Abstract
Objectives
To evaluate whether T cell activation, as reflected by levels of soluble interleukin 2 receptor (sIL2R), soluble CD30 (sCD30), IL‐10 and B cell activator of the tumour necrosis factor family (BAFF) at diagnosis and during initial follow‐up, is predictive for persistent or renewed antineutrophil cytoplasmic antibody (ANCA) positivity and clinical relapse in patients with vasculitis associated with proteinase 3‐antineutrophil cytoplasmic antibodies (PR3‐ANCA).
Methods
87 Patients with PR3‐ANCA‐associated vasculitis and at least 2 years of follow‐up were included in the study. At diagnosis, and at 3, 6, 12, 18 and 24 months after diagnosis, cytoplasmic ANCA titres were detected by indirect immunofluorescence (IIF), and PR3‐ANCA, sIL2R, sCD30, IL‐10 and BAFF levels were assessed by ELISA. 31 healthy volunteers provided plasma samples for comparison. Levels of immune markers were related to ANCA positivity and relapse during follow‐up.
Results
Plasma levels of sIL2R, sCD30 and BAFF were higher in patients than in controls at all time points. Plasma levels of sIL2R, sCD30 and IL‐10 were higher at diagnosis and relapse than during remission. At 18 months, sCD30 (p<0.001) and sIL2R levels (p = 0.01) were significantly higher in PR3‐ANCA‐positive patients (detected by ELISA) than in PR3‐ANCA‐negative patients. ANCA‐positive patients detected by ELISA or IIF at 24 months had significantly higher plasma sCD30 levels (p = 0.02 and p = 0.03, respectively) than ANCA‐negative patients.
Conclusion
Increased T cell activation in patients with ANCA‐associated vasculitis in remission during and after immunosuppressive treatment is associated with persistent or renewed ANCA positivity.
Antineutrophil cytoplasmic antibodies (ANCA) to myeloperoxidase and proteinase 3 are associated with primary vasculitides affecting small to medium‐sized vessels.1,2 With current immunosuppressive induction treatments, most patients with these diseases can be brought into stable remission. PR3‐ANCA‐positive patients, however, are particularly prone to relapse of disease during long‐term follow‐up when immunosuppressive treatment is tapered and finally stopped.3 Recently, we showed that persistence of a positive ANCA titre in these patients after induction of remission by cyclophosphamide, when treatment is switched to azathioprine, is associated with an increased risk for relapse.4 Additionally, we found that positive cytoplasmic antineutrophil cytoplasmic antibodies (C‐ANCA) and PR3‐ANCA titres at 3, 12, 18 and 24 months after diagnosis are also associated with relapse.5 We concluded from these data that ANCA positivity in patients reflects smouldering immune activation and is, therefore, associated with an increased risk for disease relapse when immunosuppressive treatment is tapered and stopped. Serological markers of immune activation are, indeed, present in patients with active ANCA‐associated vasculitis. Notably, serum levels of soluble interleukin 2 receptor (sIL2R) and soluble CD30 (sCD30) are raised during active disease, reflecting T cell activation.6,7,8,9 These have been proposed as markers of disease activity. Moreover, activated T cells can be found not only in active disease but also during remission.10 Recently, Ohlsson et al11 reported that the presence of low plasma levels of the immunosuppressive Th2 cytokine IL‐10 during remission is associated with an increased risk for subsequent relapse in ANCA‐associated vasculitis. In addition to activated T cells, patients with active ANCA‐associated vasculitis have increased numbers of activated B cells. In other autoimmune diseases, raised plasma levels of the polyclonal B cell stimulator B cell activating factor of the tumour necrosis factor family (BAFF) have been related to B cell activation.12,13,14 BAFF stimulates B cells, promoting their survival, and BAFF itself might be produced by activated T cells.15,16,17 As BAFF is produced by activated T cells, it might link activated T cells to B cell activation.
We hypothesise that patients with PR3‐ANCA‐associated vasculitis, who remain ANCA positive after induction of remission, might have ongoing smouldering immune activation with an associated increased risk for relapse. In this study, we measured serial levels of the immune markers sIL2R, sCD30, IL‐10 and BAFF in patients with PR3‐ANCA‐associated vasculitis up to 24 months after diagnosis. We assessed their association with ANCA status, disease activity and relapse rate during long‐term follow‐up. We observed that ANCA‐positive patients during remission had persisting increased T cell activation after tapering of immunosuppressants.
Patients and methods
Patients and ANCA assessment
Eighty seven patients diagnosed with PR3‐ANCA‐associated vasculitis at the University Medical Center Groningen, (Groningen, The Netherlands) between January 1991 and March 2002, and being followed up for at least 2 years, were included in this retrospective study. Table 1 shows the data of these patients.
Table 1 Characteristics of patients.
| Parameters at diagnosis | All patients (n = 87) |
|---|---|
| Sex (male:female)* | 55 (63):32 (37%) |
| Age (years)† | 55 (14–80) |
| ANCA titre (IIF) | 1/320 |
| CRP (mg/l)‡ | 126.3 (96.7) |
| Creatinine (μmol/l)† | 117 (40–918) |
| BVAS† | 25 (7–48) |
| Organ involvement* | |
| ENT, n (%) | 77 (89) |
| Lung, n (%) | 46 (53) |
| Kidney, n (%) | 67 (77) |
ANCA, antineutrophil cytoplasmic antibodies; BVAS, Birmingham Vasculitis Activity Score; CRP, C reactive protein; ENT, ear nose throat; IIF, indirect immunofluorescence.
*Values are n (%).
†Values are median (range).
‡Mean (SD).
Patients were followed up until March 2004. In all patients, the presence of PR3‐ANCA was confirmed by antigen‐specific ELISA.1 Induction treatment consisted of oral cyclophosphamide (2 mg/kg body weight) and prednisolone (1 mg/kg body weight; maximal dose 60 mg/day). Doses of cyclophosphamide were adjusted to maintain the white cell count >4×109/l. After 4–6 weeks, the daily prednisolone dose was tapered by 10 mg every 2 weeks until the dose reached 30 mg, and thereafter by 5 mg every 2–4 weeks. Once remission was achieved, the daily dose of oral cyclophosphamide was tapered by 25 mg every 3 months between 1991 and 1996. From 1997, patients were switched to azathioprine (1.5–2 mg/kg body weight daily) after 3 months of stable remission, with tapering by 25 mg every 3 months.
All patients received co‐trimoxazole (960 mg three times a week) for prevention against Pneumocystiscarinii. Thirteen patients received additional maintenance treatment with higher dosages of cotrimoxazole (960 mg twice daily). Owing to the clinical severity of their disease, 15 patients were treated additionally with plasma exchange or methylprednisolone infusions (1000 mg on three consecutive days).
Disease activity was scored at diagnosis, during follow‐up and at relapse, using the Birmingham Vasculitis Activity Score (BVAS).18 At diagnosis, at 3, 6, 12, 18 and 24 months after diagnosis, and at relapse, serum ANCA titres were determined by indirect immunofluorescence (IIF), as described previously,19 and EDTA plasma specimens were collected from each patient. Sera were considered to be positive for ANCA when a cytoplasmic staining pattern (C‐ANCA) was present at a dilution of at least 1:40. Plasma samples were centrifuged at 3000 rpm for 10 min, and supernatants were stored at −80°C until use. In plasma samples, PR3‐ANCA levels were determined by direct PR3‐ANCA ELISA, as described previously.20 Values were expressed as arbitrary units/ml; samples with values ⩾10 U/ml were considered to be positive. In all, 31 healthy laboratory workers matched for sex and age (13 women and 18 men; median age 51 (range 44–67) years) volunteered and provided EDTA plasma specimens for comparison.
Definitions
Remission: The absence of clinical signs and symptoms of active vasculitis (BVAS = 0) in combination with a normal C reactive protein (CRP) level (<10 mg/l).
Relapse: Clinical signs of vasculitic activity in combination with vasculitic disease activity, or the occurrence of nodular pulmonary lesions after exclusion of infectious or malignant diseases.
Renal vasculitic disease: Necrotising glomerulonephritis, or a combination of microscopic glomerular erythrocyturia, erythrocyte cell casts, proteinuria and a decrease in creatinine clearance.21
Detection of sIL2R, sCD30, IL‐10 and BAFF
sIL2R (R&D Systems, Oxon, UK), sCD30 (Bender MedSystems, Vienna, Austria) and IL‐10 (Mabtech, Strand, Sweden) were detected by commercial sandwich ELISAs according to the manufacturer's instructions. Lowest levels of detection of the ELISAs were 32 pg/ml for sIL2R, 0.5 U/ml for sCD30 and 0.5 pg/ml for IL‐10. Plasma BAFF levels were measured by a sandwich ELISA, developed in our laboratory, using a monoclonal antibody as a capturing antibody and a biotinylated polyclonal antibody as a detecting antibody. In brief, 96‐well plates (Nunc A/S, Roskilde, Denmark) were coated overnight at 4°C with 4 μg/ml mouse anti‐human BAFF (clone 36006.211; R&D Systems). After washing, plates were blocked with 2% bovine serum albumin and 0.05% Tween 20 in phosphate‐buffered saline for 60 min. Human plasma samples and recombinant BAFF were diluted in high‐performance ELISA buffer (Sanquin, Amsterdam, The Netherlands), added to the plate and incubated at room temperature for 1 h. After washing, bound BAFF was detected by a 1‐h incubation with biotinylated goat anti‐human BAFF (PeproTech, Rocky Hill, New Jersey, USA); samples were incubated with 0.125 μg/ml peroxidase‐conjugated streptavidin (Sanquin) for 30 min, and the colour reaction was seen with tetramethylbenzidine (Roth, Karlsruhe, Germany). The colorimetric reaction was stopped by the addition of 100 μl/well 0.5 M 2 N H2SO4. Adsorption at 450/575 nm was measured with a microplate reader. Sensitivity of this ELISA to detect BAFF was 0.15 ng/ml. Plasma concentrations were determined by interpolation from a standard curve.
Statistical analysis
For comparison of paired data the Wilcoxon signed rank test was used; for unpaired data the Mann–Whitney U test was used. For multiple comparisons the non‐parametric Kruskal–Wallis test was used, followed by Dunn's post‐test. Correlation coefficients were calculated by Spearman's test. For calculation of relapse‐free survival, survival curves were calculated using Kaplan–Meier estimates for survival distribution. Differences between groups regarding survival were analysed using the log rank test, with disease‐free survival as the dependent variable. The end point for survival analysis was the occurrence of relapse. A two‐sided p<0.05 was considered to be significant.
Results
Clinical characteristics
In all, 52 of the 87 patients (60%) included had one or more relapses of disease: 26 patients (63%) from the group that switched to azathioprine maintenance (n = 41) and 26 patients (57%) from the group taking cyclophosphamide (n = 46). Table 1 shows the clinical characteristics of patients at diagnosis. Overall actuarial relapse‐free survival was 72% at 24 months and 34% at 60 months after diagnosis. Baseline characteristics and overall relapse‐free survival did not differ between the cyclophosphamide maintenance group and the azathioprine maintenance group. Also, clinical characteristics at diagnosis did not differ between patients with (n = 52) and without relapse (n = 35) during follow‐up. Table 2 shows the clinical characteristics of patients at relapse.
Table 2 Clinical characteristics of patients at relapse.
| Parameters at relapse | Patients with relapse (n = 52) |
|---|---|
| C‐ANCA titre (IIF) | 1/320 |
| CRP (mg/l)† | 50.8 (53) |
| Creatinine (μmol/l)* | 118 (71–1243) |
| BVAS* | 12.5 (3–24) |
| Organ involvement‡ | |
| ENT | 34 (65) |
| Lung | 5 (10) |
| Kidney | 33 (63) |
BVAS, Birmingham Vasculitis Activity Score; C‐ANCA, cytoplasmic antineutrophil cytoplasmic antibodies; CRP, C reactive protein; ENT, ear nose throat; IIF, indirect immunofluorescence assay.
*Values are median (range).
†Mean (SD).
‡Values are n (%).
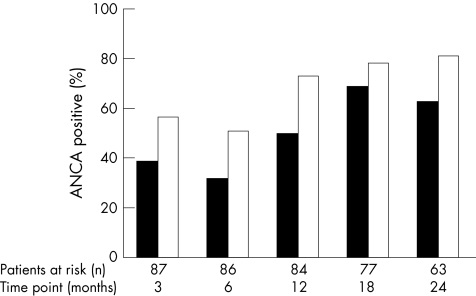
After starting immunosuppressive treatment, many patients became negative for ANCA: at 6 months, 49% were ANCA‐negative by ELISA and 69% were ANCA‐negative by IIF (fig 1). Subsequently, an increasing number again became ANCA‐positive during follow‐up (fig 1).
Figure 1 Antineutrophil cytoplasmic antibody (ANCA) status during follow‐up. Percentage of patients at risk for relapse who are positive for cytoplasmic antineutrophil cytoplasmic antibody (detected by indirect immunofluorescence; ⩾1:40; filled bar) or for proteinase 3‐antineutrophil cytoplasmic antibodies (detected by direct ELISA; ⩾10 U/ml; open bar) during follow‐up, and at 3, 6, 12, 18 and 24 months after diagnosis.
Soluble interleukin 2 receptor
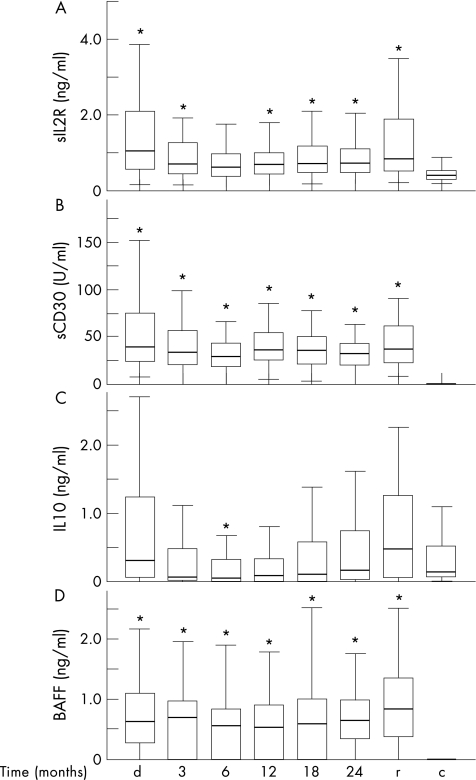
We measured levels of the T cell activation marker sIL2R during follow‐up in PR3‐ANCA‐associated vasculitis. Plasma sIL‐2R levels in patients with PR3‐ANCA‐associated vasculitis at diagnosis and during follow‐up were significantly higher than in controls (p<0.01), except at 6 months after diagnosis (fig 2A). At diagnosis and at relapse, plasma sIL‐2R levels correlated significantly with BVAS (r = 0.46, p<0.001; r = 0.40, p<0.01, respectively; table 3).
Figure 2 Plasma levels of soluble interleukin 2 receptor (sIL2R), soluble CD30 (sCD30), interleukin 10 (IL‐10) and B cell activator of the tumour necrosis factor family (BAFF). Box plots indicate 5–95% range (error bars), 25–75% range (boxes) and median value (horizontal lines) of plasma levels of (A) sIL2R, (B) sCD30, (C) IL‐10 and (D) BAFF in 87 patients with proteinase 3‐antineutrophil cytoplasmic antibodies (PR3‐ANCA)‐associated vasculitis at diagnosis (d), and during follow‐up at 3, 6, 12, 18 and 24 months after diagnosis for patients who had not yet had a relapse. In addition, levels are depicted at relapse (r; n = 52) and in controls (c; n = 31). *p<0.05 as compared with controls (Kruskal–Wallis, Dunn's post‐test).
Table 3 Correlations between plasma levels of immune markers and disease activity BVAS at diagnosis and relapse.
| Diagnosis (n = 87) | Relapse (n = 52) | |
|---|---|---|
| sIL2R | 0.46*** | 0.40** |
| sCD30 | 0.40** | 0.09 |
| IL‐10 | −0.20 | 0.12 |
| BAFF | −0.09 | 0.11 |
| CRP | 0.36** | 0.46** |
BAFF, B cell activator of the tumour necrosis factor family; CRP, C reactive protein; IL‐10, interleukin 10; sCD30, soluble CD30; sIL2R, soluble interleukin receptor.
**p<0.01, ***p<0.001.
Plasma sIL2R levels at diagnosis were significantly higher than at all time points during follow‐up (p<0.05)—except for 24 months after diagnosis, when levels did not significantly differ from sIL2R levels at diagnosis (p = 0.31; fig 2A). sIL2R levels fell after starting immunosuppressive treatment, but, subsequently, rose again. Accordingly, at 12, 18 and 24 months after diagnosis, sIL‐2R levels were significantly higher than at 6 months after diagnosis (p = 0.03, p = 0.006 and p = 0.007, respectively).
sIL2R levels as measured at several time points during the first 24 months of follow‐up did not differ between patients with a relapse within 5 years after diagnosis and those who did not have a relapse.
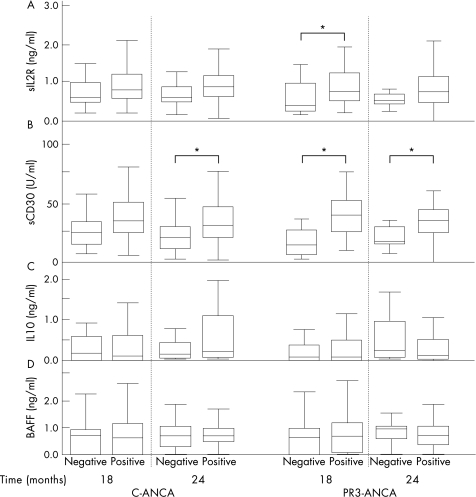
We analysed whether sIL‐2R levels differed between ANCA‐positive and ANCA‐negative patients during follow‐up. Only at 18 months after diagnosis, sIL‐2R levels were significantly higher in patients positive for PR3‐ANCA (detected by ELISA) than in PR3‐ANCA‐negative patients (p = 0.022; fig 3A). At all other time points during follow‐up—that is, at 3, 6, 12 and 24 months after diagnosis—ANCA‐positive patients did not have significantly higher levels of sIL2R than ANCA‐negative patients.
Figure 3 Plasma levels of soluble interleukin 2 receptor (sIL2R), soluble CD30 (sCD30), interleukin 10 (IL‐10) and B cell activator of the tumour necrosis factor family (BAFF) in patients positive and negative for cytoplasmic antineutrophil cytoplasmic antibody (C‐ANCA) and proteinase 3‐antineutrophil cytoplasmic antibodies (PR3‐ANCA) at 18 and 24 months of follow‐up. Box plots indicate 5–95% range (error bars), 25–75% range (boxes) and median value (horizontal lines) of plasma levels of (A) sIL2R, (B) sCD30, (C) IL‐10 and (D) BAFF (D) in C‐ANCA‐positive patients (detected by indirect immunofluorescence ) and PR3‐ANCA (detected by ELISA) as compared with patients negative for ANCA at 18 and 24 months after diagnosis. *p<0.05 (Mann–Whitney U test).
Plasma levels of sIL2R correlated significantly (p<0.01) with sCD30 levels, but not with CRP levels at diagnosis and at all time points during follow‐up.
Soluble CD30
Similar to sIL2R, sCD30 is a marker of T cell activation. We evaluated sCD30 levels during follow‐up in patients with PR3‐ANCA‐associated vasculitis. Plasma levels of sCD30 were significantly higher in patients with PR3‐ANCA‐associated vasculitis, both at diagnosis and during follow‐up, than in controls (p<0.001; fig 2B). sCD30 could be detected in only 3 of 31 controls. Patients with PR3‐ANCA‐associated vasculitis had higher sCD30 levels at diagnosis than at all time points during follow‐up (p<0.05)—except for 24 months after diagnosis, when this difference was not significant (p = 0.054). At diagnosis, sCD30 levels correlated significantly with BVAS (r = 0.40, p = 0.003; table 3). In contrast, sCD30 levels were not significantly higher at relapse than during follow‐up, and did not correlate with BVAS (r = 0.09, p = 0.56). sCD30 levels during follow‐up did not differ between patients with a relapse and those who did not have a relapse. sCD30 levels at diagnosis were, however, significantly higher in patients who later had a relapse (median 56.6, range 9.5–310.7 U/ml) than in those who did not have a relapse (median 32.0, range 7.4–106.0 U/ml). Consequently, at diagnosis, patients with an sCD30 level higher than median (39.3 U/ml) had a significantly higher risk relapse than those with sCD30 levels lower than median (relative risk (RR) 3.3, 95% confidence interval (CI) 1.1 to 4.6; p = 0.01).
Additionally, we analysed whether sCD30 levels were associated with ANCA status. Plasma levels of sCD30 did not differ significantly between C‐ANCA‐positive patients (detected by IIF) or PR3‐ANCA positive (detected by ELISA) and ANCA‐negative patients at 3, 6 and 12 months after diagnosis (fig 3B). Patients who were positive for PR3‐ANCA at 18 or 24 months had significantly raised sCD30 plasma levels compared with PR3‐ANCA‐negative patients (difference in sCD30 levels at 18 months, p<0.001; difference in sCD30 levels at 24 months, p = 0.031; fig 3B). Patients who were positive for PR3‐ANCA by ELISA at 18 months had significantly higher sCD30 levels at 12 months after diagnosis (p = 0.034). At 24 months after diagnosis, patients positive for C‐ANCA (by IIF) had significantly higher plasma sCD30 levels than C‐ANCA‐negative patients (p = 0.025).
sCD30 levels during follow‐up correlated significantly with sIL2R and CRP. At diagnosis and relapse, sCD30 levels also correlated significantly with sIL2R levels but not with CRP levels.
Interleukin 10
IL‐10 is a Th2 cytokine with immunosuppressive qualities. We studied IL‐10 levels longitudinally, and evaluated to what extent increased levels during follow‐up might protect against disease relapse in PR3‐ANCA‐associated vasculitis.
At diagnosis, IL‐10 levels were significantly higher than at 3 and 6 months after diagnosis (both p<0.01). In patients with PR3‐ANCA‐associated vasculitis, IL‐10 levels were significantly lower at 6 months after diagnosis than in controls (p<0.05; fig 2C). IL‐10 levels at relapse were significantly higher than at all time points during follow‐up (p<0.01). Plasma IL‐10 levels increased during follow‐up. At 24 months after diagnosis, when immunosuppressive treatment had been stopped, IL‐10 levels were significantly higher than at 3, 6, 12 and 18 months after diagnosis (p<0.05; fig 2C).
Patients who had a relapse within 60 months of follow‐up had significantly lower IL‐10 levels at 3 months after diagnosis than those who did not have a relapse. At 3 months after diagnosis, patients with IL‐10 levels higher than the median (61 pg/ml) were associated with a significantly reduced risk relapse compared with IL‐10 levels lower than the median (RR 0.56, 95% CI 0.31 to 0.98; p = 0.044).
Plasma IL‐10 levels at all time points during follow‐up did not differ between patients who were either positive or negative for ANCA as determined by ELISA and IIF.
B cell activator of the tumour necrosis factor family
The polyclonal B cell stimulator BAFF has been associated with autoimmunity. We measured plasma levels of BAFF in patients with PR3‐ANCA‐associated vasculitis and assessed the relationship between BAFF and disease activity, as well as between BAFF and ANCA levels.
Plasma levels of BAFF were significantly higher at all time points in patients than in controls (p<0.001; fig 2D). In patients with ANCA‐associated vasculitis, BAFF levels tended to be higher at diagnosis than at 12 months after diagnosis (p = 0.051). BAFF levels were significantly higher at 18 months than at 12 months after diagnosis (p = 0.03; fig 2D). BAFF levels in patients who had a relapse did not differ significantly from BAFF levels in patients who did not have a relapse. Plasma levels of BAFF did not differ between patients positive and negative for ANCA, as determined by ELISA or IIF.
Discussion
Several observations suggest T cell involvement in ANCA‐associated vasculitis. Activated T cells are present in lesions, and T cells are necessary to produce high‐affinity ANCA. Additionally, raised plasma levels of the T cell activation markers sIL2R and sCD30 have been reported in ANCA‐associated vasculitis, and levels correlated with disease activity.6,8,9 Plasma levels of both sIL2R and sCD30 differed between limited and generalised disease, as well as between active and inactive disease.6,8,9 Also in this study, sIL2R and sCD30 levels at diagnosis correlated with disease activity. Plasma levels of sIL2R and sCD30 were higher at diagnosis than during follow‐up. Although immunosuppressive treatment resulted in a decrease, plasma levels remained raised as compared with levels in controls. Levels of both sIL2R and sCD30 subsequently increased while tapering and eventually stopping immunosuppressive treatment, reflecting increased T cell activity in patients who had remission that is not completely suppressed or abrogated by treatment. These findings are in line with the observation that increased percentages of activated T cells are seen in patients with ANCA‐associated vasculitis in remission.10 Levels of sIL2R and sCD30, but not of BAFF, were associated with positive ANCA serology during follow‐up. At 18 and at 24 months after diagnosis, patients positive for C‐ANCA (detected by IIF) and those positive for PR3‐ANCA (detected by ELISA) had higher levels of sIL2R and sCD30 than those negative for ANCA. Therefore, ANCA positivity seems to be part of a broader, possibly T‐cell‐driven, immune activation seen in patients with ANCA‐associated vasculitis.
Obviously, clinical interpretation of levels of immune markers can be complicated by intercurrent infections. Additionally, our study is limited by its retrospective nature. Nevertheless, the association of T cell activation with the PR3‐ANCA autoantibody response suggests an initiating role for T cell activation in PR3‐ANCA‐associated vasculitis. However, levels of sIL2R and sCD30 did not differ between patients with PR3‐ANCA‐associated vasculitis who had a relapse and those who did not have a relapse during follow‐up. In contrast, Schmitt et al6 found that patients with Wegener's granulomatosis who had a relapse had significantly higher sIL2R levels before disease exacerbation than patients who did not have a relapse.
As current immunosuppressive regimens are insufficient to maintain long‐lasting remission in most patients with PR3‐ANCA‐associated vasculitis, identification of patients at risk for relapse is important. Our data suggest that the persistent presence of raised levels of markers for T cell activation, although related with ANCA status, does not identify patients at risk for relapse.
In contrast with sIL2R and sCD30, IL‐10 is a cytokine with immunosuppressive and anti‐inflammatory properties. T regulatory cell subsets, among other cells, produce IL‐10.22 Additionally, IL‐10 is associated with type 2 T helper cells and can promote polyclonal hypergammaglobulinaemia.23 In systemic lupus erythematosus (SLE), IL‐10 levels reflect disease activity.24 We found that IL‐10 levels in patients with ANCA‐associated vasculitis were raised at diagnosis and subsequently decreased. At 6 months after diagnosis, IL‐10 levels were significantly lower than in controls. Additionally, low levels of IL‐10 3 months after diagnosis were associated with increased relapse rate during the 60 months of follow‐up. Recently, Ohlsson et al11 reported on the same association between low IL‐10 levels in patients with ANCA‐associated vasculitis 3 months before relapse. In addition, in patients with Wegener's granulomatosis and those with polyangiitis, a shift towards the homozygotic AA genotype has been found in the IL‐10 (−1082) polymorphism.25 In vitro, this genotype correlates with low IL‐10 secretion by Concanavalin A‐stimulated peripheral blood mononuclear cells of healthy people.26 These data and our clinical findings suggest that low plasma levels of IL‐10 influence disease development and exacerbation.
Whereas T cells are operative in production of ANCA, other factors—for example, BAFF—may be associated with it as well. BAFF is a member of the tumour necrosis factor (TNF) ligand superfamily and is expressed by monocytes, macrophages, dendritic cells and, possibly, activated T cells.16,17,27 As BAFF stimulates all B cells and thereby promotes their survival,15 it is thought to contribute to autoimmunity by stimulating autoreactive B cells. In patients with SLE, rheumatoid arthritis and Sjögren's syndrome, raised serum levels of BAFF have been found. Levels correlated with serum immunoglobulin (Ig)G levels and anti‐double‐stranded DNA antibody titres among patients with SLE, and correlated with rheumatoid factor titres among seropositive patients with rheumatoid arthritis.12,28 Moreover, in patients with Sjögren's syndrome, BAFF levels correlated with both anti‐Sjögren's syndrome A and anti‐Sjögren's syndrome B antibodies and with rheumatoid factor. Recently, two groups reported on raised BAFF levels in Wegener's granulomatosis.29,30 We found that BAFF levels were raised in patients with PR3‐ANCA‐associated vasculitis as compared with controls. However, BAFF levels did not correlate with ANCA titres, and ANCA‐positive patients did not have higher BAFF levels than ANCA‐negative patients. Also, in previous studies, no correlation was found between B cell activation and serum ANCA levels, rheumatoid factor, IgM and IgG in patients with Wegener's granulomatosis.10 The data, therefore, suggest that raised BAFF levels are rather a result of a stimulated immune system than a factor related to the PR3‐ANCA response or to disease activity.
In summary, in PR3‐ANCA‐associated vasculitis, markers of T cell activation are increased at diagnosis and correlate with disease activity. Although immunosuppressive treatment results in a decrease of these markers, T cell activity does not normalise. On tapering and stopping immunosuppression, levels of T cell activation markers rise again, reflecting increased T cell activity. This increased T cell activity is associated with ANCA positivity, and should be viewed as a result of a T‐cell‐driven immune response, which could form the basis of this disease.
Acknowledgements
J‐SFS is an MD clinical research trainee sponsored by ZonMw.
We thank the doctors who took care of the patients included in this study: CA Stegeman, RT Gansevoort and JW Cohen Tervaert.
Abbreviations
ANCA - antineutrophil cytoplasmic antibody
BAFF - B cell activator of the tumour necrosis factor family
BVAS - Birmingham Vasculitis Activity Score
C‐ANCA - cytoplasmic antineutrophil cytoplasmic antibody
CRP - C reactive protein
IIF - indirect immunofluorescence
PR3‐ANCA - proteinase 3‐antineutrophil cytoplasmic antibodies
sCD30 - soluble CD30
sIL2R - soluble interleukin 2 receptor
SLE - systemic lupus erythematosus
Footnotes
Competing interests: None.
Patients were asked to participate in studies on assessment of serological parameters including soluble IL‐2R, CD30, IL‐10 and BAFF, as parameters for immune activation and disease activity. These studies were approved by the Local Medical Ethical Committee.
References
- 1.Tervaert J W, Goldschmeding R, Elema J D, van der G M, Huitema M G, van der Hem G K.et al Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int 199037799–806. [DOI] [PubMed] [Google Scholar]
- 2.Jennette J C, Falk R J. Small‐vessel vasculitis. N Engl J Med 19973371512–1523. [DOI] [PubMed] [Google Scholar]
- 3.Stegeman C A. Anti‐neutrophil cytoplasmic antibody (ANCA) levels directed against proteinase‐3 and myeloperoxidase are helpful in predicting disease relapse in ANCA‐associated small‐vessel vasculitis. Nephrol Dial Transplant 2002172077–2080. [DOI] [PubMed] [Google Scholar]
- 4.Slot M C, Tervaert J W, Boomsma M M, Stegeman C A. Positive classic antineutrophil cytoplasmic antibody (C‐ANCA) titer at switch to azathioprine therapy associated with relapse in proteinase 3‐related vasculitis. Arthritis Rheum 200451269–273. [DOI] [PubMed] [Google Scholar]
- 5.Sanders J S, Huitma M G, Kallenberg C G, Stegeman C A. Prediction of relapses in PR3‐ANCA‐associated vasculitis by assessing responses of ANCA titres to treatment. Rheumatology (Oxford) 200645724–729. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt W H, Heesen C, Csernok E, Rautmann A, Gross W L. Elevated serum levels of soluble interleukin‐2 receptor in patients with Wegener's granulomatosis. Association with disease activity. Arthritis Rheum 1992351088–1096. [DOI] [PubMed] [Google Scholar]
- 7.Schonermarck U, Csernok E, Trabandt A, Hansen H, Gross W L. Circulating cytokines and soluble CD23, CD26 and CD30 in ANCA‐associated vasculitides. Clin Exp Rheumatol 200018457–463. [PubMed] [Google Scholar]
- 8.Stegeman C A, Tervaert J W, Huitema M G, Kallenberg C G. Serum markers of T cell activation in relapses of Wegener's granulomatosis. Clin Exp Immunol 199391415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Hansen H, Tatsis E, Csernok E, Lemke H, Gross W L. High plasma levels of the soluble form of CD30 activation molecule reflect disease activity in patients with Wegener's granulomatosis. Am J Med 1997102517–523. [DOI] [PubMed] [Google Scholar]
- 10.Popa E R, Stegeman C A, Bos N A, Kallenberg C G, Tervaert J W. Differential B‐ and T‐cell activation in Wegener's granulomatosis. J Allergy Clin Immunol 1999103885–894. [DOI] [PubMed] [Google Scholar]
- 11.Ohlsson S, Wieslander J, Segelmark M. Low IL‐10 levels during remission is associated with a risk of subsequent relapse [abstract]. Kidney Blood Press Res 200326277 [Google Scholar]
- 12.Cheema G S, Roschke V, Hilbert D M, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune‐based rheumatic diseases. Arthritis Rheum 2001441313–1319. [DOI] [PubMed] [Google Scholar]
- 13.Mackay F, Browning J L. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol 20022465–475. [DOI] [PubMed] [Google Scholar]
- 14.Mariette X, Roux S, Zhang J, Bengoufa D, Lavie F, Zhou T.et al The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren's syndrome. Ann Rheum Dis 200362168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do R K, Hatada E, Lee H, Tourigny M R, Hilbert D, Chen‐Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 2000192953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore P A, Belvedere O, Orr A, Pieri K, LaFleur D W, Feng P.et al BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 1999285260–263. [DOI] [PubMed] [Google Scholar]
- 17.Schneider P, Mackay F, Steiner V, Hofmann K, Bodmer J L, Holler N.et al BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 19991891747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luqmani R A, Bacon P A, Moots R J, Janssen B A, Pall A, Emery P.et al Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med 199487671–678. [PubMed] [Google Scholar]
- 19.Cohen Tervaert J W, Mulder L, Stegeman C, Elema J, Huitema M, The H.et al Occurrence of autoantibodies to human leucocyte elastase in Wegener's granulomatosis and other inflammatory disorders. Ann Rheum Dis 199352115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boomsma M M, Stegeman C A, van der Leij M J, Oost W, Hermans J, Kallenberg C G.et al Prediction of relapses in Wegener's granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum 2000432025–2033. [DOI] [PubMed] [Google Scholar]
- 21.Stegeman C A, Tervaert J W, de Jong P E, Kallenberg C G. Trimethoprim‐sulfamethoxazole (co‐trimoxazole) for the prevention of relapses of Wegener's granulomatosis. Dutch Co‐Trimoxazole Wegener Study Group. N Engl J Med 199633516–20. [DOI] [PubMed] [Google Scholar]
- 22.Moore K W, de Waal M R, Coffman R L, O'Garra A. Interleukin‐10 and the interleukin‐10 receptor. Annu Rev Immunol 200119683–765. [DOI] [PubMed] [Google Scholar]
- 23.Llorente L, Zou W, Levy Y, Richaud‐Patin Y, Wijdenes J, Alcocer‐Varela J.et al Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med 1995181839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houssiau F A, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer J P, Renauld J C. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus 19954393–395. [DOI] [PubMed] [Google Scholar]
- 25.Bartfai Z, Gaede K I, Russell K A, Murakozy G, Muller‐Quernheim J, Specks U. Different gender‐associated genotype risks of Wegener's granulomatosis and microscopic polyangiitis. Clin Immunol 2003109330–337. [DOI] [PubMed] [Google Scholar]
- 26.Turner D M, Williams D M, Sankaran D, Lazarus M, Sinnott P J, Hutchinson I V. An investigation of polymorphism in the interleukin‐10 gene promoter. Eur J Immunogenet 1997241–8. [DOI] [PubMed] [Google Scholar]
- 27.Shu H B, Hu W H, Johnson H. TALL‐1 is a novel member of the TNF family that is down‐regulated by mitogens. J Leukoc Biol 199965680–683. [PubMed] [Google Scholar]
- 28.Zhang J, Roschke V, Baker K P, Wang Z, Alarcon G S, Fessler B J.et al Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 20011666–10. [DOI] [PubMed] [Google Scholar]
- 29.Edberg J C, Zhou T, Aksu K, Kimberly R P, Hoffman G S, St Clair E W.et al Levels of circulating B lymphocyte stimulator (BLyS) are elevated in patients with Wegener's granulomatosis [abstract]. Kidney Blood Press Res 200426256 [Google Scholar]
- 30.Krumbholz M, Specks U, Kalled S, Jenne D E, Meinl E. BAFF is elevated in serum of patients with Wegener's granulomatosis [abstract]. Kidney Blood Press Res 200426256. [DOI] [PubMed] [Google Scholar]