and can thereby potentially increase the power to detect linkage. The detailed clinical and radiographic data from the family members of our nodal osteoarthritis cohort allowed us to carry out and compare the data from the ASP and quantitative analyses, the findings of which we present in this paper.
Patients and methods
Characterisation of family cohort
Recruitment of families with nodal osteoarthritis from Nottinghamshire, UK, has been described previously.19 Each participant gave written consent and was interviewed and examined by a trained metrologist, and blood was collected for DNA extraction. The presence and localisation of nodes and osteoarthritis were then recorded according to standard methods. Nodes were classified at each DIP joint as grade 0 (no firm or bony swelling); grade 1 (unilateral firm or bony superolateral swelling); and grade 2 (bilateral firm or bony superolateral swelling or single posterior bar). PIP nodes were classified as present or absent. The intraobserver reproducibility for grading DIP and PIP nodes was good, with κ values of 0.9 and 0.6, respectively. Quantitative analyses were based only on the more accurately graded DIP node scores. Where possible, bilateral hand radiographs were obtained for clinically affected people. These were dorsal–palmar, both hands on one film, centred on the third metacarpal heads (Agfa film, 50 kV, 4 mA/s). The first metacarpophalangeal joint, the thumb interphalangeal joint, and the DIP and PIP joints of the index, middle, ring and little fingers were each graded for radiographic features of joint‐space narrowing (JSN; 0–3) and osteophytes (OSTs; 0–3) by a single observer, using the OsteoArthritis Research Society International photographic atlas.22
Phenotype definition and derivation of quantitative indices
The primary definition of nodal osteoarthritis used to designate people as affected for the ASP analyses was the presence of nodes on at least two or more DIP joints of each hand (96% of cases). This was extended to include a minority of people with two bilateral DIP nodes and ⩾2 x ray changes, graded ⩾2 (3% of cases), or with ⩾4 DIP nodes and bilateral involvement (1% of cases). All others were assigned an “unknown” affection status. “Nodal+x ray” was defined as nodal osteoarthritis along with either a JSN or an OST score of ⩾2 for at least one DIP or PIP joint.
For the quantitative indices, severity scores were first calculated for each person. A nodal severity score was calculated by summing the nodal grade given for each DIP joint of both hands, and JSN and OST scores were calculated by summing the number of interphalangeal joints with a grading of ⩾2. This scoring system was considered to be robust as only definite osteoarthritis‐related changes received positive severity scores. However, using this system, minor osteoarthritis‐related changes would be scored as zero. Hence, for each trait, we carried out quantitative analysis by using scores only from affected people, with definite, robustly measurable features of that trait, as well as an analysis using all available scores. For the nodal trait, people included in the affected category were those who had been designated as affected for the ASP analysis; for the JSN and OST trait, the affected category included those who had at least one joint graded ⩾2 for the respective trait.
To create the quantitative indices for analysis, the severity scores were regressed for age. The age‐regressed quantitative indices that included only affected people were named DIP (DIP node index), JSN (JSN index) and OST (OST index), and those including unaffected family members were designated U‐DIP, U‐JSN and U‐OST, respectively. Pearson's correlation coefficients between the age‐adjusted trait indices for all people were computed using STATA V.8.2, and their heritability values were calculated using the polygenic function of the sequential oligogenic linkage analysis routine.23
Genotyping of microsatellite markers
Genotypes were obtained for 448 microsatellites, with an average marker spacing of 9.28 cM. We increased the marker density to 2–5 cM to cover areas of the genome where potential osteoarthritis linkages or associations had been reported previously—for example, we included the markers D2S305 and D2S2150 that flank the matrilin‐3 locus.18 Primers were from the Applied Biosystems Linkage Marker panel sets (Applied Biosystems, Warrington, UK) or designed from sequences obtained from The Genome Database (http://www.gdb.org). The average marker heterozygosity was 0.775 and polymorphism information content was 0.747. The marker positions were obtained from Marshfield genetic maps (http://research.marshfieldclinic.org/genetics/). Pooled polymerase chain reaction products were genotyped on either an ABI 377‐automated DNA sequencer (Applied Biosystems), using software GENESCAN V.3.1 and GENOTYPER V.2.1, or a MegaBACE 500 DNA sequencer (Amersham, Little Chalfont, UK), using Genetic Profiler V.1.1 software.
Data analysis
Genotype data were compiled into a linkage format by using genetic analysis system V.2.0 (http://users.ox.ac.uk/~ayoung/gas.html). Genetic Analysis System (for the autosomes) and PEDCHECK (for the X chromosome)24 were also used to check for misinheritances caused by genotyping errors, providing an interim point of quality control for accuracy of genotyping. Single‐point, non‐parametric linkage analysis was carried out using SPLINK (http://www.mrc‐bsu.cam.ac.uk/pub/methodology/genetics), generating allele frequencies for relationship estimation and multipoint analyses. As most of the families in our cohort comprised single ASPs without parental data, we used the relationship estimation programs RELATIVE V.1.1025 and PREST V.326 on genotype data generated from 108 unlinked markers to identify probable monozygotic twins, half‐sibs or unrelated family members. Where identified, such people, and, if appropriate, their family members, were excluded.
Multipoint linkage analyses were carried out using MERLIN V.0.9.12b27 with the options npl–qtl–vc, using the processed and down‐coded genotype data, the marker map position (derived from the Marshfield genetic map), allele frequencies (calculated by MERLIN), the encoded pedigree information, and the status of affect and quantitative trait information for each person. As in previous studies, the cut‐off we used for suggestive linkage was a maximum multipoint logarithm of the odds of linkage (LOD) score of 1.5 (the value for a 10‐cM genome map), which is less than the multipoint LOD score of 1.9 for a dense map.20,28 LOD scores were adjusted for multiple testing as previously described.29
Results
The nodal osteoarthritis cohort
Members of an initial cohort of 212 families were genotyped. After an analysis of family genotypes for relatedness, 55 people and their appropriate family members were excluded, leaving a cohort of 558 people from 202 families. The mean (standard deviation (SD)) number of people per family was 2.7 (0.93; range 2–7). Of these 202 families, 195 had at least one nodal ASP with osteoarthritis. Specifically, there were 124 families with 2 affected people, 53 families with 3, 13 families with 4 and 5 families with 5 affected people. Of the 360 possible ASP combinations, 254 were female–female pairs, 98 were female–male pairs and 8 were male–male pairs. Table 1 details the subjects available for each of the ASPs and quantitative analyses.
Table 1 Summary of the subjects available for each of the analyses.
| Total | ASP analyses | Quantitative analyses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nodal OA | Nodal+x ray | U‐DIP | DIP | U‐JSN | JSN | U‐OST | OST | ||
| People | 558 | 484 | 310 | 558 | 484 | 354 | 231 | 354 | 295 |
| Women | 443 | 412 | 260 | 443 | 412 | 296 | 202 | 296 | 243 |
| Men | 115 | 72 | 50 | 115 | 72 | 58 | 29 | 58 | 52 |
| Families | 202 | 195 | 108 | 202 | 195 | 128 | 70 | 128 | 100 |
ASP, affected sibling pair; DIP, distal interphalangeal; JSN, joint‐space narrowing; OA, osteoarthritis; OST, osteophyte; U‐DIP, unaffected and distal interphalangeal; U‐JSN, unaffected and joint‐space narrowing; U‐OST, unaffected and osteophyte.
Loci identified by the ASP analysis
The multipoint ASP analysis of the nodal osteoarthritis cohort identified four loci at a nominal significance level (p<0.05; table 2), the most significant being on chromosome 11 at 116.01 cM (LOD 1.63; p = 0.003). Of the people with nodal osteoarthritis from whom hand radiographs were available, 89% had radiographic evidence of osteoarthritis. Stratification of the cohort with nodal osteoarthritis for radiographic osteoarthritis (nodal + x ray; table 1) increased the evidence for linkage on chromosome 11 (LOD 2.69), and this linkage remained suggestive after adjustment of the LOD score for the two models tested (adjusted LOD 2.39; table 2).
Table 2 Summary of the loci identified from the affected sibling pair analyses.
| Chromosome | cM | LODmax* | p Value | Span | Stratification | Reference |
|---|---|---|---|---|---|---|
| 4 | 25.9 | 1.34 | 0.007 | 20.8–36.6 | Nodal+x ray | |
| 36.1 | 0.8 | 0.03 | 31.2–37.7 | Nodal OA | ||
| 57.0 | 0.65 | 0.04 | 53.7–58.8 | Nodal OA | ||
| 7 | 135.9 | 0.92 | 0.02 | 130.4–141.6 | Nodal OA | Chapman et al30 |
| 137.8 | 0.77 | 0.03 | 137.2–139.7 | Nodal+x ray | Chapman et al30 | |
| 11 | 116.0 | 1.63 | 0.003 | 98.4–126.8 | Nodal OA | |
| 118.5 | 2.69 | <0.000 | 99.0–141.9 | Nodal+x ray | ||
| 22 | 27.4 | 1.51 | 0.004 | 14.4–41.7 | Nodal+x ray |
LODmax, maximum logarithm of the odds of linkage; OA, osteoarthritis.
*Correction for the six models tested requires the subtraction of LOD 0.30 from these values.
The highest LOD (LODmax) at each locus is shown with the corresponding cM position (Marshfield). The span indicates the extent of the region of nominal significance. Published studies on osteoarthritis linkage that have identified loci on which the position of maximal linkage is within 10 cM of the LODmax given here are referenced.
Further stratification to determine whether the linkage on chromosome 11 was primarily to a JSN or an OST phenotype was not possible, as most people in the cohort had both features, but to varying degrees. Hence, quantitative analyses were carried out. Stratification also increased the LOD scores for loci on chromosomes 4 and 22, but neither had adjusted LOD scores in the suggestive range.
Loci identified by the quantitative analysis
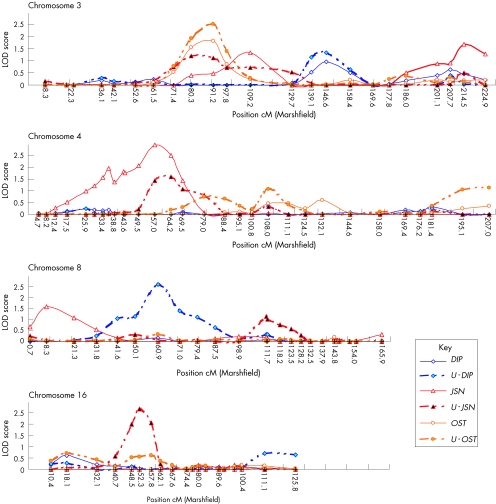
Multipoint linkage analysis was carried out with each of six quantitative indices (see Patients and methods section). Table 1 gives the details of subjects available for each of the analyses. Pairwise Pearson's correlation coefficients between the age‐adjusted trait indices were 0.47 between U‐DIP and U‐JSN, 0.48 between U‐DIP and U‐OST, and 0.64 between U‐JSN and U‐OST. The low correlation between the nodal and radiographic indices suggests that they may represent different facets of the disease, possibly with different genetic components. The U‐OST and U‐JSN traits have a higher correlation with each other, but not so high as to preclude some degree of independence. Table 3 lists the 29 loci identified at a nominal significance level (LOD>0.9; p<0.05) by one or more trait indices. These loci have been listed here as independent, as they appear at this stage to be non‐overlapping. It may emerge, however, from future studies that certain high LOD scores are from a single locus rather than from adjacent loci. For example, there may be a single locus for OST on chromosome 20 as opposed to three separate loci. Linkage to 4 of the 29 loci remained suggestive after adjustment of the LOD for the six models tested: chromosome 3 at 91.2 cM (adjusted LOD 1.7 for U‐OST); chromosome 4 at 57.0 cM (adjusted LOD 2.17 for JSN); chromosome 8 at 60.9 cM (adjusted LOD 1.78 for U‐DIP); and chromosome 16 at 52.3 cM (adjusted LOD 1.86 for U‐JSN). Figure 1 shows the comparison of the unadjusted multipoint data generated by each test at these four loci. For chromosome 16, there is a single locus for a single trait. By contrast, for chromosomes 3, 4 and 8, the patterns of linkage are more complex and may indicate the presence of more than one susceptibility gene.
Table 3 Summary of the loci identified from the quantitative analyses.
| Chromosome | cM | LODmax* | p Value | Span | Trait index | Reference |
|---|---|---|---|---|---|---|
| 2 | 103.7 | 1.04 | 0.014 | 103.2 | DIP | |
| 3 | 91.2 | 2.5 | <0.003 | 80.3–97.8 | U‐OST, OST, U‐JSN | Stefansson et al,18 Chapman et al30 |
| 109.2 | 1.32 | 0.007 | 109.2 | JSN | ||
| 146.6 | 1.33 | 0.007 | 146.6 | DIP, U‐DIP | ||
| 214.5 | 1.67 | 0.003 | 214.5–224.9 | JSN | ||
| 4 | 36.1 | 1.95 | 0.0013 | 25.9–69.5 | JSN | |
| 57.0 | 2.95 | 0.0011 | 25.9–69.5 | JSN, U‐JSN | ||
| 5 | 28.8 | 1.23 | 0.009 | 26.7–28.8 | OST | |
| 179.1 | 1.57 | 0.004 | 179.1 | JSN | ||
| 194.9 | 1.29 | 0.007 | 194.9 | DIP, U‐OST | ||
| 6 | 89.8 | 1.11 | 0.012 | 82.6–109.2 | U‐OST | Stefansson et al18 |
| 7 | 137.8 | 1.53 | 0.004 | 137.8 | OST | Chapman et al30 |
| 8 | 8.3 | 1.57 | 0.004 | 8.3–21.3 | JSN | |
| 60.9 | 2.56 | <0.003 | 41.5–79.4 | U‐DIP | ||
| 111.7 | 1.12 | 0.012 | 111.7–112.4 | U‐JSN | Leppavuori et al16 | |
| 11 | 112.3 | 1.64 | 0.003 | 108.6–123.0 | DIP | |
| 118.5 | 1.45 | 0.005 | 105.7–118.5 | JSN, U‐JSN | ||
| 12 | 75.2 | 0.94 | 0.02 | 75.2 | OST | |
| 13 | 17.2 | 1.28 | 0.008 | 17.2–25.1 | OST | Demissie et al20 |
| 93.5 | 1.13 | 0.011 | 93.5 | DIP | Demissie et al20 | |
| 14 | 40.1 | 1.44 | 0.005 | 40.1–47.5 | U‐DIP | |
| 69.2 | 1.17 | 0.01 | 69.2–76.3 | U‐JSN | ||
| 16 | 52.3 | 2.64 | <0.002 | 48.5–57.8 | U‐JSN | Ingvarsson et al,31 Forster et al32 |
| 18 | 71.3 | 1.34 | 0.007 | 71.3–84.8 | OST | |
| 19 | 72.7 | 1.25 | 0.008 | 72.7 | JSN | Demissie et al20 |
| 20 | 11.2 | 1.15 | 0.011 | 11.2 | OST | |
| 50.8 | 2.2 | <0.007 | 39.3–61.8 | OST | ||
| 75.0 | 1.36 | 0.006 | 75.0 | OST | ||
| X | 23.3 | 1.04 | 0.014 | 23.3 | JSN | |
| 42.2 | 1.05 | 0.014 | 42.2–52.5 | OST | Leppavuori et al,16 Chapman et al30 |
DIP, distal interphalangeal; JSN, joint‐space narrowing; LODmax, maximum logarithm of the odds of linkage; OST, osteophyte; U‐DIP, unaffected distal interphalangeal; U‐JSN, unaffected joint‐space narrowing; U‐OST, unaffected osteophyte.
*Correction for the six models tested requires the subtraction of LOD 0.78 from these values.
The highest LOD (LODmax) at each locus is shown with the corresponding cM position (Marshfield). The span indicates the extent of the region of nominal significance. Published studies on osteoarthritis linkage that have identified loci where the position of maximal linkage is within 10 cM of the LODmax given here are referred to.
Figure 1 Multipoint quantitative analysis for chromosomes 3, 4, 8 and 16. The x axis shows the relative position on each chromosome (cM) and the y axis shows the logarithm of the odds linkage (LOD). DIP, distal interphalangeal; JSN, joint‐space narrowing; OST, osteophyte; U‐DIP, unaffected and distal interphalangeal; U‐JSN, unaffected and joint‐space narrowing; U‐OST, unaffected and osteophyte.
Loci identified by different traits
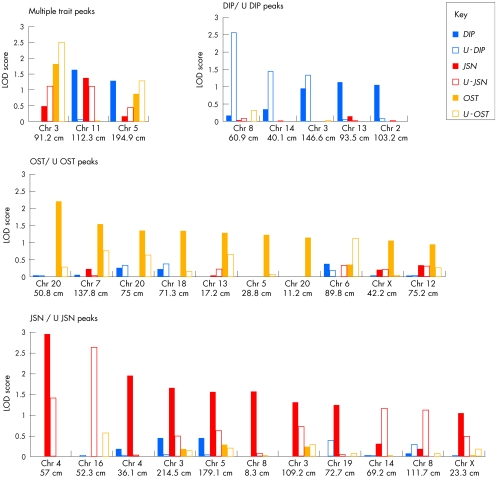
Little overlap was found in the loci detected using the different quantitative traits. Only 3 of the 29 loci identified were linked to multiple traits, whereas the remaining loci were linked to single traits (using either index; fig 2). Of the loci with a LODmax>1.5, two were linked to more than one trait, whereas nine were linked to single traits: one for DIP, six for JSN and two for OST.
Figure 2 Loci identified from the quantitative multipoint linkage analysis. The loci are grouped according to whether they were identified by more than one of the three traits (multiple‐trait loci) and those identified by a single trait (ie, by analysis using either of the two quantitative indices for each trait). In each of the groups, the chromosome and map position (cM; Marshfield) corresponding to the maximum logarithm of the odds of linkage (LOD) score for each locus is given on the x axis. The bars represent the results for each quantitative index at each locus coded as per the key and as described in the text. The y axis shows the multipoint LOD score. Chr, chromosome; DIP, distal interphalangeal; JSN, joint‐space narrowing; OST, osteophyte; U‐DIP, unaffected distal interphalangeal; U‐JSN, unaffected joint‐space narrowing; U‐OST, unaffected osteophyte.
Comparison of ASP with quantitative analyses
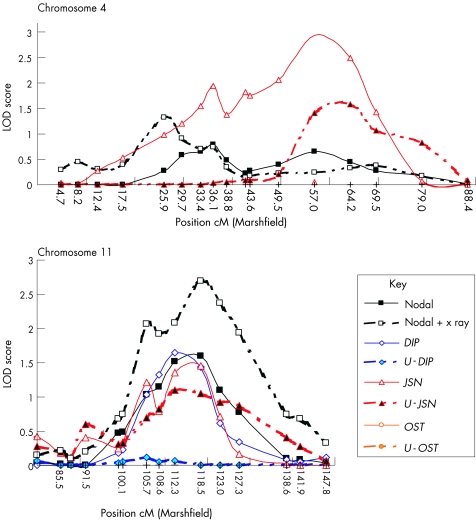
Both the ASP and quantitative analyses identified loci on chromosomes 4, 7 and 11 (tables 2, 3). The suggestive evidence for linkage on chromosome 11 for the nodal+x ray stratification was supported by the quantitative analysis for DIP and JSN (fig 3). The complex pattern of linkage on chromosome 4 for the quantitative analyses (fig 1) seems further complicated when superimposed on the ASP data (fig 3), which may again indicate that more than one susceptibility locus contributes to the linkage across this region. On chromosome 7, a single locus was detected by the ASP analysis for nodal osteoarthritis and the nodal+x ray stratification and by the quantitative analysis for OST, but in neither analysis were the corrected LOD scores suggestive of linkage.
Figure 3 Loci identified by both the affected sibling pair and quantitative multipoint analyses for chromosomes 4 and 11. The x axis shows the relative position on each chromosome (cM) and the y axis shows the logarithm of the odds of linkage (LOD). DIP, distal interphalangeal; JSN, joint‐space narrowing; OST, osteophyte; U‐DIP, unaffected distal interphalangeal; U‐JSN, unaffected joint‐space narrowing; U‐OST, unaffected osteophyte.
Comparison of data with other published genome screens for osteoarthritis
For comparison with previously published genome screens for osteoarthritis, we considered loci that were within 10 cM of the LODmax identified by the ASP or by the quantitative analyses, and potential concordances are indicated in tables 2 and 3. The suggestive locus we identified on chromosome 3 at 91.2 cM (for U‐OST, OST and U‐JSN) is potentially concordant with that identified from the analysis of genotype data of extended Icelandic families with hand osteoarthritis (stratified for nodal osteoarthritis)18 and from ASPs with large‐joint osteoarthritis (stratified for hip osteoarthritis).30 The suggestive locus we identified on chromosome 16 at 52.3 cM (for U‐JSN) also seems concordant with a locus for hip osteoarthritis reported by two independent studies,31,32 and encompasses the interleukin 4 receptor α (IL4R) gene, which has been associated with osteoarthritis in women.33 As hip and isolated hand osteoarthritis have not been strongly associated, we compared the frequency of people in our study with hip osteoarthritis in those families who contributed to the chromosome 16 linkage and those who did not, and found no difference (results not shown).
Discussion
In our study, we generated whole‐genome screen genotype data from a family cohort with hand osteoarthritis, from whom detailed clinical and radiographic information had been specifically collected, allowing both ASP and quantitative analyses to be carried out. As in previous studies, the loci identified were assessed on their likely contribution to osteoarthritis susceptibility, both in terms of their level of significance28 and their concordance with other published studies. As a lack of reproducibility between studies may have many causes,34 we have tabulated all loci with nominal evidence of linkage (acknowledging that a proportion will represent false‐positive results) for comparison with future linkage or association studies.
For the ASP analysis, the phenotype was defined by the presence of multiple bilateral DIP or PIP nodes, and the affected cohort was further stratified for definite radiographic osteoarthritis. The quantitative analyses used separate age‐regressed severity indices for DIP nodes, JSN and OST, and were carried out by including all available scores (U‐DIP, U‐JSN, U‐OST) or by using scores from only affected people (DIP, JSN, OST). Previous similar studies have combined radiographic indices (such as Kellgren–Lawrence scores) across the hand6,8,16,20 or across individual joints,35 but we found that the correlation between the U‐JSN and U‐OST indices in our study was not high. Further, evidence from the Framingham study suggests that JSN and OST may have an independent genetic basis.20 To avoid loss of power resulting from the inappropriate combination of traits, each trait was therefore considered independently. We found that most of the loci identified by the quantitative analysis (all but three) were linked to one of the DIP, OST or JSN traits (fig 2). This suggests that each of the genes at these loci affects the severity of a specific trait and it is their combined effects that result in the osteoarthritis phenotype.
Five loci were identified with suggestive linkage at a genomewide significance level after correction for multiple testing, notably on chromosomes 3, 4, 8, 11 and 16. The locus on chromosome 3 identified by the U‐OST and U‐JSN trait indices corresponds with a region previously identified for osteoarthritis of the hand18 and also of the hip.30 This locus encompasses that of a plausible candidate gene, ADAMSTS9, a metalloproteinase with aggrecanase activity that is expressed in cartilage, with a potential role in osteoarthritis pathogenesis.36 The locus on chromosome 16 identified by the U‐JSN trait index previously linked to hip osteoarthritis31,32 encompasses the IL4R gene that has been associated with hip osteoarthritis in women.33 In addition to these suggestive loci, there are further potentially concordant loci between this study and those conducted on large‐joint osteoarthritis (tables 1, 2), although epidemiological evidence indicates that hand and hip osteoarthritis may be genetically distinct.11 The identification of concordant loci for large‐joint and hand osteoarthritis suggests that specific susceptibility genes may contribute to osteoarthritic traits that are not specific to joint sites and is consistent with the “common variants–multiple disease” hypothesis of common complex genetic disorders.37 Indeed, JSN is a common feature of all forms of osteoarthritis, and the locus on chromosome 16 may confer susceptibility to this trait in both hand and hip osteoarthritis. Therefore, osteoarthritis can be influenced by a combination of alleles that confer susceptibility to osteoarthritis at a specific joint site35 or at multiple joint sites.
A comparison between the loci identified by the ASP and quantitative analysis methods showed that three of the four loci in ASP analyses were also identified by the quantitative analysis. The locus on chromosome 11 is one of the few loci identified by more than one trait, and may indicate that the action of a single gene at this locus contributes to the severity of both a nodal and a JSN phenotype. However, the possibility that the combined effect of two susceptibility genes, each contributing to a different trait, resulted in the identification of this locus by the ASP linkage analysis cannot be excluded. Similarly, the complex pattern of linkage on chromosome 4 indicates that there may be more than one susceptibility gene contributing to JSN severity.
In conclusion, our study identified novel candidate loci for osteoarthritis and loci that seem concordant with previously described loci. A per‐trait analysis of the loci identified showed that most loci are linked to a single osteoarthritis‐related trait. These findings point to a definition of osteoarthritis as the culmination of a combination of pathological changes, each modified by genetic and environmental factors that cooperatively lead to the resultant phenotype. Thus, to develop a complete picture of the contribution of genetic factors to the pathogenesis of osteoarthritis, rather than a move towards collection of data based on symptomatic or clinical criteria alone,38 it will be important to continue collecting carefully graded data on the different changes happening in the joint.39 In particular, improving the precision of quantification of the osteoarthritis phenotype will further improve the power of linkage analyses and inform association studies aimed at identifying the underlying susceptibility genes.
Acknowledgements
This work was supported by grants from the Arthritis Research Campaign, UK, and The Wellcome Trust, UK.
Abbreviations
ASP - affected sibling pair
DIP - distal interphalangeal
JSN - joint‐space narrowing
LOD - logarithm of the odds of linkage
LODmax - maximum logarithm of the odds of linkage score
OST - osteophyte
PIP - proximal interphalangeal
U‐DIP - unaffected distal interphalangeal
U‐JSN - unaffected joint‐space narrowing
U‐OST - unaffected osteophyte
Footnotes
Competing interests: None.
References
- 1.Creamer P, Hochberg M. Osteoarthritis. Lancet 1997350503–539. [DOI] [PubMed] [Google Scholar]
- 2.Doherty M.Osteoarthritis. London: Times Mirror International Publishers, 1994
- 3.Kellgren J H, Moore R. Generalised osteoarthritis and Heberden's nodes. BMJ 19521181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence J S. Generalised osteoarthritis in a population sample. Am J Epidemiol 196990381–389. [DOI] [PubMed] [Google Scholar]
- 5.Stecher R M. Heberden's nodes. Heredity in hypertrophic arthritis of the finger joints. Am J Med Sci 1941201801–809. [Google Scholar]
- 6.Spector T D, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ 1996312940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonsson H, Manolescu I, Stefansson S E, Ingvarsson T, Jonnsson H H, Manolescu A.et al The inheritance of hand osteoarthritis in Iceland. Arthritis Rheum 200348391–395. [DOI] [PubMed] [Google Scholar]
- 8.Felson D T, Couropmitree N N, Chaisson C E, Hannan M T, Zhang Y, McAlindon T E.et al Evidence for a mendelian gene in a segregation analysis of generalized radiographic osteoarthritis. Arthritis Rheum 1998411064–1071. [DOI] [PubMed] [Google Scholar]
- 9.Livshits G, Kalichman L, Cohen Z, Kobyliansky E. Mode of inheritance of hand osteoarthritis in ethnically homogeneous pedigrees. Hum Biol 200274849–860. [DOI] [PubMed] [Google Scholar]
- 10.Stern A G, Moxley G, Rao S T P, Disler D, McDowell C, Park M.et al Utility of digital photographs of the hand for assessing the presence of hand osteoarthritis. Osteoarthritis Cart 200412360–365. [DOI] [PubMed] [Google Scholar]
- 11.Loughlin J. Genetic epidemiology of primary osteoarthritis. Curr Opin Rheumatol 200113111–116. [DOI] [PubMed] [Google Scholar]
- 12.Newman B, Wallis G A. Is osteoarthritis a genetic disease? Clin Invest Med 200225139–149. [PubMed] [Google Scholar]
- 13.Loughlin J, Dowling B, Mustafa Z, Chapman K. Association of the interleukin‐1 gene cluster on chromosome 2q13 with knee osteoarthritis. Arthritis Rheum 2002461519–1527. [DOI] [PubMed] [Google Scholar]
- 14.Smith A J, Keen L J, Billingham M J, Perry M J, Elson C J, Kirwan J R.et al Extended haplotypes and linkage disequilibrium in the IL1R1‐IL1A‐IL1B‐IL1RN gene cluster: association with knee osteoarthritis. Genes Immun 20045451–460. [DOI] [PubMed] [Google Scholar]
- 15.Stern A G, de Carvalho M S, Buck G A, Adler R A, Rao T P S, Disler D.et al Association of erosive hand osteoarthritis with a single nucleotide polymorphism on the gene encoding interleukin‐1 beta. Osteoarthritis Cartilage 200311394–402. [DOI] [PubMed] [Google Scholar]
- 16.Leppavuori J, Kujala U, Kinnunen J, Kaprio J, Nissila M, Heliovaara M.et al Genome scan for predisposing loci for distal interphalangeal joint osteoarthritis: evidence for a locus on 2q. Am J Hum Genet 1999651060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loughlin J, Dowling B, Mustafa Z, Southam L, Chapman K. Refined linkage mapping of a hip osteoarthritis susceptibility locus on chromosome 2q. Rheumatology (Oxford) 200241955–956. [DOI] [PubMed] [Google Scholar]
- 18.Stefansson S E, Jonsson H, Ingvarsson T, Manolescu I, Jonsson H H, Olafsdottir G.et al Genomewide scan for hand osteoarthritis: a novel mutation in matrilin‐3. Am J Hum Genet 2003721448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillaspy E, Spreckley K, Wallis G, Doherty M, Spector T. Investigation of linkage on chromosome 2q and hand and knee osteoarthritis. Arthritis Rheum 2002463386–3387. [DOI] [PubMed] [Google Scholar]
- 20.Demissie S, Cupples L A, Myers R, Aliabadi P, Levy D, Felson D T. Genome scan for quantity of hand osteoarthritis: the Framingham study. Arthritis Rheum 200246946–952. [DOI] [PubMed] [Google Scholar]
- 21.Stankovich J, Sale M M, Cooley H M, Bahlo M, Reilly A, Dickinson J L.et al Investigation of chromosome 2q in osteoarthritis of the hand: no significant linkage in a Tasmanian population. Ann Rheum Dis 2002611081–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman R D, Hochberg M C, Murphy W A, Wolfe F, Lequesne M. Radiographic atlas for osteoarthritis of the hand, hip and knee. Osteoarthritis Cartilage 19953(Suppl A)3–70. [PubMed] [Google Scholar]
- 23.Almasy L, Blangero J. Multipoint quantitative‐trait linkage analysis in general pedigrees. Am J Hum Genet 1998621189–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connell J R, Weeks D E. “PedCheck”: a program for identifying genotype incompatibilities in linkage analysis. Am J Hum Genet 198863259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Göring H H H, Ott J. Relationship estimation in affected sib pair analysis of late‐onset diseases. Eur J Hum Genet 1997569–77. [PubMed] [Google Scholar]
- 26.McPeek M, Sun L. Statistical tests for detection of misspecified relationships by use of genome‐screen data. Am J Hum Genet 2000661076–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abecasis G R, Cherny S S, Cookson W O, Cardon L R. Merlin‐rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 20023097–101. [DOI] [PubMed] [Google Scholar]
- 28.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 199511241–247. [DOI] [PubMed] [Google Scholar]
- 29.Kidd K K, Ott J. Power and sample size in linkage studies. Cytogenet Cell Genet 198437510–511. [Google Scholar]
- 30.Chapman K, Mustafa Z, Irven C, Carr A J, Clipsham K, Smith A.et al Osteoarthritis‐susceptibility locus on chromosome 11q, detected by linkage. Am J Hum Genet 199965167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingvarsson T, Stefansson S E, Gulcher J R, Jonsson H H, Jonsson H, Frigge M L.et al A large Icelandic family with early osteoarthritis of the hip associated with a susceptibility locus on chromosome 16p. Arthritis Rheum 2001442548–2555. [DOI] [PubMed] [Google Scholar]
- 32.Forster T, Chapman K, Marcelline L, Mustafa Z, Southam L, Loughlin J. Finer linkage mapping of primary osteoarthritis susceptibility loci on chromosomes 4 and 16 in families with affected women. Arthritis Rheum 20045098–102. [DOI] [PubMed] [Google Scholar]
- 33.Forster T, Chapman K, Loughlin J. Common variants within the interleukin 4 receptor alpha gene (IL4R) are associated with susceptibility to osteoarthritis. Hum Genet 2004114391–395. [DOI] [PubMed] [Google Scholar]
- 34.Shen H, Liu Y, Liu P, Recker R R, Deng H ‐ W. Nonreplication in genetic studies of complex diseases—lessons learned from studies of osteoporosis and tentative remedies. J Bone Min Res 200429365–376. [DOI] [PubMed] [Google Scholar]
- 35.Hunter D J, Demissie S, Cupples L A, Aliabadi P, Felson D T. A genome scan for joint‐specific hand osteoarthritis susceptibility: the Framingham Study. Arthritis Rheum 2004502489–2496. [DOI] [PubMed] [Google Scholar]
- 36.Kevorkian L, Young D A, Durrah C, Donell S T, Shepstone L, Porter S.et al Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum 200450131–141. [DOI] [PubMed] [Google Scholar]
- 37.Becker K G. The common variants/multiple disease hypothesis of common complex genetic disorders. Med Hypotheses 200462309–317. [DOI] [PubMed] [Google Scholar]
- 38.Mannoni A, Briganti M P, Di Bari M, Ferrucci L, Serni U, Masotti G.et al Prevalence of symptomatic hand osteoarthritis in community‐dwelling older persons: the ICARe Dicomano study. Insufficienza Cardiaca negli Anzizni Residenti a Dicomano. Osteoarthritis Cart 20008(Suppl A)S11–S13. [DOI] [PubMed] [Google Scholar]
- 39.Buckland‐Wright J C, Verbruggen G, Haraoui P B. Imaging. Radiological assessment of hand OA. Osteoarthritis Cart 20008(Suppl A)S55–S56. [DOI] [PubMed] [Google Scholar]