Abstract
Objective
To study ethnic differences in mortality from systemic lupus erythematosus (lupus) in two large, population‐based datasets.
Methods
We analysed the national death data (1979–98) from the National Center for Health Statistics (Hyattsville, Maryland, USA) and hospitalisation data (1993–2002) from the Nationwide Inpatient Sample (NIS), the largest hospitalisation database in the US.
Results
The overall, unadjusted, lupus mortality in the National Center for Health Statistics data was 4.6 per million, whereas the proportion of in‐hospital mortality from the NIS was 2.9%. African‐Americans had disproportionately higher mortality risk than Caucasians (all‐cause mortality relative risk adjusted for age = 1.24 (women), 1.36 (men); lupus mortality relative risk = 3.91 (women), 2.40 (men)). Excess risk was found among in‐hospital deaths (odds ratio adjusted for age = 1.4 (women), 1.3 (men)). Lupus death rates increased overall from 1979 to 98 (p<0.001). The proportional increase was greatest among African‐Americans. Among Caucasian men, death rates declined significantly (p<0.001), but rates did not change substantially for African‐American men. The African‐American:Caucasian mortality ratio rose with time among men, but there was little change among women. In analyses of the NIS data adjusted for age, the in‐hospital mortality risk decreased with time among Caucasian women (p<0.001).
Conclusions
African‐Americans with lupus have 2–3‐fold higher lupus mortality risk than Caucasians. The magnitude of the risk disparity is disproportionately higher than the disparity in all‐cause mortality. A lupus‐specific biological factor, as opposed to socioeconomic and access‐to‐care factors, may be responsible for this phenomenon.
African‐American ethnicity has been studied as an independent risk factor for development of systemic lupus erythematosus (lupus),1,2,3,4,5 although not all agree that it is.4,5 Clinic‐based cohort studies show that African‐American patients with lupus have disease onset earlier in life, and have more aggressive disease course and shorter life expectancy than Caucasian patients with lupus.6 Similar ethnic differences have been observed in lupus mortality in the general population as well. Using US statistical data from 1959 to 61, Cobb7 calculated an annual lupus mortality of 4.0 per million for Caucasian women and 10.6 per million for African‐American women. A less extensive study in New York City during the period from 1955 to 64 showed annual lupus mortality of 5.5 and 15.4 per million for Caucasian and African‐American women, respectively. Such mortality risk difference continued in the subsequent 5‐year period (Caucasian women 5.2 per million and African‐American women 14.8 per million per year).8,9
Since 1977, little systematic research has been conducted on the ethnic disparities in lupus mortality at a national level. Over this time, diagnostic testing has improved in availability, and quality, leading to more widespread testing and identification of previously missed cases of lupus. The availability of better antibiotics, renal replacement therapy and cardiovascular interventions, coupled with a better understanding of the disease process, has led to improvement in prognosis of patients. Population mortality is a function of incidence and death. The effect of these changes in diagnosis of lupus on population mortality and the ethnic disparities are unknown. This topic is the focus of this article.
Data and methods
To study ethnic disparities in health outcomes, we would need population‐based data that are unlikely to be skewed by regional and centre‐to‐centre ethnicity variations that characterise hospital and clinic‐based cohorts. National mortality statistics compiled by the US government is one such data source and this has been used in previous studies, including a recent report in the Morbidity and Mortality Weekly Report.7,8,9,10 However, those studies have been criticised because they used death certificate data, the quality of which is dependent heavily on the accurate completion of death certificates by the certifying doctor. Bias may be introduced by false‐positive mentions of lupus as cause of death (based merely on a positive antinuclear antibody).11 “False negatives” or omissions of lupus from death certificates can occur, especially if the diagnosis was made in the distant past, if the disease is not recognised by the treating doctor or if the disease was (wrongly) perceived to be inactive at the time of death. As shown by Ward et al,12 these biases may also vary with education and ethnicity, adding another layer of complexity to the problem. Thus, the vagaries of coding may lead to inaccurate results if we were to examine only one source of mortality data.
Short of carrying out (expensive, difficult to execute) national‐level validation studies on death certificates, the only way to confirm the findings from national mortality data is by examining multiple sources of data using different data collection procedures. This is the raison d'etre for the method of study used here. The two datasets analysed here are available in public domain and hence no specific ethics or institutional review board approval was sought.
Data source 1: US national mortality data from the National Center for Health Statistics
We examined the county‐level US population and mortality data derived from records of all deaths that occurred in the years 1979–98 (available from the National Center for Health Statistics (NCHS) at Centers for Disease Control, Atlanta, Georgia, USA).13 The cause of death on the mortality data is the underlying cause of death, which is defined by the World Health Organization as “the disease or injury, which initiated the train of events leading directly to death, or the circumstances of the accident or violence, which produced the fatal injury”. The NCHS selects the underlying cause of death from the conditions entered by the doctor on the cause‐of‐death section of the death certificate. When the doctor enters more than one cause or condition, the underlying cause is determined by the NCHS coding specialists, who take into account the sequence of conditions on the certificate, provisions of the International Classification of Diseases, Ninth Revision (ICD‐9), and associated selection rules and modifications. Any death of a US resident in the study period was attributed to lupus if it was coded with an underlying cause of systemic lupus erythematosus (ICD‐9 code 710.0). The denominator for calculating mortality was the US Census Bureau estimates of US national, state and county resident populations. The estimates for 1979 and 1981–89 were intercensus estimates of resident populations of 1 July of these years. The 1980 and 90 population estimates were modified census counts of 1 April of these years. More details are available from the website http://wonder.cdc.gov/wonder/help/mort.html.
Data source 2: the Nationwide Inpatient Sample
The Nationwide Inpatient Sample (NIS) is the largest all‐payer hospitalisation database in the US. It contains data from approximately 7 million hospital stays, every year, from about 20% stratified samples of acute care community hospitals in the US.14 For each individual hospitalisation, data from the discharge summary are abstracted into the database after removing patient identifiers. Reasons for hospitalisation were coded either as primary diagnosis or as one of the 14 secondary diagnoses in the dataset. Patients with lupus were identified from the abstracted discharge diagnoses when 1 of the 15 possible discharge diagnoses codes was International classification of diseases, ninth revision, clinical modification15 rubric 710.0 (“systemic lupus erythematosus”). For this analysis, all adult (age ⩾18 years) African‐American and Caucasian patients with lupus hospitalised from 1993 to 2002 were included (n = 136 037). In‐hospital deaths were considered “lupus‐deaths” if lupus was one of the recorded diagnoses.
Ethnicity data
The term race is a group of people who share biological characteristics based on a common genetic background. Strictly speaking, there are few true races in the world, as the term race implies racial homogeneity. Many anthropologists have advocated elimination of the term race altogether.4 In the US, intermingling of European, African and indigenous populations has been going on for centuries. Americans tend to identify themselves not only by physical characteristics but also by culture, including ancestry, geographical origin, language, and a system of traditions, beliefs and behaviours, which provides them with a link and a sense of belonging.16,17 Thus, in reality, the commonly used term race refers to the concept of ethnicity4—the terminology we have used in this report.
The ethnic categories available for the national mortality data were Caucasian, African‐American and other ethnicities. The “other ethnicities” category includes the American Indians or Alaskan Natives and the Asian or Pacific Islanders, and was excluded for this study. Data on Hispanic ethnicity were not available for this analysis. Ascertainment of ethnicity in death certificates is usually made by the funeral directors. In the NIS, ethnicity data were available from hospitalisation records. The source is usually a self‐report at the time of admission.
Statistical analysis
Age adjustment for mortality
For the NCHS data, death rates are adjusted for age using the direct method. We used the US population for 2000 as the standard for this analysis, as it facilitates comparison with future studies.18 Further details of age adjustment are available from the website http://wonder.cdc.gov/wonder/help/mort.html
Disparities, trends and regressions
For NCHS data, mortality disparity was expressed as relative risk (ie, ratio of mortality). We also used generalised linear model (GLM) with binomial distribution to calculate odds ratios (ORs) adjusted for age (model 1), and adjusted for age and sex (model 2) for different ethnicity categories in the NIS dataset. To separate the effect of insurance status, parallel age–sex analyses were carried out in each of the three major insurance categories (government sponsored such as Medicare and Medicaid, private, and not uninsured). For both datasets, time trends in rates were graphically visualised by plotting annual death rates adjusted for age using fractional polynomial modification of ordinary linear regression. This method allows us to study non‐linear trends that ordinary linear regressions do not permit. Time trends in rate ratios were fitted with GLM regressions19 to bring out the underlying trend. Using these regressions, we tested time trends in national mortality as well as in‐hospital mortality in each ethnicity and sex subgroup separately.
Results
Ethnic disparities in mortality risk
National mortality data
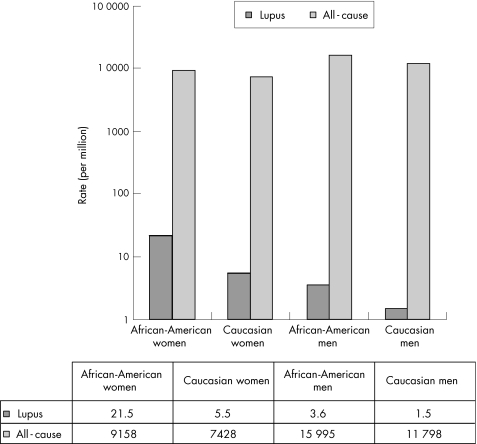
Over the study period (1979–98), 22.3 million men and 20.6 million women died in the US; 22 751 deaths over all ages were attributable to lupus during this 20‐year period. The overall crude lupus mortality was 4.61 deaths per million and mortality adjusted for age was 4.8 per million. Table 1 presents the mortality adjusted for age by ethnicity and sex. African‐American women had the highest mortality (21.5 per million) and Caucasian men and men of other ethnicities had the lowest (1.5 and 1.7 per million, respectively).
Table 1 Twenty‐year lupus mortality by sex and ethnicity, US 1979–98.
| Ethnicity | Sex | Death count | Person‐years (millions) | Crude death rate (per million) | Rate adjusted for age (per million) |
|---|---|---|---|---|---|
| African‐Americans | Women | 6160 | 318 | 19.2 | 21.5 |
| Men | 821 | 286 | 2.90 | 3.6 | |
| Caucasians | Women | 12 234 | 2121 | 5.8 | 5.5 |
| Men | 2765 | 2032 | 1.4 | 1.5 | |
| Others | Women | 665 | 90 | 7.4 | 8.2 |
| Men | 106 | 86 | 1.2 | 1.7 | |
| Total | 22 751 | 4935 | 4.6 | 4.8 |
Rates adjusted for age were 1.8 per million for all men and 7.5 per million for all women. Tables 2 and 3 give the detailed age–sex‐specific lupus mortality for both ethnicities. These data show generally higher rates for African‐Americans compared with Caucasians in both sexes and in almost every age group. Similar sex and ethnicity differences were consistently observed across the 20 years studied from 1979—98.
Table 2 Age and ethnicity‐specific lupus mortality, US 1979–98.
| Age (years) | African‐Americans | Caucasians | |||||
|---|---|---|---|---|---|---|---|
| Death count | Person‐years | Rate (per million) | Death count | Person‐years | Rate (per million) | ||
| Women | |||||||
| <1 | 1 | 6 024 199 | 0.2* | 1 | 29 908 146 | 0.0* | |
| 1–4 | 2 | 22 690 531 | 0.1* | 1 | 114 441 964 | 0.0* | |
| 5–9 | 18 | 27 569 778 | 0.7* | 9 | 139 554 938 | 0.1* | |
| 10–14 | 78 | 27 200 249 | 2.9* | 55 | 141 387 170 | 0.4* | |
| 15–19 | 238 | 28 181 188 | 8.4 | 215 | 149 455 724 | 1.4 | |
| 20–24 | 524 | 28 181 242 | 18.6 | 416 | 160 295 959 | 2.6 | |
| 25–34 | 1361 | 54 941 895 | 24.8 | 1159 | 340 638 576 | 3.4 | |
| 35–44 | 1413 | 43 652 179 | 32.4 | 1464 | 300 781 124 | 4.9 | |
| 45–54 | 1026 | 29 761 652 | 34.5 | 1645 | 228 985 726 | 7.2 | |
| 55–64 | 793 | 22 855 496 | 34.7 | 2022 | 199 796 890 | 10.1 | |
| 65–74 | 446 | 17 390 042 | 25.6 | 2563 | 174 990 432 | 14.6 | |
| 75–84 | 217 | 9 398 417 | 23.1 | 2086 | 110 112 351 | 18.9 | |
| >85 | 43 | 3 130 614 | 13.7 | 598 | 39 615 598 | 15.1 | |
| Men | |||||||
| <1 | 0 | 6 206 953 | 0.0* | 3 | 31 499 860 | 0.0* | |
| 1–4 | 0 | 23 213 081 | 0.0* | 0 | 120 513 509 | 0.0* | |
| 5–9 | 1 | 28 220 769 | 0.0* | 5 | 147 068 512 | 0.0* | |
| 10–14 | 10 | 27 796 276 | 0.4* | 18 | 149 102 562 | 0.1* | |
| 15–19 | 42 | 28 545 008 | 1.5 | 52 | 157 350 195 | 0.3 | |
| 20–24 | 81 | 26 589 722 | 3.0 | 58 | 166 266 482 | 0.3 | |
| 25–34 | 161 | 48 800 091 | 3.3 | 195 | 347 376 078 | 0.6 | |
| 35–44 | 168 | 37 472 213 | 4.5 | 279 | 299 839 777 | 0.9 | |
| 45–54 | 127 | 24 752 290 | 5.1 | 320 | 221 873 923 | 1.4 | |
| 55–64 | 103 | 18 067 594 | 5.7 | 461 | 181 183 803 | 2.5 | |
| 65–74 | 81 | 12 268 431 | 6.6 | 723 | 138 295 026 | 5.2 | |
| 75–84 | 35 | 5 416 164 | 6.5 | 522 | 66 647 446 | 7.8 | |
| >85 | 12 | 1 336 560 | 9.0 | 129 | 15 494 862 | 8.3 | |
*Rates unreliable, owing to the small number of deaths.
Table 3 Results of the logistic regressions for the association between ethnicity and risk for death.
| Model | Mortality OR for African‐Americans compared with Caucasians | 95% CI |
|---|---|---|
| Model 1: Adjusted for age and stratified by sex | ||
| Women | 1.42 | 1.32 to 1.55 |
| Men | 1.3 | 1.05 to 1.60 |
| Model 2: Adjusted for age and sex, overall | 1.41 | 1.31 to 1.52 |
| Adjusted for age and sex and stratified by insurance status | ||
| Medicare/Medicaid | 1.29 | 1.18 to 1.42 |
| Others/None | 1.98 | 1.44 to 2.74 |
| Private | 1.38 | 1.20 to 1.59 |
To assess how much the excess lupus‐mortality risk is a reflection of the raised overall mortality risk for African‐Americans, we compared mortality ratio adjusted for age (African‐Americans:Caucasians) for lupus and for all causes put together. Among women, the ethnic ratio (ie, African‐Americans:Caucasians) for lupus mortality was 3.91, whereas the corresponding figure for all‐cause mortality was 1.24 (fig 1). For men, the corresponding ratios were 2.40 and 1.36, respectively.
Figure 1 Comparing African‐American:Caucasian all‐cause and lupus mortality among men and women, US 1979–98.
Hospitalisation data
There were 136 037 people hospitalised with lupus, of which 31% were of African‐American ethnicity and 88.3% were women. The overall mean age for women was 50 years. Among African‐American women the mean age was 43 years and among Caucasian women it was 53 years (p<0.001). The mean age for men was 54 years. Mean age for African‐American hospitalisation was 43 and that for Caucasians was 58 years (p<0.001). Overall, there were 3951 hospitalisations (2.9%) that resulted in death. Mean age at death for African‐Americans was 49 years, whereas that for Caucasians was 64 years (p<0.001). In the regression models, African‐Americans were at higher mortality risk than Caucasians (table 3).
Time trends in mortality
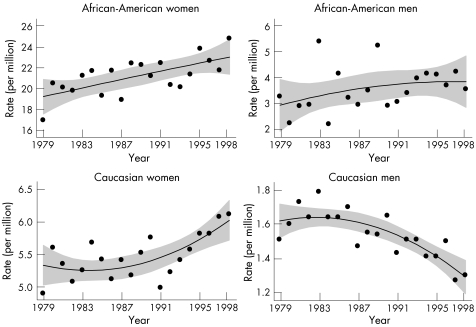
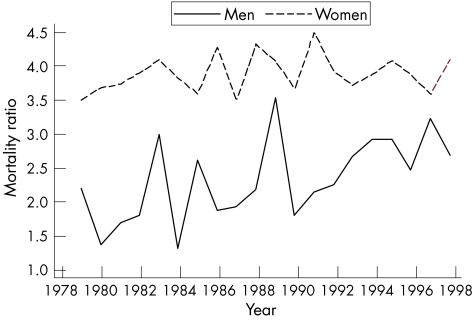
Overall, lupus death rates increased from 1979 to 98 (p<0.001). Figure 2 shows sex–ethnicity‐specific trends. Results of the GLM regressions specific to these sex–ethnicity data indicated that death rates among Caucasian men declined significantly over time (p<0.001), but death rates did not change substantially for African‐American men (p = 0.14). Although both African‐American and Caucasian women had significantly increasing death rates (p<0.001), the proportional increase was greatest among African‐Americans. A comparison of the age‐specific lupus mortality in the first and last 4 years of observation showed that among African‐Americans the increase in rates was most apparent in the groups beginning at age 35. However, among Caucasians, there was no consistent age pattern associated with the increase. Thus, the African‐American–Caucasian mortality ratio showed an increasing trend with time among men (predominantly due to declining rates in Caucasians), but little change among women (fig 3).
Figure 2 Time trends in lupus mortality. The individual year rates were fitted with fractional polynomial modification of ordinary least‐squares regression. The increasing trends in black and white women and the declining trends in white men were significant (p<0.001). The trend in black men was not significant. Note the difference in the scales used on the y axis.
Figure 3 Time trends in the mortality ratio adjusted for age in lupus. The trend test for men was significant (p<0.01), whereas that for women was not.
In the NIS data, there was a small but significant increasing trend in the proportion of African‐Americans hospitalised with lupus.
In analyses adjusted for age, the in‐hospital mortality risk decreased with successive calendar years among Caucasian women (OR for each year = 0.97, 95% confidence interval 0.95 to 0.98, p<0.001). No trends in in‐hospital mortality risks were noted among Caucasian men and all African‐Americans. To explore whether health insurance type influences the ethnicity–mortality relationship, we carried out parallel logistic regression analysis on each of the three insurance categories (Medicare/Medicaid, Private, and “Others”, including self‐pay and uninsured). In these stratified analyses, we observed higher mortality risk for African‐Americans than for Caucasians in all the strata, with OR ranging from 1.29 to 1.98 (all p<0.001; table 3).
Discussion
We have presented data from two separate data sources that use different methods for assessing race, cause of death and the diagnosis of lupus. These data show that African‐Americans are at higher mortality risk than Caucasians. The ethnic differences could be due to difference in incidence, or case fatality (disease severity)—an issue that needs a population‐based inception‐cohort study of lupus to be dealt with. The magnitude of the ethnic‐risk difference was, however, smaller in the hospitalisation data than in NCHS data (1.4 v 2.0), suggesting (1) that African‐Americans have more frequent, non‐fatal hospitalisations or (2) that they are more likely to die when out of hospital. Similar to previous decades, African‐Americans in the 1980s and 1990 have continued to have higher national lupus mortality rates between both sexes.
Previous studies have hypothesised biological and non‐biological reasons for the ethnic disparities in mortality, although teasing out the effect of each remains problematic.7 In a population‐based study of 265 patients with lupus, Cooper et al20 observed that the mean age at diagnosis among African‐Americans and other minorities was 6 years less than that of Caucasian patients (p<0.01). Discoid lupus, proteinuria, anti‐Sm and anti‐ribonucleoprotein autoantibodies were more commonly seen in African‐American patients, with ORs >3.0. These point towards an underlying biological difference between ethnic groups as the explanatory factor. In our study, we noted that the extent of racial disparity in lupus mortality is much higher than we would expect from the all‐cause mortality. This also supports the notion that ethnicity may be a biological effect modifier for risk of incidence or death from lupus.
Other factors also contribute to these ethnic disparities, including socioeconomic factors (eg, education, employment and income), social environment and access to preventive healthcare services.6,21 Although published studies have observed African‐Americans to have worse prognosis than Caucasians,2,3,4,5 they do not agree on the potential pathways that lead to this disparity. Studies by Ward et al5 and Alarcon4 reported that the ethnicity–prognosis association, although present in bivariate analyses, disappeared once adjusted, or disappeared with markers of socioeconomic status and health insurance type, suggesting an underlying confounding by the socioeconomic status and health insurance type. On the other hand, Reveille et al3 did not find any such confounding by differences in insurance status. We too, in our hospitalisation data, found no influence by insurance status.
Analysis of pathways of ethnicity–mortality link is complex and is fraught with several methodological issues. For example, poverty can reduce the access to healthcare, and therefore African‐Americans with lupus may be accessing specialist care when they are in a sicker state (such as higher levels of creatinine and lower levels of haemoglobin) than Caucasians. When these markers of worse health status are introduced into traditional multivariable regressions alongside ethnicity, they overadjust and confound the effect of true difference in disease characteristics.2 Unless this issue of colinearity of variables is not adequately explored and properly modelled, separating the effect of nature versus nurture will be difficult.
Time trends in mortality can be due to time trends in disease incidence or case fatality (a measure of healthcare). In light of improved case fatality as evinced by improving 5‐year survival in clinical studies, we propose that the observed disparities reflect higher incidence of lupus among African‐Americans.
We found some differences in findings regarding the time trends in mortality between hospitalisation and NCHS data. Preliminary explorations of the racial disparities in time trends using NCHS data have been presented earlier.10 Differences in all‐cause mortality between the two ethnicities have remained stable in the past 30 years.22 We found that there has been a decline in lupus mortality for Caucasian men and that the rates for African‐American men remained stable over time. As a result, the ethnic disparities have increased among men. Among women, the African‐American:Caucasian ratio has increased only slightly, even though in absolute terms the mortality has increased in both ethnicity categories. On the other hand, in the hospitalisation database, we observed that the in‐hospital mortality, a measure of frailty, showed no change over time, except among Caucasian women where it showed a decreasing trend.
Potential limitations and biases in our study need discussion. Attribution bias could have led to an underestimation of total lupus mortality in this study, especially among women. The potential underattribution of lupus mortality in men is because lupus is often not a common differential diagnosis made in men, especially among older men. Inaccuracies are possible with reporting of ethnicity data. These can potentially bias ethnicity data when we study minorities such as Native Americans, but probably not to a substantial extent for African‐Americans, a distinct and common ethnic category. Further, both numerator (deceased) and denominator (population) are liable for misclassification of ethnicity; the misclassification in the denominator tends to somewhat offset that in the case of lupus deaths.23
Our findings have important clinical and public health implications. African‐Americans are less likely to receive preventive health services than Caucasians.24 Therefore, many of the excess lupus‐related deaths from cardiovascular, infectious and renal complications might be preventable with aggressive preventive interventions, including lifestyle change (physical activity), control of hypertension and hyperlipidaemia, smoking cessation and management of other risk factors.25 These and similar efforts, along with better education of doctors and patients, may serve to reduce the higher lupus mortality among African‐Americans.
Abbreviations
GLM - generalised linear model
NCHS - National Center for Health Statistics
NIS - Nationwide Inpatient Sample
Footnotes
Competing interests: None.
References
- 1.Uribe A G, Alarcon G S. Ethnic disparities in patients with systemic lupus erythematosus. Curr Rheumatol Rep 20035364–369. [DOI] [PubMed] [Google Scholar]
- 2.Ginzler E M, Diamond H S, Weiner M, Schlesinger M, Fries J F, Wasner C.et al A multicenter study of outcome in systemic lupus erythematosus. I. Entry variables as predictors of prognosis. Arthritis Rheum 198225601–611. [DOI] [PubMed] [Google Scholar]
- 3.Reveille J D, Bartolucci A, Alarcon G S. Prognosis in systemic lupus erythematosus. Negative impact of increasing age at onset, black race, and thrombocytopenia, as well as causes of death. Arthritis Rheum 19903337–48. [DOI] [PubMed] [Google Scholar]
- 4.Alarcon G S. Of ethnicity, race and lupus. Lupus 200110594–596. [DOI] [PubMed] [Google Scholar]
- 5.Ward M M, Pyun E, Studenski S. Long‐term survival in systemic lupus erythematosus. Patient characteristics associated with poorer outcomes. Arthritis Rheum 199538274–283. [DOI] [PubMed] [Google Scholar]
- 6.Liang M H, Partridge A J, Daltroy L H, Straaton K V, Galper S R, Holman H R. Strategies for reducing excess morbidity and mortality in blacks with systemic lupus erythematosus. Arthritis Rheum 1991341187–1196. [DOI] [PubMed] [Google Scholar]
- 7.Cobb S. The frequency of rheumatic diseases. Vital & health statistics monographs, American Public Health Association. Cambridge: Harvard University Press, 1971
- 8.Kaslow R A, Masi A T. Age, sex, and race effects on mortality from systemic lupus erythematosus in the United States. Arthritis Rheum 197821473–479. [DOI] [PubMed] [Google Scholar]
- 9.Gordon M F, Stolley P D, Schinnar R. Trends in recent systemic lupus erythematosus mortality rates. Arthritis Rheum 198124762–769. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease C o n t r o, Prevention ( C D C ) Trends in deaths from systemic lupus erythematosus—United States, 1979–1998. MMWR Morb Mortal Wkly Rep 200251371–374. [PubMed] [Google Scholar]
- 11.Uramoto K M, Michet C J, Jr, Thumboo J, Sunku J, O'Fallon W M, Gabriel S E. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum 19994246–50. [DOI] [PubMed] [Google Scholar]
- 12.Ward M M. Education level and mortality in systemic lupus erythematosus (SLE): evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthritis Rheum 200451616–624. [DOI] [PubMed] [Google Scholar]
- 13.Sacks J J, Helmick C G, Langmaid G. Deaths from arthritis and other rheumatic conditions, United States, 1979–1998. J Rheumatol 2004311823–1828. [PubMed] [Google Scholar]
- 14.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract 20025143–151. [PubMed] [Google Scholar]
- 15.Hart A, Hopkins C.ICD‐9 CM professional for physicians. 6th edn. Salt Lake City, UT: Ingenix, 2004
- 16.Huth E J. Identifying ethnicity in medical papers. Ann Intern Med 1995122619–621. [DOI] [PubMed] [Google Scholar]
- 17.Fustinoni O, Biller J. Ethnicity and stroke: beware of the fallacies. Stroke 2000311013–1015. [DOI] [PubMed] [Google Scholar]
- 18.Hoyert D L, Anderson R N. Age‐adjusted death rates: trend data based on the year 2000 standard population. Natl Vital Stat Rep 2001491–6. [PubMed] [Google Scholar]
- 19.Dobson A.An introduction to generalized linear models. Boca Raton, FL: Chapman & Hall, 2002
- 20.Cooper G S, Parks C G, Treadwell E L, St Clair E W, Gilkeson G S, Cohen P L.et al Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus 200211161–167. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease C o n t r o, Prevention ( C D C ) Health disparities experienced by black or African Americans—United States. MMWR Morb Mortal Wkly Rep 2005541–3. [PubMed] [Google Scholar]
- 22.Anderson R. United States life tables, 1998. National vital statistics reports. Vol 48. No 18. Hyattesville, MD: National Center for Health Statistics, 2001 [PubMed]
- 23.Rosenberg H M, Maurer J D, Sorlie P D, Johnson N J, MacDorman M F, Hoyert D L.et al Quality of death rates by race and Hispanic origin: a summary of current research, 1999. Vital Health Stat 199921–13. [PubMed] [Google Scholar]
- 24.Shi R, Berkel H, Sartor O. Comparison of utilization of preventive health care services between two racial populations. Ann Epidemiol 200010454. [DOI] [PubMed] [Google Scholar]
- 25.Petri M, Perez‐Gutthann S, Spence D, Hochberg M C. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med 199293513–519. [DOI] [PubMed] [Google Scholar]