Abstract
Background
The diagnostic value of molecular analysis of the familial Mediterranean fever (FMF) gene (Mediterranean fever (MEFV)) has been well established only in patients selected on the basis of ethnic background or clinical criteria. Genetic diagnosis for other hereditary periodic fever syndromes has been poorly evaluated.
Objective
To determine the diagnostic contribution of genetic tests for hereditary periodic syndromes in a large, unselected series of patients.
Methods
A retrospective study was conducted on 1941 patients referred to us for FMF genetic tests between 1997 and 2005. MEFV genotypes were compared with clinical data to appraise criteria for FMF diagnosis. Genetic tests for tumour necrosis factor receptor‐associated periodic syndrome (TRAPS), hyperimmunoglobulinaemia D syndrome (HIDS) and cryopyrin‐associated periodic syndromes (CAPS) were also reviewed.
Results
71% of the 1574 patients with enough data had a clinical diagnosis of FMF according to the widely used Israeli criteria. Two MEFV mutations were found in only 409 patients of this subgroup (sensitivity = 37%) and in 15 (3.3%) of the patients with an improbable clinical diagnosis of FMF (specificity = 97%). Molecular diagnosis for alternate hereditary periodic syndromes was carried out in 456 of the patients having a non‐conclusive FMF genetic test. A positive diagnosis was obtained in 31 of these patients (TRAPS (n = 19), HIDS (n = 4) and CAPS (n = 8)).
Conclusions
First‐line MEFV mutation screening in patients with clinically typical FMF may be appropriate only in particular areas. To optimise genetic diagnosis, we propose a decision tree, which, with the advice of an expert practitioner, could help redirect test indications towards non‐FMF hereditary periodic syndromes.
Familial Mediterranean fever (FMF) is an autoinflammatory disease that shows autosomal recessive transmission. FMF is the most frequent of all hereditary periodic fever syndromes, mainly affecting populations from the Mediterranean basin, such as Sephardic Jews, Armenians, Turks and Arabs, with a prevalence ranging from 1 in 200 to 1 in 1000.1,2 Its major clinical features are recurrent episodes of fever, accompanied by abdominal pains reflecting acute peritoneal inflammation. The main complication of FMF is renal amyloid A amyloidosis. As this severe complication can be effectively prevented by treatment with colchicine, it is critical that diagnosis of FMF is established early.3 Owing to the absence of specific biological tests, diagnosis of FMF has long been exclusively based on phenotypic grounds, and a set of currently widely used clinical criteria was proposed in Israel in 1997.4 The identification in the same year of MEFV, the gene responsible for FMF, allowed the development of a new diagnostic tool.5,6 MEFV is located on the short arm of chromosome 16 and encodes pyrin marenostrin, a protein associated with innate immunity.7 To date, >70 MEFV mutations have been associated with symptoms of FMF, although only five of them (M694V, V726A, M694I, M680I and E148Q) account for 70–80% of the cases in Mediterranean countries.8,9 The value of molecular diagnosis of FMF has been well established in patients classically affected by this disease or fulfilling a particular clinical criteria—that is, in selected populations.10,11,12,13,14 In unselected cases, the diagnosis of other hereditary periodic syndromes is only rarely considered, and except in expert centres requests for genetic diagnosis almost exclusively focus on the FMF gene. To date, only two studies have been carried out to evaluate the diagnostic value of molecular tests for FMF in unselected populations, even though in clinical practice such an evaluation could be of great interest in atypical cases.2,15 We thus retrospectively reviewed data, from a large series of 1941 unselected patients, that had been referred to our laboratories for genetic diagnosis of FMF between 1997 and 2005. MEFV genotypes were correlated with the presence or absence of symptoms fulfilling recognised clinical criteria.4 When only one or no MEFV mutation was detected, and if the clinical presentation of the patient was suggestive, we carried out molecular diagnosis for one or more of the three other hereditary periodic fevers: tumour necrosis factor receptor‐associated syndrome (TRAPS),16 hyperimmunoglobulinaemia D syndrome (HIDS),17,18 or cryopyrin‐associated syndromes (CAPS), which comprise familial cold urticaria (FCU), Muckle–Wells syndrome and chronic infantile neurological cutaneous and articular syndrome.19,20
Methods
Samples
We included in this study all patients referred to our laboratories for genetic diagnosis of FMF up to December 2005. We did not require that patients fulfil published clinical criteria as a condition for molecular testing. For each patient, epidemiological data (including sex, ethnic background, familial history and age of onset of inflammation signs) and main clinical data (including fever, abdominal, articular, thoracic, cutaneous and neurosenorial signs, duration and frequency of episodes, presence of amyloidosis, and response to colchicine) were recorded by the prescribing doctor using a standard form.
We retrospectively assess the simplified clinical criteria set for the diagnosis of FMF for each patient.4 We chose to use the simplified criteria as they were easily retrieved from our standard medical chart, show similar diagnostic value to the extensive ones,4 and are more specific than the Tel Hashomer criteria.21 These criteria are divided into five main types (1–4, typical episodes of peritonitis, pleuritis or pericarditis, monoarthritis, fever alone; 5, incomplete abdominal episodes) and 4 minor criteria (1–2, incomplete episodes involving chest or joints; 3, exertional leg pain; 4, favourable response to colchicine). The requirements for diagnosis of FMF are ⩾1 major criteria, or ⩾2 minor criteria. A comprehensive description of these criteria is provided in the supplementary material (available at http://www.annrheaumdis.com/supplemental). All patients gave written informed consent before blood sampling, the research protocol was approved by the ethics committee of the CHU of Montpellier, France.
Detection of mutation
Genetic diagnosis of FMF has been available in our laboratories since the cloning of the MEFV gene. A specific examination of E148Q (in exon 2) and a generic search for mutations in exon 10 were systematically conducted. When one mutation was identified in this screening step, rare mutations in exons 3, 5 and sometimes 2 were subsequently searched for. We used various combinations of restriction enzyme digestions, an amplification‐refractory mutation system and denaturing gradient gel electrophoresis of polymerase chain reaction (PCR) fragments, as previously described.22 Direct sequencing of both strands was carried out where necessary. A test was considered positive when at least two mutations were found in the MEFV gene. It was considered non‐conclusive in all other cases.
Genetic diagnosis of TRAPS and HIDS has been available in our laboratories since 2003, and that of CAPS since 2004. DNA samples, dating back to 1997 from patients with an inconclusive MEFV test, were reanalysed for non‐FMF periodic fever genes as we estimated, on the basis of the clinical charts, that complementary tests could be relevant. Tumour necrosis factor receptor superfamily type 1A (TNFRSF1A) exons 2, 3 and 4, mevalonate kinase exons 2 and 9, cold‐induced autoinflammatory syndrome 1 exon 3, and the corresponding intronic boundaries of these three genes, were amplified using the M13‐tagged primers (supplementary material; available at http://www.annrheumids.com/supplemental). In all, 50 ng genomic DNA, 2.5 mM magnesium chloride, 0.25 µM each of forward and reverse primers, 0.025 U of Taq Gold polymerase and 0.2 mM dNTP were used for amplification reactions as follows: an initial denaturation at 95°C for 9 min, followed by 30 cycles of denaturing at 94°C for 30 s, annealing at 58°C and extension at 72°C for 1 min, with a final 9‐min extension at 72°C. PCR efficiencies were verified on a 5% acrylamide gel, and 1–4 µl of amplified product was directly submitted to sequencing in both directions with M13 universal primers using the Big Dye Terminator v3.1 kit (Applied Biosystems, Applera, France), following the manufacturer's instructions. Sequencing products were run on an ABI Prism Genetic Analyzer 3100 (Applied Biosystems). Mevalonate kinase exon 11 was amplified using 1176 and 1381 primers,23 and the PCR fragments were digested with BsmA1 to examine V377I, the most frequent HIDS mutation.
Statistics
Statistical comparisons were carried out using the χ2 method or Fisher's exact test. A p value >0.05 was considered significant.
Results
Samples from 1941 patients were sent to our laboratories for genetic diagnosis of FMF between 1997 and 2005. FMF was genetically confirmed in 27% patients. The 1941 patients came from 1512 unrelated families, which were used to calculate the genotype distribution. In patients genetically confirmed to have FMF, M694V/M694V was the most frequent genotype (39.2%), followed by M694V/V726A (9.0%), E148Q/M694V (7.6%) and M680I/M694V (5.9%). No other genotype frequency exceeded 5%. In 16.5% of the patients, only one mutation was identified, which in 43% of such patients was M694V. No mutation was found in 56.5% of the patients.
The referring doctors were from diverse specialties (internal medicine 33%, paediatrics 33%, genetics 8%, gastroenterology 7%, infectiology 5%, each of the others <5%). Patients came from various ethnic groups: non‐Ashkenazi Jews 20.1%, Arabs 13.4%, Turks 8.7%, Armenians 5.5%, French not from these four groups 14.9%, other 29.8% and not determined 7.6%, with a 0.93 male:female ratio. Patients primarily had recurrent episodes of fever (82.6%) and abdominal pain (77.5%). The other signs of inflammation reported were synovitis (59.1%), pleuritis (31.5%) and erysipelas‐like erythema (19.7%). Age of disease onset was <20 years in 66.7% of the patients. Amyloid A amyloidosis was documented in 1.1% patients. Treatment with colchicine had been tested in only 41.5% patients, with a positive response seen in 70.8% and no or little effect observed in the others. We had sufficient phenotypic data to retrospectively determine clinical criteria in 1574 of 1941 patients.4 FMF was clinically defined in 1118 of 1574 (71%) patients.
These clinical results were compared with the genetic diagnostic data (table 1). Among the expected results obtained was the finding that the genetic diagnosis was positive in 409 of 1118 (overall sensitivity = 37%) patients with a clinical diagnosis of FMF, and that no mutation was found in 441 of 456 (overall specificity = 97%) patients with an improbable clinical diagnosis of FMF. On the other hand, genetic testing failed to confirm diagnosis in 709 of 1118 (63%) patients with a clinical diagnosis of FMF, although 15 of 456 (3.3%) genetically confirmed patients were not detected when using Livneh's criteria MEFV. Table 2 shows the characteristics of the 15 patients with a genetic diagnosis of FMF, but with an improbable clinical diagnosis.
Table 1 Comparison of clinical and genetic criteria of familial Mediterranean fever.
| Reference | Study n* | Clinical criteria† | Genetic criterion‡ | Sensitivity (%)§ | Specificity (%)¶ | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Samuels et al2 | 100 (52; 48) | Yes | 24 | 62 | 28 | 100 |
| No | 0 | 14 | ||||
| Tchernitchko et al24 | 208 (0; 208) | Yes | 0 | 208 | 0 | ND |
| No | ND | ND | ||||
| This study | 1574 (752; 822) | Yes | 409 (351; 58) | 709 (235; 474) | 37 (60; 11) | 97 (91.6; 99.7) |
| No | 15 (14; 1) | 441 (152; 289) | ||||
FMF, familial Mediterranean fever; MEFV, Mediterranean fever; ND, not done.
*n, number in the total population, followed in brackets by the number of patients in the subgroups from (the four mainly affected ethnic groups; patients from other origin).
†Clinical criteria as defined by Livneh.
‡Genetic criterion as defined by the identification of at least two MEFV mutations.
§Percentage of MEFV‐positive patients among those with a clinical FMF.
¶Percentage of MEFV‐negative patients among those negative for the clinical criteria.
Table 2 Characteristics of the 15 patients with a positive genetic diagnosis of familial Mediterranean fever and an improbable clinical diagnosis according to Livneh's criteria.
| Ethnic background | Sex | Age at inclusion in the study | Major criteria | Minor criteria | Other signs | Genotype |
|---|---|---|---|---|---|---|
| Arab | M | 14 | No | Incomplete joint episodes | Familial FMF, atypical abdominal signs | M694I/M694I |
| Arab | M | 11 | No | No | Splenomegaly, myalgia, atypical articular signs | M694I/M694I |
| Arab | M | 20 | No | Incomplete chest episodes | No | M694I/M694I |
| Arab | F | 38 | No | Incomplete chest episodes | Atypical abdominal signs | M694I/V726A |
| Armenian | F | 36 | No | No | Familial FMF, atypical articular signs | R761H/E148Q |
| Jewish | M | 21 | No | No | Familial FMF, Crohn's disease | M694V/M694V |
| Jewish | F | 34 | No | Incomplete joint episodes | No | M694V/R761H |
| Jewish | M | 13 | No | Incomplete chest episodes | No | M694V/M694V |
| Jewish | F | 23 | No | Favourable response to colchicine | Atypical abdominal and articular signs | E148Q/M694V |
| Jewish | M | 4 | No | Incomplete joint episodes | Familial FMF | M694V/M694V |
| Turkish | M | 55 | No | Favourable response to colchicine | Splenomegaly, atypical abdominal and articular signs | E148Q/M694V |
| Turkish | M | 9 | No | No | Familial FMF, myalgia, atypical abdominal and articular signs | M680I/M694V |
| Turkish | M | 6 | No | Incomplete chest episodes | Familial FMF | M694V/V726A |
| Turkish | M | 8 | No | Incomplete joint episodes | No | M694V/M694V |
| French | F | 52 | No | Incomplete joint episodes | No | E148Q/E148Q |
F, female; FMF, familial Mediterranean fever; M, male.
The diagnosis value of MEFV testing according to ethnicity was also dealt with (table 1). The sensitivity of the molecular analysis of the MEFV gene, and to a lesser extent, the specificity, dramatically varied according to the origin of the patient (p<10−4). As expected, the highest rates of positive genetic diagnosis were obtained in patients from the four mainly affected populations (48.5%), but molecular diagnosis also pinpointed 16.2% patients from other Mediterranean countries (Italy, Greece and Spain, but not Portugal). A small number of French patients positive for MEFV, with no known Mediterranean ancestry, were also identified (5/234, 2.1%).
The possibility that an alternative hereditary periodic fever syndrome was present was investigated for a subset of the patients with a non‐conclusive genetic diagnosis of FMF (n = 456). One or more additional genes was analysed after the request of the referring practitioner or on an internal decision for 225 and 231 patients, respectively. In 231 patients, we did not use available clinical diagnostic criteria (HIDS25 and FCU26) as a basis for selecting patients. Instead, a complementary procedure was carried out when at least one typical sign was recorded—that is, periorbital oedema, myalgia, late onset, long episodes or effectiveness of corticoids for TRAPS; aphtosis, post‐vaccinal reactivity or cervical adenopathy for HIDS; neurosensorial symptoms or urticaria for CAPS. A diagnosis was genetically confirmed in 19 of 289 (6.6%) patients tested for TRAPS, 4 of 202 (2%) patients tested for HIDS and 8 of 96 (8.3%) patients tested for CAPS. A mutation in both TNFRSF1A and cold‐induced autoinflammatory syndrome 1 was identified in a female patient and her mother (this family is described in a companion paper,27 see page 1530). Note that 17 of these 31 patients initially referred for FMF genetic diagnosis were eventually identified in our laboratories as having an alternate recurrent fever. Tables 3–5 show the epidemiological, clinical and genetic characteristics of these 31 patients.
Table 3 Characteristics of the 19 patients with a genetic diagnosis of TRAPS.
| Patients* | Ethnic background | Sex | Age at onset (years) | Episode duration (days) | Episode frequency (per month) | Fever | Abd | Art | Tho | Cut | Myalgia | Peri‐orbital oedema | Other signs | Cortico‐ steroids efficacy | TNFRSFIA genotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | French | F | <1 | 1–2 | <1 | + | + | + | + | – | – | – | – | + | C30R |
| 1b | French | M | <1 | 1–2 | <1 | + | + | + | – | – | – | – | – | + | C30R |
| 2a | Greek | F | 4 | 14 | 1 | + | + | + | – | – | – | – | – | NT | C73W |
| 2b | Greek | F | 2 | 2 | 2 | + | + | + | – | – | + | – | Pharyngitis | NT | C73W |
| 2c | Greek | F | 2 | 10 | 1–2 | + | + | + | – | – | + | – | – | NT | C73W |
| 3 | French | F | 1 | 15 | 1–2 | + | + | + | – | + | + | – | Conjunctivitis | – | H69fs |
| 4 | Spaniard | F | 6 | ND | >2 | + | + | + | + | – | + | – | – | – | L39F |
| 5 | Arab | M | 2 | 3–4 | 1 | + | + | + | – | + | – | – | Splenomegaly | NT | P46L |
| 6 | Turkish | M | 10 | ND | >2 | – | + | + | – | – | + | – | – | + | P46L |
| 7 | French | M | 43 | 5 | 2 | + | – | + | – | – | – | – | – | NT | P46L |
| 8a | Sefardi‐Jews | F | ND | Chronic | Chronic | – | – | + | – | – | – | – | Aphtosis | NT | R92Q |
| 8b | Sefardi‐Jews | M | 10 | 1 | <1 | – | + | – | – | – | – | – | Conjunctivitis | NT | R92Q |
| 9 | Italian | M | 26 | 10 | <1 | + | – | + | – | – | + | – | Aphtosis | + | R92Q |
| 10 | French | M | 19 | 3 | <1 | + | + | + | – | – | + | – | – | – | R92Q |
| 11 | French | M | 32 | Chronic | Chronic | + | – | + | + | + | – | – | – | NT | R92Q |
| 12 | Jewish | F | 12 | 2 | <1 | + | + | + | + | – | – | – | – | ND | R92Q |
| 13 | French | M | ND | 3–5 | <1 | + | + | + | – | – | + | – | – | NT | R92Q |
| 14 | French | M | 10 | 10 | <1 | + | – | + | + | – | – | – | – | NT | R92Q |
| 15 | French | M | 24 | 4 | 1–2 | ND | – | – | + | – | + | – | Aphtosis | NT | R92Q |
Abd, abdominal signs; Art, articular signs; Cut, cutaneous signs; F, female; M, male; ND, not determined; NT, not treated; Tho, thoracic signs; +, presence of symptoms; −, absence of symptoms.
*Patients' relationships: 1a and 1b are siblings; 2a is the mother of 2b and 2c; 8a and 8b are siblings.
Table 4 Characteristics of the four patients with a genetic diagnosis of hyperimmunoglobulinaemia D syndrome.
| Patients | Ethnic background | Sex | Age at onset (years) | Episode duration (days) | Episode frequency (per month) | Fever | Abd | Art | Tho | Cut | ADP | Aphtosis | Post‐vaccinal reactivity | Other signs | IgD (UI/L) | MVK genotypes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | French | M | 5 | 3 | <1 | + | + | + | + | – | – | – | – | – | >400 | I268T/V377I |
| 2 | French | M | 3 | 3–5 | <1 | + | + | – | – | – | – | – | – | Pharyngitis | ND | I268T/V377I |
| 3 | French | F | <1 | 2 | 1–2 | + | – | + | – | – | – | ND | – | – | >800 | V377I/S329N |
| 4 | French | F | <1 | 3–4 | 1–2 | + | + | + | – | + | – | – | – | Conjunctivitis | normal | V377I/V377I |
Abd, abdominal signs; ADP, cervical adenopathies; Art, articular signs; Cut, cutaneous signs; F, female; M, male; MVK, mevalonate kinase; ND, not determined; Tho, thoracic signs; +, presence of symptoms; −, absence of symptoms.
Table 5 Characteristics of the eight patients with a genetic diagnosis of cryopyrin‐associated periodic syndrome.
| Patient* | Ethnic background | Sex | Age at onset (years) | Episode frequency (per month) | Episode duration (days) | Fever | Abd | Art | Tho | Urticaria | Deafness | Meningitis | Other signs | CIAS1 mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | French | M | 3 | <1 | >7 | + | + | + | – | + | – | – | Oedema | V198M |
| 1b | French | F | 5 | <1 | >7 | – | – | + | – | + | – | – | Oedema | V198M |
| 3a | Italian | F | 15 | 2 | 1–2 | + | + | + | + | + | – | – | Myalgia, Uveitis | R260W |
| 3b | Italian | M | 7 | 1–2 | 1–2 | + | – | + | – | + | + | – | Myalgia, UveitisBi‐polar aphtosis | R260W |
| 4 | Italian | F | 8 | 1–2 | 1 | + | – | + | – | + | – | – | – | R260W |
| 5 | French | F | 0–1 | 1 | 14 | + | – | + | – | + | + | – | Conjunctivitis | T348M |
| 6 | French | F | <1 | >2 | 1 | + | – | + | – | + | – | – | – | D303N |
| 7 | French | F | <1 | 1–2 | 1–2 | + | – | + | – | + | – | – | Conjunctivitis | D303N |
Abd, abdominal signs; Art, articular signs; F, female; M, male; Tho, thoracic signs; +, presence of symptoms; −, absence of symptoms.
*Patients' relationships: 1b is the mother of 1a; Sibships: 3a and 3b.
Although no clear phenotype–genotype correlation can be drawn from this relatively small set of patients, several interesting observations can be made. Firstly, none of the patients with non‐FMF periodic fever had renal amyloidosis. Secondly, none of the 19 patients with TRAPS showed pathognomonic periorbital oedema. Thirdly, 10 of these 19 patients have an episode duration ⩽5 days, considerably shorter than usual report durations. Finally, none of the patients with HIDS mutations had cervical adenopathies or post‐vaccinal hyper‐reactivity, two symptoms frequently associated with this syndrome.
Discussion
Until the identification of the MEFV gene, FMF was a diagnosis of exclusion that was made based on clinical grounds. The diagnostic value of molecular analysis of MEFV has been mainly investigated in selected populations that fulfil clinical criteria or come from classically affected ethnic groups, with genetic confirmation rates ranging from 55% to 89%.10,11,12,13,14 Molecular tests allowing the routine diagnosis of FMF have now been developed in many laboratories in non‐classically affected countries, such as France. We determined the diagnostic contribution of genetic tests for hereditary periodic syndromes in patients referred to our laboratories up to December 2005. We are aware that such a laboratory‐based retrospective study has limitations, especially because we used a clinical form filled out most of the times by non‐expert practitioners. However, the aim of the study was to assess unselected patients, and this form was comprehensive and standardised. The epidemiological and clinical characteristics of our 1941 patients show the heterogeneity of cases referred by doctors from various specialities. Their requests for genetic diagnosis of FMF seem to have been justified in more than two thirds of the cases, as 71% of the informative patients had clinical FMF according to published criteria.4
We genetically confirmed the diagnosis of FMF in 27% of the patients. Probably the routine strategy that we and others use—that is, a partial examination of the MEFV gene focusing on mutational hot spots—cannot account for the inconclusive FMF test results obtained in many patients. Indeed, this low value is consistent with two previous studies, conducted in unselected populations, in which the authors yielded a positive diagnostic value in 24 of 100 (24%) and 133 of 303 (44%) patients, respectively.2,15 As expected, both we and Grateau et al15 observed higher genetic confirmation rates in patients with a definite diagnosis of FMF. Grateau et al's study used the Tel Hashomer criteria, which are more sensitive but less specific. The genetic approach for diagnosis of FMF was also more effective in patients belonging to the 4 (48.5%) classic ancestries, and most of all in clinically and ethnically typical patients (60%). These values of clinically and ethnically typical patients fall within ranges previously reported for selected populations.10,11,12,13,14 Genetic diagnosis was also helpful in 3.3% cases that did not fulfil clinical criteria, and in 7.2% of our patients from other ancestries. Tchernitcko et al24 reported a series of 208 western European Caucasian patients meeting Livneh's criteria, including 142 French. None of them had two demonstrable MEFV mutations. We found 5 of 234 positive western European Caucasian (French) patients, but the difference between the two studies was not significant. Restricting genetic diagnosis to those cases that strictly fulfil the clinical criteria or that are from classically affected ethnic backgrounds may not be rational, as a diagnosis of FMF would have been missed in 15 and 58 of our patients, respectively. We concede, however, that genetic confirmation of FMF was not made in 441 of 456 (97%) patients without a clinical diagnosis of FMF.
In patients with a non‐conclusive genetic diagnosis of FMF, other hereditary periodic fevers should be considered as a preferred strategy, especially in patients with non‐Mediterranean ancestry. We carried out complementary molecular tests in 456 patients with phenotypes compatible with TRAPS (n = 289), HIDS (n = 202) or CAPS (n = 96), and found TRAPS, HIDS and CAPS mutations in 6.6%, 2% and 8.3% of the tested patients, respectively. Altogether, we found that 31 of 456 (6.8%) of the patients referred to us for genetic diagnosis of FMF actually had a different hereditary periodic syndrome. The significance of some sequence variants such as E148Q in MEFV, R92Q and P46L in TNFRSF1A receptor,28 or V198M in CAPS is, however, currently debated because they are frequent in the general population. They may correspond to functional polymorphisms, and are recognised at least as low‐penetrance mutations. In our family with both TRAPS and CAPS mutations, the two patients with combined mutations presented with quite atypical, overlapping and severe symptoms, whereas the three patients with only one variant were either totally asymptomatic or had a much milder disease.27 This suggests that the phenotype of the patient may depend on his or her genetic background or result from digenic inheritance, which is consistent with the transmission pattern observed in our family. Accumulation of clinical data related to these variants will help elucidate their true significance. An accurate diagnosis is critical for targeting novel and effective therapeutic agents to these conditions, such as anti‐interleukin 1 in CAPS, anti‐tumour necrosis factor in TRAPS, and possibly simvastatine and anti‐interleukin 1 in HIDS.29,30,31,32 The diagnosis of patients in whom we could identify no mutation remains unclear. They may have been carrying a rare mutation that escaped our screening strategy, a mutation in a yet unknown gene, or may have had another inflammatory condition. The low number of genetically confirmed diagnosis of periodic diseases was probably caused by the fact that samples were freely sent to our laboratories. As observed by Simon et al,24 a low yield is reached when tests are carried out on the basis of a minimal clinical suspicion.
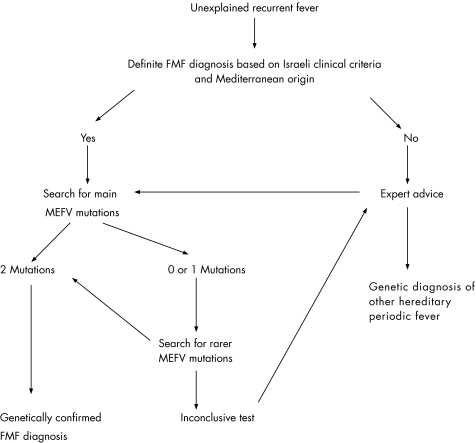
In conclusion, molecular screening of MEFV mutations proved to be of positive diagnostic value in 27% of 1941 unselected patients having an inflammatory disorder of unknown origin who were referred for routine genetic diagnosis of FMF. Even if this value had been increased to 60% by testing only the most typical patients, 73 of our clinically (n = 15) or ethnically (n = 58) atypical patients would have been missed had they not been genotyped. At the same time, consistent with previous studies conducted in Western countries,2,15,24 the diagnosis of FMF was not genetically confirmed in most of our patients, strongly suggesting that most of them had another disease that sometimes meets the clinical diagnostic criteria of FMF. It is important for clinicians to realise that there is a considerable number of patients with definite autoinflammatory syndromes without genetic diagnosis. This suggests that the widely used Israeli clinical criteria may only be appropriate in particular areas. We thus propose that in such cases, molecular tests should be carried out only after an expert's advice. This expert consultation could point to the use, when appropriate, of molecular tests for non‐FMF conditions including TRAPS, HIDS and CAPS, potentially allowing a correct diagnosis to be obtained in as many as 6.8% more patients. In light of these results, we propose a decision tree for determining the indications of molecular diagnosis in patients with periodic fever in uncommonly affected areas (fig 1).
Figure 1 Decision tree for genetic testing in patients with unexplained recurrent fever.
Supplementary Material
Acknowledgements
We thank Drs Cordier, Garnier, Lachaux, Longy, Manna, Monnier, Mourad, Sailler, Serratrice, Stich, Swiader, Villard and Vougiouka for contributing patients, and B Dumont and C Lault for excellent technical assistance. This work was supported by the Centre Hospitalo‐Universitaire (CHU) de Montpellier, France.
Abbreviations
CAPS - cryopyrin‐associated periodic syndrome
FCU - familial cold urticaria
FMF - familial Mediterranean fever
HIDS - hyperimmunoglobulinaemia D syndrome
MEFV - Mediterranean fever
PCR - polymerase chain reaction
TRAPS - tumour necrosis factor receptor‐associated periodic syndrome
Footnotes
Competing interests: None.
References
- 1.Ben‐Chetrit E, Levy M. Familial Mediterranean fever. Lancet 1998351659–664. [DOI] [PubMed] [Google Scholar]
- 2.Samuels J, Aksentijevich I, Torosyan Y, Centola M, Deng Z, Sood R.et al Familial Mediterranean fever at the millenium. Clinical spectrum, ancient mutations, and a survey of 100 American referrals to the National Institutes of Health. Medicine 199877268–297. [DOI] [PubMed] [Google Scholar]
- 3.Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni J. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med 19863141001–1005. [DOI] [PubMed] [Google Scholar]
- 4.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T.et al Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum 1997401879–1885. [DOI] [PubMed] [Google Scholar]
- 5.The French FMF Consortium A candidate gene for familial Mediterranean fever. Nat Genet 19971725–31. [DOI] [PubMed] [Google Scholar]
- 6.The International FMF Consortium Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 199790797–807. [DOI] [PubMed] [Google Scholar]
- 7.Stehlik C, Reed J C. The PYRIN connection: novel players in innate immunity and inflammation. J Exp Med 2004200551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touitou I. The spectrum of familial Mediterranean fever (FMF) mutations. Eur J Hum Genet 20019473–483. [DOI] [PubMed] [Google Scholar]
- 9.Touitou I, Lesage S, McDermott M, Cuisset L, Hoffman H, Dode C.et al Infevers: an evolving mutation database for auto‐inflammatory syndromes. Hum Mut. 2004;24: 194–8, http://fmf.igh.cnrs.fr/infevers/ [DOI] [PubMed]
- 10.Drenth J P, van der Meer J W. Hereditary periodic fever. N Engl J Med 20013451748–1757. [DOI] [PubMed] [Google Scholar]
- 11.Cazeneuve C, Sarkisian T, Pecheux C, Dervichian M, Nedelec B, Reinert P.et al MEFV‐gene analysis in armenian patients with familial Mediterranean fever: diagnostic value and unfavorable renal prognosis of the M694V homozygous genotype‐genetic and therapeutic implications. Am J Hum Genet 19996588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F.et al Turkish FMF Study Group. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study, Medicine 2005841–11. [DOI] [PubMed] [Google Scholar]
- 13.Ben‐Chetrit E, Urieli‐Shoval S, Calko S, Abeliovich D, Matzner Y. Molecular diagnosis of FMF: lessons from a study of 446 unrelated individuals. Clin Exp Rheumatol 200220(Suppl 26)S25–S29. [PubMed] [Google Scholar]
- 14.Zaks N, Shinar Y, Padeh S, Lidar M, Mor A, Tokov I.et al Analysis of the three most common MEFV mutations in 412 patients with familial Mediterranean fever. Isr Med Assoc J 20035585–588. [PubMed] [Google Scholar]
- 15.Grateau G, Pecheux C, Cazeneuve C, Cattan D, Dervichian M, Goossens M.et al Clinical versus genetic diagnosis of familial Mediterranean fever. QJM 200093223–229. [DOI] [PubMed] [Google Scholar]
- 16.McDermott M F, Aksentijevich I, Galon J, McDermott E M, Ogunkolade B W, Centola M.et al Germline mutations in the extra‐cellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndrome. Cell 199997133–144. [DOI] [PubMed] [Google Scholar]
- 17.Drenth J P, Cuisset L, Grateau G, Vasseur C, van de Velde‐Visser S D, de Jong J G.et al Mutations in the gene encoding mevalonate kinase cause hyper‐IgD and periodic fever syndrome. International Hyper‐IgD Study Group. Nat Genet 199922178–181. [DOI] [PubMed] [Google Scholar]
- 18.Houten S M, Kuis W, Duran M, de Koning T J, Royen‐Kerkhof A, Romeijn G J.et al Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet 199922175–177. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman H M, Mueller J L, Broide D H, Wanderer A A, Kolodner R D. Mutation of a new gene encoding a putative pyrin‐like protein causes familial cold autoinflammatory syndrome and Muckle‐Wells syndrome. Nat Genet 200129301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldmann J, Prieur A M, Quartier P, Berquin P, Certain S, Cortis E.et al Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet 200271198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pras M. Familial Mediterranean fever: from the clinical syndrome to the cloning of the pyrin gene. Scand J Rheumatol 19982792–97. [DOI] [PubMed] [Google Scholar]
- 22.Bernot A, da Silva C, Petit J L, Cruaud C, Caloustian C, Castet V.et al Non‐founder mutations in the MEFV gene establish this gene as the cause of familial Mediterranean fever (FMF). Hum Mol Genet 199871317–1325. [DOI] [PubMed] [Google Scholar]
- 23.Cuisset L, Drenth J P, Simon A, Vincent M F, van der Velde Visser S, van der Meer J W, et al; International Hyper‐IgD Study Group Molecular analysis of MVK mutations and enzymatic activity in hyper‐IgD and periodic fever syndrome. Eur J Hum Genet 20019260–266. [DOI] [PubMed] [Google Scholar]
- 24.Tchernitchko D, Moutereau S, Legendre M, Delahaye A, Cazeneuve C, Lacombe C.et al MEFV analysis is of particularly weak diagnostic value for recurrent fevers in Western European Caucasian patients. Arthritis Rheum 2005523603–3605. [DOI] [PubMed] [Google Scholar]
- 25.Simon A, van Der Meer J W, Vesely R, Myrdal U, Yoshimura K, Duys P.et al Approach to genetic analysis in the diagnosis of hereditary autoinflammatory syndromes. Rheumatology (Oxford) 200645269–273. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman H M, Wanderer A A, Broide D H. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol 2001108615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Touitou I, Perez C, Dumont B, Federici L, Jergensen C. Refractory auto‐inflammatory syndrome associated with digenic transmission of low‐penetrance tumour necrosis factor receptor‐associated periodic syndrome and cryoprin‐associated periodic syndrome mutations. Ann Rheum Dis 2006651530–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravet N, Rouaghe S, Dode C, Bienvenu J, Stirnemann J, Levy P.et al Clinical significance of P46L and R92Q substitutions in the Tumor Necrosis Factor Superfamily 1A gene. Ann Rheum Dis 2006651158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drewe E, McDermott E M, Powell P T, Isaacs J D, Powell R J. Prospective study of anti‐tumour necrosis factor receptor superfamily 1B fusion protein, in tumour necrosis factor receptor associated periodic syndrome (TRAPS): clinical and laboratory findings in a series of seven patients. Rheumatology 200342235–239. [DOI] [PubMed] [Google Scholar]
- 30.Simon A, Drewe E, van der Meer J W, Powell R J, Kelley R I, Stalenhoef A F.et al Simvastatin treatment for inflammatory attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Clin Pharmacol Ther 200475476–483. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman H M, Rosengren S, Boyle D L, Cho J Y, Nayar J, Mueller J L.et al Prevention of cold‐associated acute inflammation in familial cold autoinflammatory syndrome by interleukin‐1 receptor antagonist. Lancet 20043641779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodar E J, van der Hilst J C, Drenth J P, van der Meer J W, Simon A. Effect of etanercept and anakinra on inflammatory attacks in the hyper‐IgD syndrome: introducing a vaccination provocation model. Neth J Med 200563260–264. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.