Abstract
Objective
To use power Doppler sonography (PDS) to evaluate changes in synovial perfusion induced by adalimumab in the wrist joints of patients with rheumatoid arthritis.
Methods
48 wrists of 24 patients (18 women and 6 men) were examined. Despite prior treatment with disease‐modifying antirheumatic drugs, including methotrexate, patients with clinically active rheumatoid arthritis were recruited in two rheumatological centres to receive 40 mg adalimumab subcutaneously every other week. Clinical, laboratory and PDS assessments were carried out at 0, 2, 6 and 12 weeks. Clinical and laboratory measurements of disease activity included physician's global assessment of disease activity, erythrocyte sedimentation rate and serum levels of C reactive protein. The Disease Activity Score for 28 joints (DAS28) was calculated. PDS signal was scored from 0 to 3 according to the overall expression of PDS findings at the wrists.
Results
A significant reduction in both clinical (p<0.001) and PDS findings (p<0.001) was found at all follow‐up examinations. A tendency to positive correlation (Spearman's r = 0.382; p = 0.067) was shown between reduction in PDS score and improvement in DAS28 at week 2 examination.
Conclusion
PDS detected a rapid and significant reduction in synovial perfusion at the wrist joints of patients with rheumatoid arthritis receiving adalimumab. Ongoing follow‐up will provide further information regarding the persistence of considerable reduction in PDS signal score and its correlation with DAS28.
Biological agents are changing the landscape of the treatment of patients with rheumatoid arthritis, resulting in considerable and rapid clinical improvement in patients with active disease.1
Treatment monitoring is a challenge in daily rheumatological practice, and the development of new tools for assessing activity of rheumatoid synovitis is a relevant topic of research in rheumatology.2 Among the most interesting tools is power Doppler sonography (PDS).
PDS is a safe, inexpensive and non‐invasive imaging modality, which provides a quick and sensitive method of visualisation of synovial hyperperfusion in joints with inflammatory disease.2 The value of PDS in the assessment of synovial inflammation has been recently tested by comparing its findings with those obtained using magnetic resonance imaging, histology and arthroscopy, and markedly positive correlations have been reported.3,4,5,6 More recently, PDS has proved to be a feasible and promising tool for short‐term monitoring of changes in synovial perfusion induced by steroids and biological agents (etanercept and infliximab) in patients with rheumatoid arthritis.2,7,8,9,10
Our study aimed at PDS evaluation of the changes in synovial perfusion induced by adalimumab in the wrist joints of patients with rheumatoid arthritis. The wrist joint was selected for PDS evaluation because it is almost invariably affected in patients with rheumatoid arthritis.11
Patients and methods
Patients
In all, 24 consecutive patients were recruited from the Rheumatology Clinic of the Università Politecnica delle Marche, Ancona, Italy, and 48 wrists examined.
Patients satisfying inclusion criteria were aged ⩾18 years and had rheumatoid arthritis fulfilling the American College of Rheumatology (formerly the American Rheumatism Association) criteria.12 Active disease was defined by a Disease Activity Score for 28 joints (DAS28) not less than 3.2, despite prior treatment with disease‐modifying antirheumatic drugs (DMARDs), including methotrexate.
All women of childbearing potential had a negative pregnancy test (serum human chorionic gonadotrophin) before starting treatment with adalimumab. All patients with procreative capacity were asked to use a reliable method of contraception while receiving adalimumab, and for at least 70 days after the end of the treatment. Exclusion criteria included those used in trials of other biological agents and those reported in the ARMADA (Anti‐TNF Research Study Program of the Antibody D2E7 in Patients with Rheumatoid Arthritis) trial.1 Patients were excluded if they had received intra‐articular steroid injections at the wrist or if they had changed their dose of DMARDs in the preceding 3 months.
Study protocol
The clinical and PDS examinations were carried out at the Rheumatology Department of the Università Politecnica delle Marche in Ancona, Italy, according to local regulations and the Declaration of Helsinki. Patients were recruited to receive 40 mg adalimumab subcutaneously every other week. Permitted concomitant drugs included stable doses of non‐steroidal anti‐inflammatory drugs, including acetylsalicylic acid, and steroids (maximum daily dose of 10 mg of prednisone or equivalent orally). No intra‐articular steroid injections were permitted in either the wrist joints examined or in other joints. No changes in the dose of DMARDs were permitted during the study.
Clinical, laboratory and PDS assessments were carried out on the same day at 0, 2, 6 and 12 weeks. An experienced rheumatologist (AC) carried out all the physical examinations. Clinical and laboratory measurements of disease activity included physician's global assessment of disease activity, erythrocyte sedimentation rate (ESR) using the Westergren method and serum levels of C reactive protein (CRP; upper reference level 4 mg/l). At week 2, serum levels of CRP were not measured. Rheumatoid factor was negative if serum levels of CRP were <20 IU/ml. DAS28 was calculated.13
PDS evaluation
PDS was carried out by an experienced operator (EF) blinded to both clinical and laboratory findings. An AU5 Harmonic (Esaote Biomedica, Genoa, Italy) with a 10–14‐MHz linear probe was used. PDS settings were standardised with a pulse repetition frequency of 1000 Hz and a colour mode frequency of 7 MHz. The wall filters and the colour gain of the PDS equipment were identical to those proposed by Rubin et al.14 Each wrist was scanned over the dorsal surface from lateral to medial aspects in both longitudinal and transverse planes.
Representative pictures of the highest expression of intra‐articular PDS signals were obtained. A score from 0 to 3 was assigned according to the overall expression of PDS signals at the wrist level. The semiquantitative visual scale was as follows: 0, normal or minimal degree; 1, mild degree; 2, moderate degree; and 3, marked degree.
The same investigator carried out follow‐up examinations using the same ultrasound equipment, the same values of the setting parameters and the same scanning technique as that used for baseline assessment.
To evaluate intraobserver reproducibility, the first set of representative sonographic pictures taken in the first 12 patients consecutively enrolled in the study was evaluated twice by the same sonographer (EF): the first time soon after the ultrasound examination and the second time 1 week after.
Statistical analysis
Data were statistically analysed using the package MedCalc software (V.7.2 for Windows). Wilcoxon's rank sum test was used for the comparison of the findings at baseline and at follow‐up. Correlation between changes of PDS scores and clinical data was analysed by Spearman's rank correlation test. Correlation between binary data was calculated using the χ2 test. The level of significance was p<0.05.
Results
Table 1 summarises the clinical, laboratory and PDS data obtained at baseline and at follow‐up visits. Of the 24 patients, 13 received adalimumab as monotherapy and 11 patients were taking a stable dose of DMARD (8 took methotrexate and 3 took hydroxychloroquine) in addition to adalimumab.
Table 1 Demographic, clinical, laboratory and sonography data of patients (n = 24).
| Number | Sex | Age (years) | Disease duration (years) | RF (IU/ml) | VAS (0–100) | ESR (mm/h) | CRP (mg/l) | DAS28 | PDS right wrist† | PDS left wrist† | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T2 | T6 | T12 | T0 | T2 | T6 | T12 | T0 | T2 | T6 | T12 | T0 | T2 | T6 | T12 | T0 | T2 | T6 | T12 | T0 | T2 | T6 | T12 | |||||
| 1 | M | 48 | 16 | 96 | 59 | 40 | 40 | 19 | 12 | 4 | 6 | 6 | 11.1 | ND | <3.5 | <3.5 | 4.7 | 2.5 | 3.5 | 2.8 | 3 | 1 | 1 | 2 | 3 | 1 | 0 | 1 |
| 2 | M | 54 | 17 | 1030 | 40 | 45 | 26 | 19 | 8 | 2 | 3 | 7 | 19.9 | ND | 7.7 | 20.4 | 4.4 | 2.6 | 2.4 | 2 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 1 |
| 3 | F | 68 | 17 | 74 | 37 | 14 | 10 | 0 | 22 | 12 | 20 | 13 | 26.7 | ND | 17 | <3.5 | 4.4 | 2.2 | 2.1 | 1.8 | 3 | 2 | 1 | 0 | 3 | 2 | 1 | 0 |
| 4 | F | 57 | 8 | 429 | 79 | 10 | 10 | 0 | 21 | 12 | 7 | 10 | 13.4 | ND | <3.5 | <3.5 | 5.6 | 4 | 1.7 | 1.6 | 3 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| 5 | F | 64 | 12 | <20 | 21 | 7 | 3 | 1 | 19 | 17 | 11 | 15 | 6.9 | ND | <3.5 | <3.5 | 3.7 | 2.1 | 1.6 | 2.4 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 6 | F | 67 | 5 | <20 | 30 | 23 | 8 | 13 | 23 | 29 | 25 | 27 | 30.9 | ND | 16.6 | 33.7 | 5.4 | 3.9 | 3 | 3.7 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 |
| 7 | F | 66 | 18 | 422 | 79 | 76 | 52 | 41 | 28 | 25 | 23 | 26 | 45.8 | ND | 10 | 10.6 | 6.6 | 6.2 | 5.7 | 5.6 | 3 | 2 | 1 | 1 | 3 | 2 | 2 | 2 |
| 8 | M | 55 | 30 | <20 | 75 | 59 | 49 | 40 | 71 | 31 | 25 | 11 | 71 | ND | <3.5 | 4 | 7.4 | 6.1 | 6.3 | 5.1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 |
| 9 | F | 54 | 7 | 186 | 60 | 30 | 19 | 10 | 58 | 18 | 12 | 7 | 9.2 | ND | <3.5 | <3.5 | 5.9 | 3.7 | 3.5 | 3.3 | 3 | 1 | 1 | 1 | 2 | 1 | 0 | 0 |
| 10 | M | 64 | 4 | 1150 | 67 | 70 | 45 | 18 | 36 | 34 | 27 | 41 | 26.3 | ND | 11 | 20.4 | 5.9 | 6.1 | 5 | 4.6 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 0 |
| 11 | M | 64 | 5 | 74 | 48 | 36 | 28 | 24 | 23 | 13 | 15 | 25 | 5.9 | ND | 32.1 | <3.5 | 5.7 | 4.5 | 4.5 | 4.6 | 2 | 1 | 2 | 3 | 2 | 2 | 2 | 2 |
| 12 | F | 61 | 5 | 182 | 46 | 30 | 13 | 5 | 11 | 7 | 8 | 9 | <3.5 | ND | <3.5 | <3.5 | 4.7 | 4.1 | 3.6 | 2.6 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 13 | F | 40 | 5 | <20 | 38 | 20 | 12 | 8 | 28 | 14 | 13 | 19 | 18.6 | ND | <3.5 | <3.5 | 4.8 | 3.4 | 2.9 | 2.9 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 14 | F | 67 | 25 | <20 | 58 | 49 | 39 | 14 | 19 | 12 | 7 | 10 | 26.2 | ND | 6.6 | <3.5 | 5.6 | 4.7 | 4 | 3 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 1 |
| 15 | F | 39 | 11 | 31 | 48 | 37 | 20 | 20 | 9 | 5 | 4 | 4 | <3.5 | ND | <3.5 | <3.5 | 5.7 | 4.2 | 3.9 | 3.9 | 2 | 1 | 0 | 0 | 2 | 1 | 1 | 1 |
| 16 | F | 70 | 21 | 1660 | 83 | 72 | 58 | 58 | 51 | 34 | 25 | 25 | 55 | ND | 7.4 | 12.4 | 7.7 | 7.1 | 6.2 | 6.1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 17 | F | 48 | 9 | 60 | 42 | 33 | 20 | 16 | 10 | 7 | 6 | 2 | 7.8 | ND | <3.5 | <3.5 | 4.8 | 4.4 | 3.9 | 2.4 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 18 | M | 69 | 13 | 586 | 60 | 50 | 32 | 23 | 6 | 4 | 2 | 7 | 7.5 | ND | <3.5 | 4.7 | 5.3 | 4.8 | 3.5 | 3.5 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| 19 | F | 45 | 16 | 59 | 65 | 52 | 65 | 40 | 19 | 14 | 9 | 15 | 25.9 | ND | 6.7 | 11.5 | 6.3 | 5.5 | 5.2 | 4.9 | 2 | 1 | 0 | 0 | 2 | 2 | 2 | 2 |
| 20 | F | 72 | 23 | 554 | 80 | 40 | 30 | 20 | 40 | 34 | 16 | 19 | 18.5 | ND | 3.9 | <3.5 | 7.5 | 4.8 | 4.1 | 3.6 | 3 | 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| 21 | F | 62 | 2 | 22 | 45 | 32 | 15 | 7 | 16 | 5 | 5 | 8 | 13.6 | ND | <3.5 | <3.5 | 4.7 | 3.4 | 2.5 | 2.1 | 3 | 1 | 0 | 0 | 2 | 1 | 0 | 0 |
| 22 | F | 41 | 5 | 136 | 73 | 59 | 40 | 18 | 39 | 8 | 7 | 8 | 40.7 | ND | <3.5 | <3.5 | 7.4 | 5.4 | 4.3 | 3.5 | 0 | 0 | 0 | 0 | 3 | 2 | 1 | 0 |
| 23 | F | 68 | 6 | 660 | 52 | 25 | 15 | 9 | 9 | 4 | 2 | 6 | 9.3 | ND | <3.5 | 5.2 | 5.3 | 3.8 | 2.6 | 3.1 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 0 |
| 24 | F | 45 | 1 | <20 | 65 | 35 | 28 | 23 | 42 | 17 | 39 | 28 | 7.1 | ND | <3.5 | <3.5 | 6.6 | 4.7 | 4.5 | 4.3 | 2 | 0 | 0 | 0 | 2 | 1 | 1 | 2 |
| Median | 61.5 | 10 | — | 58.5 | 36.5 | 27 | 18 | 21.5 | 12.5 | 10 | 10.5 | 16.05 | — | <3.5 | <3.5 | 5.6 | 4.3 | 3.75 | 3.4 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | |
| SD | 10.6 | 7.9 | 17.1 | 18.9 | 17.1 | 14.3 | 17.1 | 10.6 | 9.8 | 9.7 | 17.4 | 6.7 | 7.6 | 1.1 | 1.3 | 1.3 | 1.2 | 0.9 | 0.7 | 0.7 | 0.9 | 0.8 | 0.6 | 0.6 | 0.8 | |||
| P25–75 | 48–67 | 5–17 | 43.5–70 | 27.5–51 | 14–40 | 8.5–23 | 11.5–37.5 | 6–21.5 | 6–21.5 | 7–22 | 7.65–26.5 | <3.5–7.55 | <3.5–7.9 | 4.75–6.45 | 3.55–5.1 | 2.75–4.5 | 2.5–4.45 | 2–3 | 1–1.5 | 0–1 | 0–1 | 2–3 | 1–1.5 | 0–1 | 0–1 | |||
CRP, C reactive protein; DAS28, Disease Activity Score for 28 joints; ESR, erythrocyte sedimentation rate; F, female; M, male; ND, not done; P25–75, between 25th and 75th centile; PDS, power Doppler sonography; RF, rheumatoid factor; T0, baseline; T2, week 2; T6, week 6; T12, week 12; VAS, visual analogue scale.
*VAS for global assessment of disease activity ranges from 0 (no disease activity) to 100 (extreme disease activity).
†Score system ranging from 0 to 3.
Clinical findings
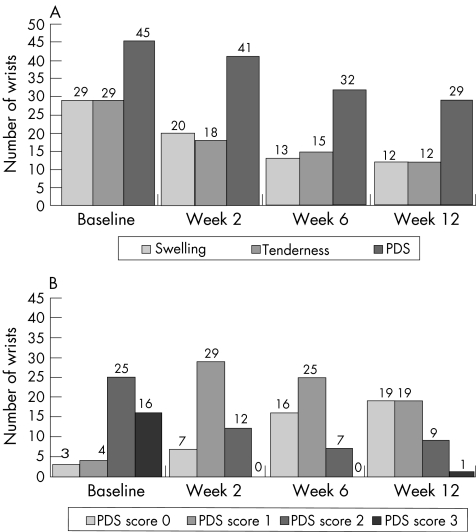
With respect to baseline findings, at all follow‐up examinations (weeks 2, 6 and 12), a significant reduction in all clinical (p<0.001) and laboratory (p<0.001) parameters of disease activity was found (table 1, fig 1A). No patients required a change in their maintenance steroid dose during the 12 weeks of the study.
Figure 1 (A) Number of wrists showing swelling, tenderness and power Doppler sonography (PDS) signal at baseline and at follow‐up examinations. (B) Distribution of the number of wrists with different PDS score at baseline and at follow‐up examinations.
PDS reproducibility
The intraobserver κ value for agreement for scoring PDS signals at the wrist level in 12 patients with rheumatoid arthritis was 0.894 (standard error (SE) 0.153).
PDS findings at baseline
At baseline, PDS detected abnormal synovial perfusion in 45 of 48 wrists (93.7%) of 23 of 24 patients (95.8%) with at least one wrist affected. Most of these wrists showed a high degree of synovial perfusion: 16 wrists (33.3%) had a PDS score of 3 and 25 wrists (52.1%) had a score of 2. In 3 wrists (6.3%), no PDS signal was detected (fig 1).
PDS findings at week 2
At week 2, a significant reduction in PDS score was found (p<0.001). No wrists showed a PDS score of 3, the number of wrists with a PDS score of 2 was less than half of the number at baseline and the number of those with a PDS scores of 0 had more than doubled from that at baseline.
A decrease of at least one point in PDS score was found in 38 wrists (79.2%). PDS score decreased by 2 points in 11 wrists (22.9%; fig 2A,B). In 10 wrists (20.8%), no changes in PDS signals were detected and no wrists showed an increase in PDS score.
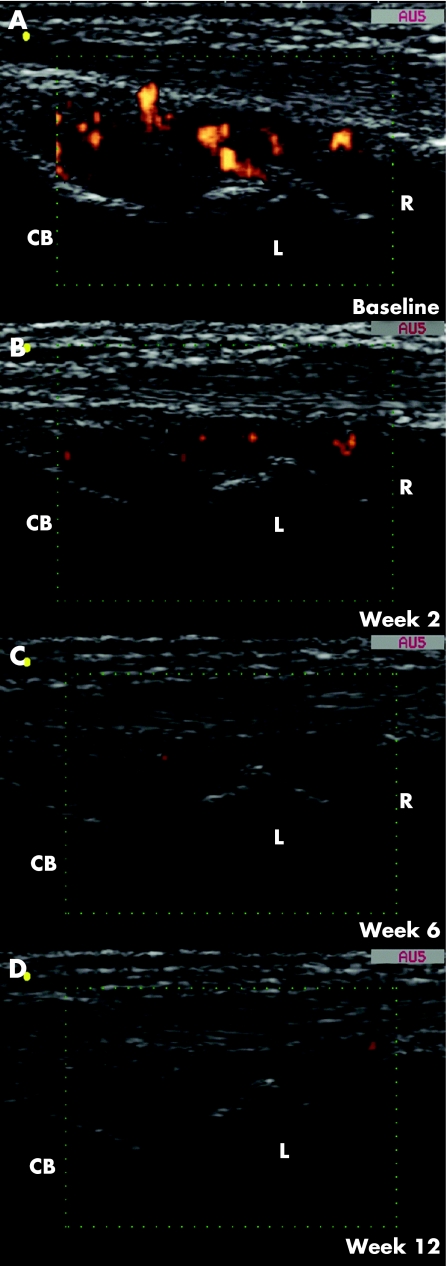
Figure 2 Representative power Doppler sonography (PDS) images obtained on longitudinal dorsal scan of the dominant wrist in a 62‐year‐old woman with a 2‐year history of disease (patient number 21). (A) Baseline PDS examination showed a marked increment in synovial tissue perfusion (PDS score 3). Follow‐up examinations at weeks (B) 2, (C) 6 and (D) 12 detected a clearly evident decrease in PDS signals (PDS score was 1, 0 and 0, respectively). CB, capitate bone; L, lunate bone; R, radius.
PDS findings at week 6
At week 6, a significant change from baseline was found in PDS signals (p<0.001). PDS scores showed a decrease from the baseline value of at least 1 point in 39 wrists (81.3%) and decreased by at least 2 points in 22 wrists (45.8%). In 3 wrists (6.3%), PDS score decreased by 3 points (fig 2A,C). No PDS signal changes were detected in 7 wrists (14.6%). In 2 wrists (4.2%), PDS signals increased by 1 point.
PDS findings at week 12
After 12 weeks of treatment, PDS signals continued to maintain a significant reduction (p<0.001). With respect to baseline data, PDS score was decreased in 38 wrists (79.2%), remained unchanged in 7 wrists (14.6%) and increased (by 1 point) in 3 wrists (6.3%). In 5 wrists (10.4%), PDS score decreased by 3 points (fig 2A,D).
In 19 wrists (39.6%) of 12 of 24 patients (50%), PDS score was 0. Ongoing synovitis was evident, as detected by PDS in 29 wrists (60.4%).
Relationship between PDS and clinical data
In total 192 clinical and PDS examinations were conducted. Table 2 shows the relationship between PDS, and both joint swelling and tenderness.
Table 2 Frequency table and χ2 test for correlation between presence and absence of power Doppler sonography signals, and both joint swelling* (A) and joint tenderness† (B).
| PDS signal | |||
|---|---|---|---|
| Absent | Present | Total n(%) | |
| Joint swelling | |||
| Absent | 32 | 86 | 118 (61.5%) |
| Present | 13 | 61 | 74 (38.5%) |
| Total n (%) | 45 (23.4%) | 147 (76.6%) | 192 |
| Joint tenderness | |||
| Absent | 35 | 89 | 124 (64.6%) |
| Present | 10 | 58 | 68 (35.4%) |
| Total n (%) | 45 (23.4%) | 147 (76.6%) | 192 |
PDS, power Doppler sonography.
*χ2 = 1.810; p = 0.178.
†χ2 = 3.752; p = 0.053.
We found a tendency to positive correlation between reduction in PDS score obtained in the dominant wrists and improvement in DAS28 score at week 2 (Spearman's r = 0.382; p = 0.067), but not at week 6 (Spearman's r = 0.178; p = 0.4) and at week 12 (Spearman's r = 0.256; p = 0.22).
Discussion
Over the past few years, PDS has proved to be a sensitive tool for assessing disease activity at small‐joint level, by detecting short‐term reduction in synovial perfusion and showing underestimated persistence of active synovitis in patients with chronic arthritis receiving steroids or biological treatment.7,8,9,10
In a recent study,7 our group evaluated PDS signals in the small joints of hands, wrists and feet in 20 patients with chronic arthritis, and showed a high positive interobserver reproducibility (the interobserver κ value for agreement between the two examiners for PDS findings was 0.953 (SE 0.159) at baseline and 0.901 (SE 0.201) at follow‐up examinations, with κ = 1 showing the perfect agreement). A good interobserver reproducibility has also been reported by other investigators.6,15
A limitation of this study is the fact that PDS signals were not assessed in the periarticular soft tissues. This relates to the methods used, which relies on the previous experience reporting a good interobserver reproducibility only in assessing intra‐articular PDS signals.7
To the best of our knowledge, this is the first study aimed at assessing the ability of PDS to evaluate changes in synovial perfusion induced by adalimumab in patients with rheumatoid arthritis. The potential value of PDS in treatment monitoring has been evaluated in only a limited number of patients with rheumatoid arthritis treated with steroids or other anti‐tumour necrosis factor α agents.7,8,9,10
At follow‐up examinations after 2 weeks of treatment, a decrease of at least 1 point in PDS score was detected in 79% of wrists. This improvement was comparable with that induced by an intra‐articular steroid injection in the small joints of patients with chronic synovitis.7
Interestingly, at week 12, the number of wrist joints with a PDS score of 0 was six times that at baseline. Although 60% of the wrists still showed the presence of PDS signals, only one wrist had a PDS score of 3, and 66% of wrists positive for the presence of intra‐articular PDS signal had a PDS score of 1.
The correlation between PDS score and changes in DAS28, although positive, is not strong. In fact, there were patients with a consistent improvement in DAS28 score, whose synovial tissue at the wrist level still showed the presence of PDS signals. This can be explained by the fact that PDS is a sensitive tool for showing even minimal increases in synovial perfusion at the small‐joint level, which can be missed by both clinical and laboratory evaluations.
Our study suggests that PDS is a feasible and sensitive imaging tool for assessing the response to treatment of synovitis at the small‐joint level. In particular, we have shown that the wrist joint is a suitable anatomical site to be assessed by PDS for detecting changes in synovial perfusion induced by systemic drug treatment.
Ongoing follow‐up will add further insight into the persistence of considerable reductions in PDS scores and their correlation with DAS28. In particular, long‐term follow‐up will provide information on the predictive value of rapid PDS signal reduction for sustained remission of the disease at the small‐joint level.
Abbreviations
CRP - C reactive protein
DAS28 - Disease Activity Score for 28 joints
DMARD - disease‐modifying antirheumatic drug
ESR - erythrocyte sedimentation rate
PDS - power Doppler sonography
Footnotes
Funding: This study was supported by the Università Politecnica delle Marche grant number 1104 (29 March 2001).
Competing interests: None declared.
References
- 1.Weinblatt M E, Keystone E C, Furst D E, Moreland L W, Weisman M H, Birbara C A.et al Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 20034835–45. [DOI] [PubMed] [Google Scholar]
- 2.Grassi W, Filippucci E. Is power Doppler sonography the new frontier in therapy monitoring? Clin Exp Rheumatol 200321424–428. [PubMed] [Google Scholar]
- 3.Szkudlarek M, Court‐Payen M, Strandberg C, Klarlund M, Klausen T, Østergaard M. Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum 2001442018–2023. [DOI] [PubMed] [Google Scholar]
- 4.Walther M, Harms H, Krenn V, Radke S, Faehndrich T P, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 200144331–338. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt W A, Volker L, Zacher J, Schlafke M, Ruhnke M, Gromnica‐Ihle E. Colour Doppler ultrasonography to detect pannus in knee joint synovitis. Clin Exp Rheumatol 200018439–444. [PubMed] [Google Scholar]
- 6.Fiocco U, Ferro F, Cozzi L, Vezzu M, Sfriso P, Checchetto C.et al Contrast medium in power Doppler ultrasound for assessment of synovial vascularity: comparison with arthroscopy. J Rheumatol 2003302170–2176. [PubMed] [Google Scholar]
- 7.Filippucci E, Farina A, Carotti M, Salaffi F, Grassi W. Grey scale and power Doppler sonographic changes induced by intra‐articular steroid injection treatment. Ann Rheum Dis 200463740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hau M, Kneitz C, Tony H P, Keberle M, Jahns R, Jenett M. High resolution ultrasound detects a decrease in pannus vascularisation of small finger joints in patients with rheumatoid arthritis receiving treatment with soluble tumour necrosis factor a receptor (etanercept). Ann Rheum Dis 20026155–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor P C, Steuer A, Gruber J, Cosgrove D O, Blomley M J K, Marsters P A.et al Comparison of ultrasonographic assessment of synovitis and joint vascularity with radiographic evaluation in a randomized, placebo‐controlled study of infliximab therapy in early rheumatoid arthritis. Arthritis Rheum 2004501107–1116. [DOI] [PubMed] [Google Scholar]
- 10.Ribbens C, Andre B, Marcelis S, Kaye O, Mathy L, Bonnet V.et al Rheumatoid hand joint synovitis: gray‐scale and power Doppler US quantifications following anti‐tumor necrosis factor‐alpha treatment: pilot study. Radiology 2003229562–569. [DOI] [PubMed] [Google Scholar]
- 11.Gordon D A, Hastings D E. Rheumatoid arthritis and spondyloarthropathy. Clinical features: early, progressive and late disease, In: Klippel JH, Dieppe PA, eds. Rheumatology. London: Mosby, 19985.5.4
- 12.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 13.Prevoo M L L, van't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B A, van Riel P L C M. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 14.Rubin J M, Adler R S, Fowlkes J B, Spratt S, Pallister J E, Chen J F.et al Fractional moving blood volume: estimation with power Doppler US. Radiology 1995197183–190. [DOI] [PubMed] [Google Scholar]
- 15.Szkudlarek M, Court‐Payen M, Jacobsen S, Klarlund M, Thomsen H S, Østergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum 200348955–962. [DOI] [PubMed] [Google Scholar]