Abstract
Background
Annexin A5 is thought to have a role in the pathophysiology of the antiphospholipid syndrome (APS)—a syndrome characterised by recurrent thrombosis and pregnancy morbidity.
Objective
To investigate whether anti‐annexin A5 immunoglobulin (Ig)M or IgG antibodies, or the −1C→T polymorphism of annexin A5, is a risk factor for thrombosis or miscarriage, and whether the −1C→T polymorphism is correlated with APS.
Methods
A cohort study was carried out with a population of 198 patients with primary APS, systemic lupus erythematosus or lupus‐like disease. For the detection of anti‐annexin A5 antibodies and the measurement of annexin A5 plasma levels, ELISA‐type methods were used. The annexin A5 −1C→T mutation was detected by restriction fragment length polymorphism.
Results
71 patients were positive for annexin A5 IgM or IgG antibodies, of whom 53 patients were positive for anti‐annexin A5 IgG antibodies and 27 of 198 patients were positive for anti‐annexin A5 IgM antibodies. The prevalence of IgM or IgG anti‐annexin A5 antibodies was not significantly associated with thrombosis or miscarriage on multivariate analysis. The prevalence of the −1C→T mutation in the annexin A5 gene (46/198 patients) was significantly associated with miscarriage (odds ratio 2.7, 95% confidence interval 1.1 to 6.7, independent risk factor).
Conclusion
The detection of anti‐annexin A5 antibodies does not seem relevant for estimating the risk for thrombosis or miscarriage in APS. The −1C→T mutation was an independent risk factor for miscarriage, which is independent of APS.
The antiphospholipid syndrome (APS) is a systemic autoimmune disease characterised by the combined presence of antiphospholipid antibodies in plasma of patients with thrombosis or specific pregnancy morbidity.1 The term antiphospholipid antibody is in fact a misnomer because these antibodies are directed towards plasma proteins with affinity for negatively charged phospholipids, of which β2‐glycoprotein I (β2‐GPI) seems to be the most important.2 Owing to its high affinity for negatively charged phospholipids and its proposed role in haemostasis, it is hypothesised that, besides β2‐GPI, annexin A5 also has a role in the pathophysiology of APS.3
Annexin A5 was discovered more than 20 years ago, but its physiological function is still unclear.4 Several functions have been assigned to annexin A5 on the basis of its ability to bind with high affinity to negatively charged phospholipids. A recent in vitro study showed that annexin A5 exhibits anticoagulant properties in vitro by forming a two‐dimensional crystal on negatively charged spots in the cellular membrane, creating a sort of “anticoagulant shield”.5 Antibodies with reactivity for β2‐GPI can disturb this anticoagulant shield resulting in increased thrombin generation.6 This increase in thrombin generation is in agreement with results of earlier publications on findings with cultured trophoblasts and endothelial cells.7
A possible role for annexin A5 in the pathology of APS was further strengthened by publications describing significant correlations between the presence of anti‐annexin A5 antibodies in patients' plasma samples and a history of pregnancy morbidity. However, data are conflicting.8,9,10,11,12,13 On the basis of immunohistochemical procedures, several studies found a reduction in annexin A5 on the placental villi from placentas of patients with APS compared with those from women who had miscarriages in the absence of antiphospholipid antibodies, but results are contradictory.14,15,16,17
The most recent suggestion that endogenous annexin A5 is associated with the regulation of haemostasis came from studies on the annexin A5 gene. Gonzalez‐Conejero et al18 reported that a common polymorphism (−1C→T) in the Kozak sequence (the sequence in front of the coding sequence that facilitates translation) of the annexin A5 gene has a protective effect against arterial thrombosis in young adults (<45 years). Changes in the Kozak sequence may have important consequences for the translation of genes. So far, the results of Gonzales‐Conejero et al have not been confirmed.19,20,21
Altogether, the role of annexin A5 in the pathology of APS is controversial. Therefore, we used a cohort of 198 patients with systemic lupus erythematosus (SLE), lupus‐like disease (LLD) or primary APS to investigate whether anti‐annexin A5 immunoglobulin (Ig)M or IgG antibodies are a risk factor for thrombosis or miscarriage. Furthermore, we investigated whether the presence of these antibodies affected plasma annexin A5 levels and determined the prevalence of the −1C→T mutation in our patient cohort.
Patients and methods
Patients
One hundred and ninety eight patients with various autoimmune diseases were included in this study. These patients were seen consecutively at the lupus clinic of the University Medical Center Utrecht, Utrecht, The Netherlands. All patients gave informed consent. Patients with SLE met at least four American College of Rheumatology criteria for the classification of SLE, and patients with LLD met one to three of these criteria.22 Patients with primary APS had antiphospholipid antibodies and a history of thrombosis or miscarriage in the absence of other signs for a systemic autoimmune disease.23
Blood samples were collected by venepuncture, using plastic tubes containing 3.8% trisodium citrate (0.129 mol/l) as the anticoagulant (9:1, vol/vol). To obtain platelet‐poor plasma, the samples were centrifuged twice at 2000 g for 10 min and subsequently stored at −50°C until use. The number of objectively verified thromboembolic events was assessed by reviewing the patient records as described before.24 Pregnancy morbidity was determined in accordance with the clinical criteria of APS.23 For isolation of DNA, EDTA was used as an anticoagulant. Mononuclear cells were isolated by the Ficoll‐Isopaque method and suspended in RPMI with 10% fetal calf serum; dimethylsulphoxide with 40% fetal calf serum was added and stored in liquid nitrogen until DNA extraction.
Coagulation assays
Lupus anticoagulant
Lupus anticoagulant (LAC) was measured using a partial thromboplastin time‐lupus anticoagulant (PTT‐LA) test and a dilute Russell's viper venom time (RVVT) test. Patients were classified as positive for LAC when they had a positive PTT‐LA or a positive dilute RVVT test. The PTT‐LA (Diagnostica Stago, Gennevillier, France) test was carried out as described before.24 The dilute RVVT test was carried out according to the instructions of the manufacturer (Gradipore, North Ryde, New South Wales, Australia) and considered positive when LAC screen/LAC confirm >1.20.
Serological assays
Anticardiolipin antibody ELISA
Anticardiolipin antibodies were measured using ELISA as described previously.25 IgG/IgM calibrators were used to report anticardiolipin antibody levels as G phospholipid or M phospholipid units. Concentrations >10 G phospholipid or 10 M phospholipid units were considered positive.
Anti‐β2‐GPI antibody ELISA
Antibodies against β2‐GPI were measured using ELISA as described previously.26 Plasma‐purified β2‐GPI (10 μg/ml diluted in Tris‐buffered saline (TBS)) was incubated in a high‐binding ELISA plate (Costar, New York, USA). After 1 h the plates were washed with 0.1% Tween in TBS (washing solution) and incubated with 4% bovine serum albumin in TBS for 1 h (blocking buffer). The plates were washed again, followed by incubation with plasma from the patient (1:50 diluted in blocking buffer) for 1 h. Then the plates were washed and incubated with a goat‐anti‐human alkaline‐phosphatase‐labelled antibody (diluted 1:1000, Biosource, Camarillo, California, USA), followed by staining with p‐nitrophenylphosphate (0.4 mg/ml). A sample was considered positive if the optical density was >3 standard deviations (SD) above the mean optical density obtained with plasma from 40 healthy volunteers (controls).
Antiprothrombin antibody ELISA
For the detection of antiprothrombin antibodies, plasma‐purified prothrombin (50 μg/ml in TBS/5 mM CaCl2) was added to the plates and incubated for 1 h (Costar).26 After washing the plates, blocking was carried out with 0.1% Tween/1% bovine serum albumin/5 mM CaCl2/TBS for 1 h. The plates were washed and plasma (1:50 diluted in blocking buffer) was incubated for 1 h. Subsequently, the plates were washed and incubated with a goat‐anti‐human alkaline‐phosphatase‐labelled antibody (diluted 1:1000, Biosource). Staining was carried out using p‐nitrophenylphosphate as described above. Plasma samples were considered positive when the measured absorption values were higher than the mean value (3 SD) measured with 42 normal plasma samples.
Anti‐annexin A5 antibody ELISA
For the detection of IgG/IgM antibodies directed against annexin A5 we used a prototype commercial ELISA assay (Diagnostica Stago; fig 1). The source of annexin A5 coated on to the ELISA plates was human recombinant annexin A5. Plasma (200 μl/well, diluted 1:101 in dilution buffer) and anti‐annexin A5 IgM/IgG calibrators were incubated in the preblocked ELISA plate for 1 h at room temperature. The ELISA plates were washed five times with 300 μl/well washing solution (provided with the ELISA plates) between each incubation. To detect the anti‐annexin A5 antibodies bound to the coated annexin A5, either a peroxidase‐labelled anti‐human IgG antibody or anti‐human IgM antibody (200 μl/well) was incubated in the plate for 1 h at room temperature. Colouring was carried out by the addition of 200 μl 3,3′,5,5′‐tetramethylbenzidine substrate; the reaction was stopped by the addition of 50 μl 1 M H2SO4. Absorbance was read at 450 nm. Cut‐off levels (mean absorbance (3 SD)) were determined by measuring the absorbance of 40 controls.
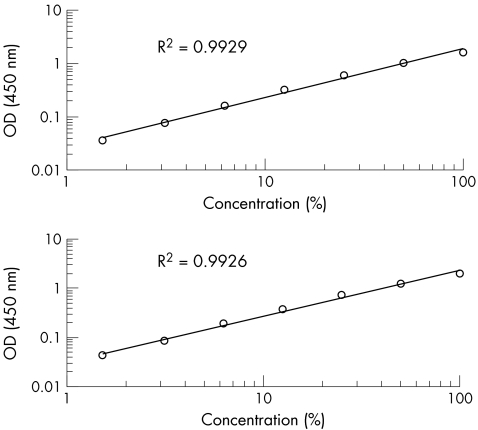
Figure 1 Analytical sensitivity of the anti‐annexin A5 antibody ELISA. Ig, immunoglobulin; OD, optical density. To test the quality of every ELISA plate used, of either the IgG or IgM type, we used a high‐level positive internal control sample on the plate. We incubated several dilutions of a positive control sample, and furthermore used a negative internal control sample. For the detection of antibodies bound to the plate, peroxidase‐labelled anti‐human IgG or IgM antibodies were used. By polynomial first‐order equation, an average R2 of 0.9929 was calculated for the anti‐annexin A5 IgG ELISA, and 0.9926 for the anti‐annexin A5 IgM ELISA.
Annexin A5 levels in plasma
Annexin A5 levels were measured with “a sandwich‐ELISA‐type” assay as described previously.27 In short, a polyclonal antibody directed towards annexin A5 was coated to the plate, and annexin A5 from plasma was detected by a peroxidase‐conjugated monoclonal antibody (RU‐Wac2a). Recombinant annexin A5 was used for the calibration curve.
Detection of −1C→T polymorphism by restriction fragment length polymorphism
Exon 2 of the annexin A5 gene was amplified from the genomic DNA by polymerase chain reaction (PCR). The PCR was carried out in a final volume of 50 μl reaction mixture containing 1 μl genomic DNA (50 ng), 1.5 U Taq DNA polymerase (Pharmacia, Markham, Ontario, Canada), 5 μl PCR reaction buffer containing 1.5 mM MgCl2, 0.2 mM of each deoxyribonucleotide triphosphate and 10 pmol of forward (CGCTAAGCCCGAGGTTTCT) and reverse (CGCAGCATACAAAGTTGTGG) primers. The PCRs were carried out in a Perkin‐Elmer 9700 thermocycler (PE Applied Biosystems, Foster City, California, USA). Cycling conditions for PCR were as follows: an initial denaturation at 94°C for 10 min followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 61°C for 30 s and elongation at 72°C for 30 s. The last cycle was followed by an additional elongation step of 72°C for 7 min and a cooling step to 4°C. The amplicons were subsequently cut with the restriction enzyme NcoI. The digests were analysed by polyacrylamide gel electrophoresis (7%) after staining by ethidium bromide. Impaired cleavage occured in the presence of the −1C→T polymorphism.
Statistics
In this cross‐sectional study, we used multivariate logistic regression to relate the prevalence of thrombosis to serological findings. We used SPSS V.11.0 for the calculations of the odds ratios (ORs), 95% confidence intervals and the significance of the calculated ORs. Student's t test or the Mann–Whitney U test was used to calculate differences between two groups; p⩽0.05 was considered significant.
Results
Characterisation of patients
The total population consisted of 198 patients: 176 patients had SLE, 6 patients had primary APS and 16 patients had LLD. One hundred and eighty patients were women. The whole population had a median age of 34 years. Table 1 shows the prevalence of the −1C→T polymorphism and all serological factors. Sixty patients had a history of either venous or arterial thrombosis. Of these 60 patients, 50 had antiphospholipid antibodies (positive in the anticardiolipin ELISA (n = 46) or LAC assay (n = 36)) in their plasma. Seventy three of 180 female patients had been pregnant and 24 patients met the obstetric criteria of APS. Of these, 19 had antiphospholipid antibodies (positive in the anticardiolipin ELISA (n = 16) or LAC assay (n = 10)) in their plasma.
Table 1 Distribution of serological factors and −1C→T polymorphism in the study population.
| Thrombosis (arterial or venous) | Arterial thrombosis | Venous thrombosis | Pregnancy morbidity | |
|---|---|---|---|---|
| Total population, n = 198 | 60 | 32 | 38 | 24 |
| DRVVT, n = 61 | 41 | 21 | 27 | 11 |
| APTT, n = 58 | 36 | 19 | 22 | 10 |
| LAC, n = 63 | 41 | 21 | 27 | 11 |
| aCL IgG, n = 102 | 44 | 21 | 29 | 15 |
| aCL IgM, n = 66 | 32 | 16 | 20 | 12 |
| aCL IgG/IgM, n = 112 | 46 | 22 | 30 | 16 |
| Anti‐β2‐GPI IgG, n = 52 | 32 | 16 | 21 | 12 |
| Anti‐β2‐GPI IgM, n = 28 | 17 | 9 | 10 | 9 |
| Anti‐β2‐GPI IgG/IgM, n = 62 | 36 | 19 | 22 | 14 |
| Antiprothrombin IgG, n = 73 | 31 | 14 | 24 | 10 |
| Antiprothrombin IgM, n = 42 | 21 | 8 | 14 | 6 |
| Antiprothrombin IgG/IgM, n = 95 | 38 | 18 | 27 | 12 |
| Anti‐annexin A5 IgG, n = 53 | 19 | 10 | 14 | 10 |
| Anti‐annexin A5 IgM, n = 27 | 13 | 4 | 9 | 4 |
| Anti‐annexin A5 IgG/IgM, n = 71 | 27 | 12 | 20 | 12 |
| Annexin A5 −1C→T polymorphism, n = 46 | 15 | 10 | 8 | 10 |
aCL, anticardiolipin antibody; APTT, activated partial thromboplastin time; β2‐GPI, β2‐glycoprotein I; DRVVT, dilute Russell's viper venom time; Ig, immunoglobulin; LAC, lupus anticoagulant; RVVT, Russell's viper venom time.
APTT interassay coefficient of variation (CV): 2.0%; aCL CV: 6.9%; anti‐β2‐GPI antibody CV: 11.5%; RVVT CV: 3.2%.
Prevalence and clinical significance of anti‐annexin A5 IgG/IgM antibodies
Fifty three patients (26.8%) were positive for anti‐annexin A5 IgG antibodies and 27 (13.6%) for anti‐annexin A5 IgM antibodies (table 1). The OR calculated by multivariate logistic regression for anti‐annexin A5 IgG antibodies and miscarriage is 2.0 (95% CI 0.8 to 5.3; table 2). When the cut‐off level for anti‐annexin A5 IgG antibodies was raised from the mean (3 SD) to mean (10 SD) of 40 controls, the OR increased to 2.7 (95% CI 0.9 to 8.0). We found no significant association between anti‐annexin A5 IgM antibodies and miscarriage (OR 0.8, 95% CI 0.2 to 3.7; table 2).
Table 2 Odds ratio for anti‐annexin A5 IgG/IgM antibodies or −1C→T polymorphism for miscarriage and thrombosis.
| Miscarriage | Thrombosis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Lower | Upper | Lower | Upper | |||
| Anti‐annexin A5 IgG | 2.0 | 0.8 | 5.3 | 1.0 | 0.4 | 2.3 |
| Anti‐annexin A5 IgM | 0.8 | 0.2 | 3.7 | 2.1 | 0.6 | 6.8 |
| −1C→T polymorphism of annexin A5 | 2.7* | 1.0 | 6.7 | 1.1 | 0.4 | 2.6 |
| aCL IgG | 0.9 | 0.3 | 2.8 | 1.2 | 0.5 | 3.1 |
| aCL IgM | 0.6 | 0.1 | 2.7 | 1.3 | 0.5 | 3.6 |
| Anti‐β2‐GPI IgG | 2.5 | 0.8 | 7.9 | 3.3* | 1.4 | 7.9 |
| Anti‐β2‐GPI IgM | 6.1* | 1.2 | 29.7 | 1.1 | 0.3 | 3.8 |
| Antiprothrombin IgG | 0.8 | 0.3 | 2.3 | 1.1 | 0.5 | 2.6 |
| Antiprothrombin IgM | 0.6 | 0.2 | 2.5 | 0.8 | 0.3 | 2.3 |
aCL, anticardiolipin antibody; β2‐GPI, β2‐glycoprotein I; Ig, immunoglobulin.
For the anti‐annexin A5 IgM/IgG antibody ELISA, the cut‐off levels (mean absorbance (3 SD)) were determined by measuring the absorbance of 40 healthy volunteers. ORs were calculated for the IgG class antibodies and the IgM class antibodies by multivariate logistic regression.
*Significant association with recurrent miscarriage or thrombosis (p<0.05).
We did not find an association between thrombosis and anti‐annexin A5 IgG antibodies (OR 1.0, 95% CI 0.4 to 2.3), or between anti‐annexin A5 IgM antibodies and thrombosis (OR 2.1, 95% CI 0.6 to 6.8; table 2).
Annexin A5 levels in plasma
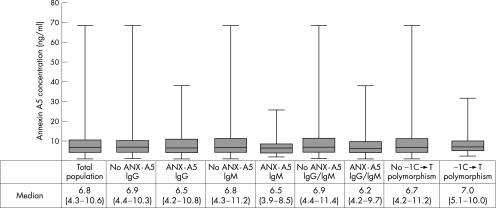
The median (interquartile range (IQR)) annexin A5 plasma concentration was 6.8 ng/ml (4.3–10.6; fig 2).26 Patients with anti‐annexin A5 antibody concentrations of either IgG or IgM class had non‐significant lower annexin A5 concentrations in plasma than patients without these antibodies (median (IQR) 6.2 ng/ml (4.2–9.7) v 6.9 ng/ml (4.4–11.4), respectively, p = 0.24). When we separated anti‐annexin A5 antibodies into anti‐annexin A5 IgM and IgG antibodies, the presence of either class of antibodies resulted in non‐significant lower annexin A5 concentrations (for IgG: IgG− median (IQR) 6.9 ng/ml (4.4–10.3) v IgG+ 6.5 ng/ml (4.2–10.8), p = 0.57; for IgM: IgM− median (IQR) 6.8 ng/ml (4.3–11.2) v IgM+ median 6.5 ng/ml (3.9–8.5), p = 0.44). Patients who had miscarriages did not have significantly different annexin A5 concentrations from patients without pregnancy morbidity (median (IQR) 7.1 ng/ml (4.6–13.8) v 6.8 ng/ml (4.3–10.3), respectively, p = 0.44).
Figure 2 Annexin A5 (ANX‐A5) plasma concentration in measured plasma. ANX‐A5 levels were measured with “a sandwich‐ELISA‐type” assay. ANX‐A5 plasma concentrations are displayed as median (interquartile range). No significant differences were found between groups. Ig, immunoglobulin.
Annexin A5 polymorphism
We found a frequency of the −1C→T transition of 0.23 in the total population of 198 patients. We found no difference in annexin A5 plasma level between patients with the −1C→T polymorphism (n = 46) and patients without this polymorphism (n = 152; median 6.9 v 6.7 ng/ml; fig 2).
In the total population of 198 patients, we found a significant association between the −1C→T polymorphism in annexin A5 and a history of pregnancy morbidity (OR 2.7, 95% CI 1.0 to 6.7; table 2). We did not find a significant relationship between the −1C→T polymorphism and a history of thrombosis (OR 1.1, 95% CI 0.4 to 2.6; table 2). The same holds for arterial thrombosis (OR 1.5, 95% CI 0.6 to 3.7). Furthermore, we did not find a correlation between the presence of anti‐annexin A5 antibodies (IgG, IgM or both) and −1C→T polymorphism.
Discussion
Recently, it has been suggested that annexin A5 has a central role in the pathophysiology of APS.3,7,12,14,28 Owing to contradicting results, there is still a lot of debate about the clinical relevance of anti‐annexin A5 antibodies.8,9,10,11 We found a (non‐significant) trend between the presence of anti‐annexin A5 IgG antibodies and pregnancy morbidity when we raised the cut‐off level of the anti‐annexin A5 IgG assay from the mean (3 SD) to the mean (10 SD) of controls. This may indicate that only high levels of anti‐annexin A5 IgG antibodies in plasma of patients are associated with a risk for miscarriage. Furthermore, we did not find an association between anti‐annexin A5 IgM antibodies and a history of thrombosis (table 2).
As a comment on the study of Gonzalez‐Conejero et al, Kozak concluded from mutagenesis studies that the identity of base −1 has little effect on translation if it would have an effect at all, which is in harmony with the results we found and reported before in controls.18,19,20 We found that the −1C→T mutation in the annexin A5 gene was significantly associated with miscarriage (OR 2.7, 95% CI 1.1 to 6.7; table 2) as an independent risk factor. This association is intriguing and difficult to explain because the −1C→T mutation is not situated in the coding sequence of annexin A5, but in the Kozak sequence that regulates the translation of annexin A5. It might well be that annexin A5 has an important role in placental formation, as the intracellular levels of annexin A5 in trophoblasts are high. In general, annexins regulate membrane aggregation and even membrane fusion, and as such annexin A5 may regulate the formation of syncytium.
Recently, our group reported that both patients with SLE and those with APS had a significant increase in plasma annexin A5 levels compared with controls.27 In this study, we used the same plasma samples to measure the prevalence of anti‐annexin A5 antibodies. In the group of patients with anti‐annexin A5 antibodies there was a non‐significant lower plasma annexin A5 level than that in the group of patients without these antibodies, indicating a higher clearance of annexin A5 by the presence of anti‐annexin A5 antibodies.
From this study, we conclude that the −1C→T mutation is an independent risk factor for pregnancy morbidity, which is probably not related to APS. In the future, more research should be conducted to unravel the exact role of the −1C→T polymorphism in association with recurrent miscarriage. Only the detection of high‐titre IgG anti‐annexin A5 seems to be relevant to detect patients at risk for pregnancy morbidity in APS. The detection of IgG/IgM antibodies with reactivity towards annexin A5 does not seem relevant for the detection of patients at risk for thrombosis.
Acknowledgements
This study was supported by a grant from the Netherlands Organisation for Health Research and Development (ZonMw grant number 902‐26‐290).
Abbreviations
APS - antiphospholipid syndrome
β2‐GPI - β2‐glycoprotein I
IQR - interquartile range
LAC - lupus anticoagulant
LLD - lupus‐like disease
PCR - polymerase chain reaction
PTT‐LA - partial thromboplastin time‐lupus anticoagulant
RVVT - Russell's viper venom time
SLE - systemic lupus erythematosus
TBS - Tris‐buffered saline
Footnotes
Competing interests: All authors except BJW certify that they have no affiliation with or financial involvement in any organisation, or entity with a direct financial interest in the subject matter or materials discussed in this manuscript (eg, employment, consultancies, stock ownership, honoraria), except as disclosed in an attachment or cover letter. BJW is an employee of Stago R&D. He provided the anti‐annexin A5 kits and was of technical assistance in several experiments. Being employed by Stago R&D had no influence on BJW's collection, presentation and interpretation of the data. Any research or project support is identified in the Acknowledgements.
References
- 1.Hughes G R. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. BMJ 19832871088–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil H P, Simpson R J, Chesterman C N, Krilis S A. Anti‐phospholipid antibodies are directed against a complex antigen that includes a lipid‐binding inhibitor of coagulation: beta2‐glycoprotein I (apolipoprotein H). Proc Natl Acad Sci USA 1990874120–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Heerde W L, Lap P, Schoormans S, de Groot P G, Reutelingsperger C P M, Vroom T M. Localization of annexin A5 in human tissue. Annexins 2004137–43. [Google Scholar]
- 4.Volker G, Moss S E. Annexins: from structure to functions. Physiol Rev 200282331–371. [DOI] [PubMed] [Google Scholar]
- 5.Rand J H. Molecular pathogenesis of the antiphospholipid syndrome. Circ Res 20021129–37. [DOI] [PubMed] [Google Scholar]
- 6.Rand J H, Wu X X, Quinn A S, Chen P P, McCrae K R, Bovill E G.et al Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers: evidence from atomic force microscopy and functional assay. Am J Pathol 20031631193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rand J H, Wu X X, Andree H A, Lockwood C J, Guller S, Scher J.et al Pregnancy loss in the antiphospholipid‐antibody syndrome—a possible thrombogenic mechanism. N Engl J Med 199717154–160. [DOI] [PubMed] [Google Scholar]
- 8.Nojima J, Kuratsune H, Suehisa E, Futsukaichi Y, Yamanishi H, Machii T.et al Association between the prevalence of antibodies to beta(2)‐glycoprotein I, prothrombin, protein C, protein S, and annexin V in patients with systemic lupus erythematosus and thrombotic and thrombocytopenic complications. Clin Chem 2001471008–1015. [PubMed] [Google Scholar]
- 9.Arnold J, Holmes Z, Pickering W, Farmer C, Regan L, Cohen H. Anti‐beta 2 glycoprotein 1 and anti‐annexin V antibodies in women with recurrent miscarriage. Br J Haematol 2001113911–914. [DOI] [PubMed] [Google Scholar]
- 10.Gris J C, Quere I, Sanmarco M, Boutiere B, Mercier E, Amiral J.et al Antiphospholipid and antiprotein syndromes in non‐thrombotic, non‐autoimmune women with unexplained recurrent primary early foetal loss. The Nimes Obstetricians and Haematologists Study—NOHA. Thromb Haemost 200084228–236. [PubMed] [Google Scholar]
- 11.Siaka C, Lambert M, Caron C, Amiral J, Hachulla E, Hatron P Y.et al Low prevalence of anti‐annexin V antibodies in antiphospholipid syndrome with fetal loss. Rev Med Interne 199920762–765. [DOI] [PubMed] [Google Scholar]
- 12.Van Eerden P, Wu X, Chazotte C, Rand J H. The role of annexins in pregnancy. Annexins 2004190–98. [Google Scholar]
- 13.Lakos G, Kiss E, Regeczy N, Tarjan P, Soltesz P, Zeher M.et al Antithrombin and antiannexin V antibodies imply risk of thrombosis in patients with systemic autoimmune diseases. J Rheumatol 200027924–929. [PubMed] [Google Scholar]
- 14.Rand J H, Wu X X, Guller S, Gil J, Guha A, Scher J.et al Reduction of annexin‐V (placental anticoagulant protein‐I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol 19941711566–1572. [DOI] [PubMed] [Google Scholar]
- 15.Krikun G, Lockwood C J, Wu X X, Zhou X D, Guller S, Calandri C.et al The expression of the placental anticoagulant protein, annexin V by villous trophoblasts: immunolocalization and in vitro regulation. Placenta 199415601–612. [DOI] [PubMed] [Google Scholar]
- 16.La Rosa L, Meroni P L, Tincani A, Balestrieri G, Faden D, Lojacono A.et al Beta 2 glycoprotein I and placental anticoagulant protein I in placentae from patients with antiphospholipid syndrome. J Rheumatol 1994211684–1693. [PubMed] [Google Scholar]
- 17.Lakasing L, Campa J S, Poston R, Khamashta M A, Poston L. Normal expression of tissue factor, thrombomodulin, and annexin V in placentas from women with antiphospholipid syndrome. Am J Obstet Gynecol 1999181180–189. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez‐Conejero R, Corral J, Roldan V, Martinez C, Marin F, Rivera J.et al A common polymorphism in the annexin V Kozak sequence (−1C>T) increases translation efficiency and plasma levels of annexin V, and decreases the risk of myocardial infarction in young patients. Blood 2002152081–2086. [PubMed] [Google Scholar]
- 19.van Heerde W L, Kenis H, Schoormans S, Lap P, Reutelingsperger C P. The −1C>T mutation in the annexin A5 gene does not affect plasma levels of annexin A5. Blood 2003154223–4224. [DOI] [PubMed] [Google Scholar]
- 20.Kozak M, Neufeld E. Not every polymorphism close to the AUG codon can be explained by invoking context effects on initiation of translation. Blood 20031011202–1203. [DOI] [PubMed] [Google Scholar]
- 21.Kenis H, Doggen C J, Vos H L, Reutelingsperger C P, van Heerde W L. The C‐1T mutation in the annexin A5 Kozak sequence slightly increases the risk of myocardial infarction in men. J Thromb Haemost 200312688–2689. [DOI] [PubMed] [Google Scholar]
- 22.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 23.Wilson W A, Gharavi A E, Koike T, Lockshin M D, Branch D W, Piette J C.et al International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum 1999421309–1311. [DOI] [PubMed] [Google Scholar]
- 24.De Laat H B, Derksen R H W M, Urbanus R T, Roest M, de Groot P G. β2‐glycoprotein I dependent lupus anticoagulant highly correlates with thrombosis in the antiphospholipid syndrome. Blood 20041043598–3602. [DOI] [PubMed] [Google Scholar]
- 25.Derksen R H, Hasselaar P, Blokzijl L, Gmelig Meyling F H, de Groot P G. Coagulation screen is more specific than the anticardiolipin antibody ELISA in defining a thrombotic subset of lupus patients. Ann Rheum Dis 198847364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horbach D A, van Oort E, Donders R C J M, Derksen R H W M, de Groot P G. Lupus anticoagulant is the strongest risk factor for both venous and arterial thrombosis in patients with systemic lupus erythematosus. Thromb Haemost 199676916–992. [PubMed] [Google Scholar]
- 27.Van Heerde W L, Reutelingsperger C P, Maassen C, Lux P, Derksen R H, de Groot P G. The presence of antiphospholipid antibodies is not related to increased levels of annexin A5 in plasma.J Thromb Haemost 20031532–536. [DOI] [PubMed] [Google Scholar]
- 28.Rand J H, Wu X X, Guller S, Scher J, Andree H A, Lockwood C J. Antiphospholipid immunoglobulin G antibodies reduce annexin‐V levels on syncytiotrophoblast apical membranes and in culture media of placental villi. Am J Obstet Gynecol 1997177918–923. [DOI] [PubMed] [Google Scholar]