Abstract
Background
Although age is the strongest predictor of osteoarthritis, the exact mechanism underlying this disorder remains elusive.
Objective
To examine the association between leucocyte telomere length (LTL), a bio‐indicator of ageing, and radiographic hand osteoarthritis.
Methods
An unselected, predominantly female sample from the TwinsUK Adult Twin Registry (Twin Research and Genetic Epidemiology Unit, St Thomas Hospital, London, UK) was studied. Radiographs of both hands were obtained with a standard posteroanterior view and assessed for radiographic osteoarthritis according to the Kellgren/Lawrence (K/L) score. Individual radiographic features including osteophytes and joint space narrowing (JSN) were also assessed on a four‐point scale using a standard atlas. Hand osteoarthritis was defined radiographically as having ⩾3 osteoarthritis‐affected joints of both hands (K/L score⩾2). Severity of hand osteoarthritis was indicated semiquantitatively by total K/L scores, osteophytes, JSN scores and proportion of joints affected. Mean LTL was measured by the terminal restriction fragment length using the Southern blot.
Results
A total of 1086 Caucasian subjects (mean (SD) age 55 (8.0) years) were studied. LTL was 6.95 (0.64) kb and was inversely correlated with age. After adjustment for age, sex, body mass index and smoking, LTL was significantly shorter by 178 bp in subjects with hand osteoarthritis (n = 160) than in those without (n = 926; p = 0.04). LTL was also significantly associated with semicontinuous measures of osteoarthritis (eg, total K/L score, JSN score, osteophyte score and proportion of joints affected) after adjustment (all p⩽0.02) in a dose–response fashion.
Conclusion
Shorter LTL equivalent to around 11 years of annual loss in normal people is associated with radiographic hand osteoarthritis and disease severity, suggesting potential shared mechanisms between osteoarthritis and ageing, and implicating oxidative stress and low‐level chronic inflammation in both conditions.
Osteoarthritis is the most common form of arthritis and a leading cause of musculoskeletal disability in middle‐aged and older people.1 The hand is one of the most frequent osteoarthritis‐affected sites.2 Hand osteoarthritis is more common in women3 and is markedly associated with functional impairment and reduced independence.4 Although hand osteoarthritis has been associated with genetic factors,5,6 environmental factors such as occupation,7 and hormone replacement therapy,8 age is the strongest predictor.9,10 However, the mechanism causing osteoarthritis is still unclear. Given that the prevalence and severity of osteoarthritis increase dramatically with age, it is possible that people who exhibit osteoarthritis are “biologically” older than their chronological peers. To explore this supposition, we examined the mean of leucocyte telomere length (LTL) in subjects with hand osteoarthritis versus controls.
Telomeres are tandem repeats at chromosomal ends and form with an assortment of protein‐specialised structures that are essential for chromosomal replication and stability.11,12 In human somatic cells, telomeres shorten with cell proliferation owing to a host of factors, including the “end replication problem”13,14 and insufficient repair of oxidative damage.15,16 LTL is a complex genetic trait, reflecting both genetic17,18 and environmental factors.15,19 Telomeres appear to serve as a “mitotic clock” at least in cultured cells.20 In vivo, short LTL has been linked to a variety of ageing‐related diseases in the general population,21,22,23,24 perhaps because chronic oxidative stress and inflammation, two postulated key factors in ageing,25,26,27 may accelerate telomere erosion in leucocytes.
The average telomere length in cartilage chondrocytes decreases with age.28 Moreover, greater shortening of the telomere length is observed in chondrocytes from the osteoarthritic lesion site than in the more intact regions of cartilage in the same joint,29 and lifespan in vitro of cultured osteoarthritic chondrocytes can be extended by exogenous telomerase,30 suggesting that telomere shortening may be responsible for senescence in cartilage chondrocytes. However, there have been no in vivo data regarding telomere length and osteoarthritis, owing to the difficulty in assessing telomere length of chondrocytes in vivo. Investigation of telomere length in other proliferative and accessible somatic cells is an alternative to in vivo studies as there is a high correlation between telomere lengths in different proliferative somatic cell types.23,31 The aim of our study, therefore, was to examine the association between LTL as a general marker of telomere status in replicating cells and radiographic hand osteoarthritis in a population‐based study.
Methods
Study participants
Participants were derived from the TwinsUK Adult Twin Registry (Twin Research and Genetic Epidemiology Unit, St Thomas Hospital, London, UK), a volunteer sample previously developed to study the heritability and genetics of age‐related diseases. These twins, without selecting for particular diseases or traits, were recruited from the general population through a series of national media campaigns in the UK,32 and shown to be comparable with age‐matched population singletons in terms of disease‐related and lifestyle characteristics.33 The study was approved by St Thomas' Hospital Research Ethics Committee and all twins provided informed written consent.
x Rays
Radiographs of both hands were obtained with a standard posteroanterior view. The distal interphalangeal, proximal interphalangeal, metacarpophalangeal and first carpometacarpal joints of the thumb were assessed for radiographic osteoarthritis according to the Kellgren/Lawrence (K/L) score using a standard atlas.34 Individual radiographic features, including osteophytes and joint space narrowing (JSN), were also assessed on a four‐point scale using a standard atlas.35 A joint was defined as osteoarthritis‐affected if it had a K/L score ⩾2. Hand osteoarthritis was defined as ⩾3 joints of both hands affected with osteoarthritis. Severity of hand osteoarthritis was indicated by total K/L scores, osteophytes, JSN scores and the proportion of joints affected. All radiographs were independently assessed by two trained observers (DJ Hunter and DJ Hart) who were blinded to the telomere length, and in cases of disagreement a third adjudicator was used. The intraobserver and interobserver reproducibility of the scoring were tested on a subgroup of 50 hands with a (κ) statistic of approximately 0.68 for all sites and features.
LTL measurements
A venous blood sample was taken after an overnight fast and the mean leucocyte terminal restriction fragment (TRF) length was measured as described previously.36 Briefly, DNA samples were checked for integrity on 0.8% agarose gel. They were digested overnight with restriction enzymes HinfI (10 U) and RsaI (10 U) (Roche, Indianapolis, Indiana, USA). DNA samples (3 µg each) and four DNA ladders (1 kb DNA ladder plus λ DNA/HindIII fragments; Invitrogen, Carlsbad, California, USA) were resolved on 0.6% agarose gel (20 cm×20 cm) at 50 V (GNA‐200, Pharmacia Biotech, Piscataway, New Jersey, USA). After 16 h, the DNA was depurinated for 15 min in 0.25 N hydrochloric acid, denatured for 30 min in 0.5 mol/l sodium hydroxide/1.5 mol/l sodium chloride and neutralised for 30 min in 0.5 mol/l Tris, pH 8/1.5 M sodium chloride. The DNA was transferred for 1 h to a positively charged nylon membrane (Roche) using a vacuum blotter (Appligene, Oncor, Illkirch, Graffenstanden, France). The membranes were hybridised at 65°C with the telomeric probe (digoxigenin 3′‐end labelled 5′‐(CCTAAA)3) overnight in 5×SSC (0.3 M trisodium citrate and 3.0 M sodium chloride in high‐purity dH2O, pH 7.0), 0.1% SarkosyI, 0.02% sodium dodecyl sulphate and 1% blocking reagent (Roche). The membranes were washed three times at room temperature in 2×SSC and 0.1% sodium dodecyl sulphate each for 15 min, and once in 2×SSC for 15 min. The digoxigenin‐labelled probe was detected by the digoxigenin luminescent detection procedure (Roche) and exposed on x ray film. The autoradiographs were scanned and the TRF length signal was digitised at molecular weight 1–20 kb. The mean TRF length was then calculated accordingly. Conversion of the optical density versus DNA migration distance to optical density adjusted for background)/molecular weight versus molecular weight yielded a new histogram from which the mean TRF length was calculated. Each DNA sample was resolved in duplicate (on different gels). If the difference between the duplicates was >5%, a third measurement was carried out and the mean of two results <5% apart was taken. The coefficient of variation of the TRF in this study was 0.92%.
Statistics
Mean LTL between people with and without hand osteoarthritis was compared before and after adjustment for age, sex, body mass index (BMI) and smoking status by unpaired t test. The interaction between age and hand osteoarthritis on LTL was assessed by analysis of covariance. Because of the skewed distribution of the severity measures (eg, total K/L score, JSN score, osteophyte score and proportion of affected joints) and non‐independent observations in twin pairs, robust regression modelling was used to assess the association between LTL and severity or amount of hand osteoarthritis before and after adjustment for age, sex, BMI and smoking status. The Stata's cluster‐correlated robust estimate of standard errors was calculated and used for testing significance.37 Because all the participants with osteoarthritis were aged ⩾50 years in the study, the subanalysis in the same manner was also carried out in subjects aged ⩾50 years. A value of p<0.05 (two‐tailed) was considered to be significant. All statistical analyses were carried out on STATA/SE V.9 for Windows.
Results
A total of 1086 Caucasian subjects aged between 31 and 79 years were included in the study. Most of them were women (1044 women v 42 men). Of these, 100 subjects were singletons and 986 were twin pairs (73 monozygotic pairs and 420 dizygotic pairs). Hand osteoarthritis was prevalent in 15% of the participants and all the subjects with osteoarthritis were aged ⩾50 years. The mean (standard deviation (SD)) scores were 4.2 (7.7), 0.6 (2.4) and 1.6 (3.9) for total K/L, total JSN and total osteophyte scores, respectively. Table 1 presents the characteristics of the study participants. In all, 32% participants had a BMI>25, and 12% had a BMI⩾30. The controls were 9 years younger and their LTL was 281 bp longer than that of the cases on average. There was no difference in BMI and smoking status between cases and controls.
Table 1 Characteristics of participants with and without hand osteoarthritis.
| With hand OA (n = 160) | Without hand OA (n = 926) | p Values | |
|---|---|---|---|
| Age, years (range 30–79) | 62.87 (5.67) | 53.88 (7.64) | <0.001 |
| Sex (female %) | 99 | 96 | 0.06 |
| BMI (kg/m2) | 25.63 (4.03) | 25.09 (4.47) | 0.15 |
| Ever smoker (%) | 20 | 27 | 0.09 |
| Current smoker (%) | 17 | 15 | 0.79 |
| Leucocyte telomere length (kb) | 6.71 (0.59) | 7.00 (0.64) | <0.001 |
OA, osteoarthritis.
Values are mean (SD) for continuous variables and prevalence for dichotomous variables.
Hand OA was defined as ⩾3 joints of both hands affected (K/L⩾2).
Unpaired t test or χ2 test was used wherever appropriate.
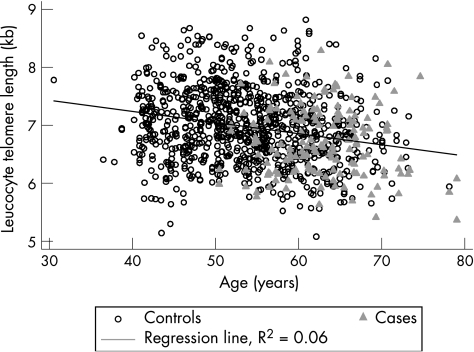
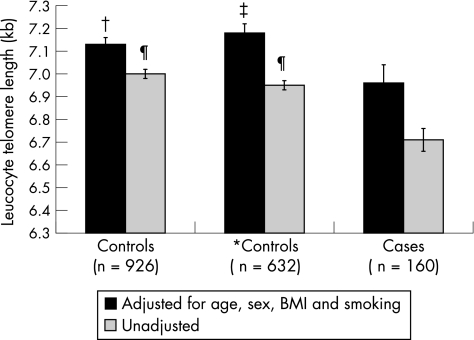
LTL declined with age as expected (fig 1). A highly significant (p<0.001) effect of age was observed on mean LTL of the total population, with an average decrease in length equivalent to 16 (standard error (SE) 2) bp/year of life in subjects without hand osteoarthritis. Subjects with hand osteoarthritis had substantially shorter LTL than those without, with a crude mean difference between cases and controls of 281 bp (p<0.001; fig 2). After adjustment for potential confounders including age, sex, BMI and smoking status, the mean difference was reduced by 37% to 178 bp, but the significance persisted (fig 2). When considering only subjects aged ⩾50 years, the adjusted difference between subjects with osteoarthritis and controls was also significant at 218 bp (fig 2). When stratifying older participants into two further subgroups, aged 50–65 years and >65 years, the adjusted difference in LTL between subjects with and without hand osteoarthritis was 249 bp (p = 0.01) and 128 bp (p = 0.146) for younger and older groups, respectively. The results remained the same after excluding the small number of men.
Figure 1 Relationship between leucocyte telomere length (LTL) and age in the total sample.
Figure 2 Association between hand osteoarthritis (OA) and leucocyte telomere length. Hand OA was defined as ⩾3 hand joints affected (Kellgren/Lawrence Score ⩾2). BMI, body mass index. Bars depict standard error of the telomere length. *Excluded subjects <50 years of age. Compared to subjects with hand OA, p values were 0.04†, 0.01‡, and <0.001 ¶, respectively.
A significant dose–response relationship was observed between LTL and total K/L score, total osteophyte score, and total JSN score (all p for trend <0.05 after adjustment for age, sex, BMI and smoking). The LTL significantly decreased with an increase in the osteoarthritis feature scores. The mean difference in LTL between subjects without hand osteoarthritis and those with most severe disease ranged from 307 to 371 bp.
In the univariate and multivariate analysis (table 2), LTL was significantly associated with the amount or severity of hand osteoarthritis as indicated by various measures, including total K/L score, JSN, osteophyte score and proportion of osteoarthritis‐affected joints before and after adjustment for potential confounders. The effect size was reduced by 35–57% after adjustment for age, sex, BMI and smoking status, but still remained highly significant. Most of the attenuation in effect size occurred after adjustment for age.
Table 2 Association between hand osteoarthritis and leucocyte telomere length (n = 1086)*.
| Unadjusted | Adjusted for age and sex | Adjusted for age, sex, BMI and smoking | ||||
|---|---|---|---|---|---|---|
| β (SE) | p Value | β (SE) | p Value | β (SE) | p Value | |
| Total K/L score | −2.05 (0.41) | <0.001 | −0.88 (0.36) | 0.01 | −0.86 (0.36) | 0.01 |
| Total JSN score | −0.48 (0.12) | <0.001 | −0.31 (0.11) | <0.01 | −0.31 (0.11) | <0.01 |
| Total osteophyte score | −0.97 (0.21) | <0.001 | −0.47 (0.19) | 0.01 | −0.46 (0.19) | 0.01 |
| Proportion of joints affected | −2.14 (0.46) | <0.001 | −0.95 (0.41) | 0.02 | −0.92 (0.41) | 0.02 |
β, regression coefficient; BMI, body mass index; K/L, Kellgren/Lawrence; JSN, joint space narrowing; SE, Stata's cluster‐correlated robust estimate of standard error.
*Robust regression modelling was used.
The results remained the same when the analyses were carried out in the independent sample of singletons selected randomly from each twin pair (data not shown) and also when occupation was adjusted for in those with complete data (n = 600, data not shown).
Discussion
To our knowledge, this is the first clinical study that relates telomere length to subjects with osteoarthritis. LTL is a putative marker of exposure to chronic inflammation and biological ageing and has been associated with age‐related diseases, such as dementia,23,24 atherosclerosis21 and myocardial infarction.22 Here we document that LTL is inversely associated with hand osteoarthritis, with a dose–response relationship between LTL and the amount or severity of hand osteoarthritis. After adjustment for age, sex, BMI and smoking status, the difference in LTL between subjects with and without hand osteoarthritis was 178 bp.
Potential limitations
There are several potential limitations in this study.
The sample consisted of twins, and this could theoretically limit its generalisability. However, a previous study has shown that the participants in the TwinUK cohort are comparable to age‐matched population singletons in terms of disease‐related and lifestyle characteristics, including osteoarthritis,33 suggesting that this is not a major concern. Women comprised the overwhelming majority of the study population and therefore we cannot extrapolate our results to men. Twins are also related, which could bias the levels of significance. However, we adjusted for this using robust regression methods.
The classification of hand osteoarthritis may be problematic. We used a fairly strict definition, thereby providing more reliability. Our results were robust when the individual features were used instead in the composite K/L score. In addition, occupation might confound our results as it could be associated with both hand osteoarthritis and LTL. However, adjustment for this variable in a subsample with available social class status information did not alter the results.
We note that different assay methods may affect the results because of the different reliability and reproducibility of the methods. Real‐time polymerase chain reaction method has a coefficient of variation >5%38 and does not generate results that are expressed in absolute values, which makes comparisons difficult not only among studies but also between individuals within a study, suggesting that it is less suitable for epidemiological studies. We used the Southern blot method, which, although laborious, is highly reproducible with a coefficient of variance of <1% and provides telomere length in absolute values.
There may potentially be a difference in telomere length between leucocyte subsets,39 and age‐related reconfiguration of cell subsets may confound telomere analysis. However, the reconfiguration is mostly engaged in lymphocyte subsets and the relative number of neutrophils changes little with age40,41; thus, using leucocyte subsets in the telomere analysis would reduce the confounding effect of the proportions of lymphocyte subsets. In addition, telomere length is synchronised (equivalent) in different tissues of newborns42 and partially synchronised in adults.31,43 Therefore, people with relatively long (or short) telomeres in one tissue are expected to have relatively long (or short) telomeres in others. Thus, if the mechanisms that shape telomere dynamics (telomere length and attrition rate) in leucocytes mirror those in chondrocytes, the association we observed might also hold for chondrocytes. We therefore believe that despite direct data on chondrocytes or osteoblasts, measuring LTL rather than the rarer cell subsets is the preferred method for answering the research question in such a large sample.
Possible mechanism
Our results show that LTL is associated with hand osteoarthritis. The shorter the LTL, the more severe the hand osteoarthritis. In vitro studies show that the mean chondrocyte telomere length declined with donor's age28 and that the mean telomere length was shorter in chondrocytes near the osteoarthritic lesion than in the distal sites in the same joint,29 suggesting that age‐related telomere erosion may lead to chondrocyte senescence. Senescent chondrocytes synthesise smaller aggrecans and fewer functional link proteins and seem to have a reduced response to anabolic cytokines,44,45,46 predisposing people to osteoarthritis. Furthermore, in cultured chondrocytes, telomere length, replicative capacity and glycosaminoglycan are decreased by treatment with reactive oxygen species. In contrast, treatment with an anti‐oxidative agent results in a tendency to elongate telomere length and replicative lifespan in cultured chondrocytes.47 Excessive stress on articular surfaces due to acute joint trauma or post‐traumatic joint instability, incongruity or malalignment increases reactive oxygen species, which may accelerate chondrocyte senescence.48
The association between LTL and hand osteoarthritis persisted after adjustment for age. At any age, telomere length in a given tissue reflects its length at birth and its attrition rate thereafter. Leucocyte telomere attrition results from cell replication and factors that affect the replicative rate, including inflammation and oxidative stress, which may affect the magnitude of telomeric loss per replication.49 LTL might hence register, at least in part, the cumulative burden of oxidative stress and inflammation during the individual's lifetime. As both oxidative stress25 and inflammation50 are involved in ageing, subjects with osteoarthritis are probably exposed to these potentially biologically ageing mechanisms more than controls.
Finally, LTL is genetically determined with heritability estimates of 36–78%.17,18 It is also known that there is a significant genetic component in osteoarthritis, particularly hand osteoarthritis.5,51,52 The observed association in the current study might partly be because they share common genetic components; but given that the overall correlation is low, it is more likely to be due to acquired factors.
In conclusion, loss in LTL is associated with mild radiographic hand osteoarthritis and increases with disease severity, suggesting potential shared mechanisms between osteoarthritis and ageing, and implicating acquired factors, such as oxidative stress and low‐level chronic inflammation, in both conditions.
Acknowledgements
We thank all the participants in the study and Gabriela L Surdulescu for the DNA preparation. We also thank Dr Bernet Kato for statistical support.
This study is supported by The Wellcome Trust, The Arthritis Research Campaign. AA ageing research is supported by the Healthcare Foundation of New Jersey and NIH grants AG021593 and AG020132.
Abbreviations
BMI - body mass index
JSN - joint space narrowing
K/L - Kellgren/Lawrence
LTL - leucocyte telomere length
SSC - 0.3 M trisodium citrate and 3.0 M sodium chloride in high‐purity dH2O, pH 0.7
TRF - terminal restriction fragment
Footnotes
Competing interests: None declared
References
- 1.Buckwalter J A, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res 2004427(Suppl)S6–15. [DOI] [PubMed] [Google Scholar]
- 2.van Saase J L, van Romunde L K, Cats A, Vandenbroucke J P, Valkenburg H A. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis 198948271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srikanth V K, Fryer J L, Zhai G, Winzenberg T M, Hosmer D, Jones G. A meta‐analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 200513769–781. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Niu J, Kelly‐Hayes M, Chaisson C E, Aliabadi P, Felson D T. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am J Epidemiol 20021561021–1027. [DOI] [PubMed] [Google Scholar]
- 5.Spector T D, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ 1996312940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stecher R. Heberden's nodes. Heredity in hypertrophic arthritis of the finger joints. Am J Med Sci 1941201801 [Google Scholar]
- 7.Hadler N M, Gillings D B, Imbus H R, Levitin P M, Makuc D, Utsinger P D.et al Hand structure and function in an industrial setting. Arthritis Rheum 197821210–220. [DOI] [PubMed] [Google Scholar]
- 8.Cooley H M, Stankovich J, Jones G. The association between hormonal and reproductive factors and hand osteoarthritis. Maturitas 200345257–265. [DOI] [PubMed] [Google Scholar]
- 9.Oliveria S A, Felson D T, Reed J I, Cirillo P A, Walker A M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum 1995381134–1141. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence J S, Bremner J M, Bier F. Osteo‐arthrosis. Prevalence in the population and relationship between symptoms and x ray changes. Ann Rheum Dis 1966251–24. [PMC free article] [PubMed] [Google Scholar]
- 11.de Lange T. Protection of mammalian telomeres. Oncogene 200221532–540. [DOI] [PubMed] [Google Scholar]
- 12.Wai L K. Telomeres, telomerase, and tumorigenesis—a review. Medscape Gen Med 2004619. [PMC free article] [PubMed] [Google Scholar]
- 13.Watson J D. Origin of concatemeric T7 DNA. Nat New Biol 1972239197–201. [DOI] [PubMed] [Google Scholar]
- 14.Olovnikov A M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 197341181–190. [DOI] [PubMed] [Google Scholar]
- 15.Valdes A M, Andrew T, Gardner J P, Kimura M, Oelsner E, Cherkas L F.et al Obesity, cigarette smoking, and telomere length in women. Lancet 2005366662–664. [DOI] [PubMed] [Google Scholar]
- 16.Von Zglinicki T, Martin‐Ruiz C M. Telomeres as biomarkers for ageing and age‐related diseases. Curr Mol Med 20055197–203. [DOI] [PubMed] [Google Scholar]
- 17.Andrew T, Aviv A, Falchi M, Surdulescu G L, Gardner J P, Lu X.et al Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet 200678480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slagboom P E, Droog S, Boomsma D I. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet 199455876–882. [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner J P, Li S, Srinivasan S R, Chen W, Kimura M, Lu X.et al Rise in insulin resistance is associated with escalated telomere attrition. Circulation 20051112171–2177. [DOI] [PubMed] [Google Scholar]
- 20.Boukamp P. Ageing mechanisms: the role of telomere loss. Clin Exp Dermatol 200126562–565. [DOI] [PubMed] [Google Scholar]
- 21.Benetos A, Gardner J P, Zureik M, Labat C, Xiaobin L, Adamopoulos C.et al Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension 200443182–185. [DOI] [PubMed] [Google Scholar]
- 22.Brouilette S, Singh R K, Thompson J R, Goodall A H, Samani N J. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 200323842–846. [DOI] [PubMed] [Google Scholar]
- 23.Von Zglinicki T, Serra V, Lorenz M, Saretzki G, Lenzen‐Grossimlighaus R, Gessner R.et al Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest 2000801739–1747. [DOI] [PubMed] [Google Scholar]
- 24.Panossian L A, Porter V R, Valenzuela H F, Zhu X, Reback E, Masterman D.et al Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol Aging 20032477–84. [DOI] [PubMed] [Google Scholar]
- 25.Finkel T, Holbrook N J. Oxidants, oxidative stress and the biology of ageing. Nature 2000408239–247. [DOI] [PubMed] [Google Scholar]
- 26.Roubenoff R, Harris T B, Abad L W, Wilson P W, Dallal G E, Dinarello C A. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci 199853M20–M26. [DOI] [PubMed] [Google Scholar]
- 27.Ershler W B, Keller E T. Age‐associated increased interleukin‐6 gene expression, late‐life diseases, and frailty. Annu Rev Med 200051245–270. [DOI] [PubMed] [Google Scholar]
- 28.Martin J A, Buckwalter J A. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci 200156172–179. [DOI] [PubMed] [Google Scholar]
- 29.Price J S, Waters J G, Darrah C, Pennington C, Edwards D R, Donell S T.et al The role of chondrocyte senescence in osteoarthritis. Aging Cell 2002157–65. [DOI] [PubMed] [Google Scholar]
- 30.Piera‐Velazquez S, Jimenez S A, Stokes D. Increased life span of human osteoarthritic chondrocytes by exogenous expression of telomerase. Arthritis Rheum 200246683–693. [DOI] [PubMed] [Google Scholar]
- 31.Martens U M, Zijlmans J M, Poon S S, Dragowska W, Yui J, Chavez E A.et al Short telomeres on human chromosome 17p. Nat Genet 19981876–80. [DOI] [PubMed] [Google Scholar]
- 32.Spector T D, MacGregor A J. The St. Thomas' UK Adult Twin Registry. Twin Res 20025440–443. [DOI] [PubMed] [Google Scholar]
- 33.Andrew T, Hart D J, Snieder H, de L M, Spector T D, MacGregor A J. Are twins and singletons comparable? A study of disease‐related and lifestyle characteristics in adult women. Twin Res 20014464–477. [DOI] [PubMed] [Google Scholar]
- 34.Kellgren J H, Lawrence J S.Atlas of standard radiographs of arthritis. The epidemiology of chronic rheumatism. Oxford: Blackwell Scientific Publications, 1963
- 35.Burnett S, Hart D J, Cooper C, Spector T D.A radiographic atlas of osteoarthritis. London: Springer Verlag, 1994
- 36.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J.et al Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 200137381–385. [DOI] [PubMed] [Google Scholar]
- 37.Williams R L. A note on robust variance estimation for cluster‐correlated data. Biometrics 200056645–646. [DOI] [PubMed] [Google Scholar]
- 38.Cawthon R M. Telomere measurement by quantitative PCR. Nucleic Acids Res 200230e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rufer N, Brummendorf T H, Kolvraa S, Bischoff C, Christensen K, Wadsworth L.et al Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 1999190157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper M D, Alder M N. The evolution of adaptive immune systems. Cell 2006124815–822. [DOI] [PubMed] [Google Scholar]
- 41.Lord J M, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunosenescence. Mech Ageing Dev 20011221521–1535. [DOI] [PubMed] [Google Scholar]
- 42.Okuda K, Bardeguez A, Gardner J P, Rodriguez P, Ganesh V, Kimura M.et al Telomere length in the newborn. Pediatr Res 200252377–381. [DOI] [PubMed] [Google Scholar]
- 43.Butler M G, Tilburt J, DeVries A, Muralidhar B, Aue G, Hedges L.et al Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet Cytogenet 1998105138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbero A, Grogan S, Schafer D, Heberer M, Mainil‐Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post‐expansion chondrogenic capacity. Osteoarthritis Cartilage 200412476–484. [DOI] [PubMed] [Google Scholar]
- 45.Guerne P A, Blanco F, Kaelin A, Desgeorges A, Lotz M. Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Rheum 199538960–968. [DOI] [PubMed] [Google Scholar]
- 46.Loeser R F, Shanker G, Carlson C S, Gardin J F, Shelton B J, Sonntag W E. Reduction in the chondrocyte response to insulin‐like growth factor 1 in aging and osteoarthritis: studies in a non‐human primate model of naturally occurring disease. Arthritis Rheum 2000432110–2120. [DOI] [PubMed] [Google Scholar]
- 47.Yudoh K, Nguyen T, Nakamura H, Hongo‐Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther 20057380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin J A, Brown T D, Heiner A D, Buckwalter J A. Chondrocyte senescence, joint loading and osteoarthritis. Clin Orthop Relat Res 2004427(Suppl)96–103. [DOI] [PubMed] [Google Scholar]
- 49.Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 200227339–344. [DOI] [PubMed] [Google Scholar]
- 50.Finch C E, Crimmins E M. Inflammatory exposure and historical changes in human life‐spans. Science 20043051736–1739. [DOI] [PubMed] [Google Scholar]
- 51.Felson D T, Couropmitree N N, Chaisson C E, Hannan M T, Zhang Y, McAlindon T E.et al Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: The Framingham Study. Arthritis Rheum 1998411064–1071. [DOI] [PubMed] [Google Scholar]
- 52.Kellgren J H, Lawrence J S. Genetic factors in generalized osteoarthrosis. Ann Rheum Dis 196322237–255. [DOI] [PMC free article] [PubMed] [Google Scholar]