Abstract
Objectives
To characterise and quantify the CD4+ CD25+ T cell population in patients with systemic lupus erythematosus (SLE) and to detect the possible influence of treatments and clinical manifestations.
Methods
Characterisation of CD25low and CD25high CD4+ T cells from healthy controls and from patients with SLE was carried out using flow cytometry, analysing the expression of activation and differentiation markers. The percentage of both circulating cell subsets was determined in 56 controls and 110 unselected patients with SLE. Data were related to treatment during the past 3 months and to various clinical manifestations.
Results
CD4+ CD25high lymphocytes from controls expressed low levels of CD69, CD154 or CD30, but also expressed glucocorticoid‐induced tumour necrosis factor receptor, high levels of intracellular cytotoxin T lymphocyte‐associated antigen 4, CD45RO and diminished amounts of CD4, all of which are phenotypic characteristics of natural regulatory T cells. CD4+ CD25low cells, on the other hand, expressed the highest levels of activation markers, indicating that they represent recently activated effector cells. Similarly, analysis of cells from patients with SLE showed the same two phenotypically distinguishable CD4+ CD25low and CD4+ CD25high populations, although both expressed slightly increased levels of activation markers. Quantitative analysis showed a considerably raised percentage of CD25low and, especially, CD25high cells in patients with SLE compared with controls. This increment was unrelated to clinical manifestations, but correlated with glucocorticoid treatment. Patients treated with glucocorticoids presented raised levels of CD25high cells, whereas untreated patients and those with anti‐malarial or immunosuppressive drugs had levels similar to those in controls.
Conclusions
The percentage of CD4+ CD25high cells was not altered in non‐steroid‐treated patients, whereas glucocorticoid treatment increased their frequency in patients with SLE.
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease characterised by B cell activation and T helper cell‐dependent production of autoantibodies resulting in immune complex‐mediated tissue damage. Growing evidence suggests that naturally acquired immunological self‐tolerance, in addition to clonal deletion, anergy and ignorance, is accounted for by a population of CD4+ T cells, called natural regulatory T (Treg) cells, which actively suppress the activation and expansion of self‐reactive T cells. These are produced by the normal thymus as functionally mature cells and seed into the periphery, creating a distinct subpopulation of CD4+ T lymphocytes with immunosuppressive qualities.1,2 On isolation and polyclonal T cell stimulation, human CD4+ CD25+ Treg cells were anergic and able to suppress proliferation and cytokine production from both CD4+ and CD8+ T cells in a cell contact‐dependent manner. These Treg cells, naturally present in normal people, can be detected in human peripheral blood by their constitutively high expression of the interleukin (IL)2 receptor α chain (CD25). Furthermore, they are mainly CD45RO, do not present activation markers and constitutively express the tumour necrosis factor (TNF) receptor family member glucocorticoid‐induced tumour necrosis factor receptor (GITR) and high levels of intracellular cytotoxin T lymphocyte‐associated antigen 4 (CTLA4).1,2,3
Although it has been shown in animal models that the depletion of the CD4+ CD25+ Treg population causes autoimmune diseases, their role in the pathogenesis of human autoimmunity has not yet been thoroughly proved. Phenotypic identification and quantification and suppressor functional studies of the CD4+ CD25+ regulatory T cell population have been investigated in several autoimmune diseases, presenting conflicting results in most cases. A higher proportion of functional CD4+ CD25+ cells was observed in the peripheral blood of patients with primary Sjögren's syndrome4 and in the synovial fluid of patients with rheumatoid arthritis5,6 than in controls. However, normal, increased and diminished numbers of Treg cells have been reported in the peripheral blood of patients with rheumatoid arthritis or those with other chronic rheumatic diseases.5,6,7 Similarly, various studies on patients with type 1 diabetes showed differences in the number of CD4+ CD25+ cells and in the level of CD25 expression.8,9,10,11 Normal numbers of Treg cells with normal or diminished suppressor function were reported in patients with multiple sclerosis,12,13 autoimmune polyglandular syndrome14 and myasthenia gravis,15 whereas diminished amounts of the CD4+ CD25+ Treg cell population were reported in patients with autoimmune liver disease.16 Only two studies have analysed Treg cells in patients with SLE, with no conclusive results. Crispin et al17 reported a decreased percentage of CD4+ CD25+ T cells in 10 untreated patients with active disease, whereas Liu et al18 found a normal percentage of CD25+ T cells among CD4+ lymphocytes, but a reduced level in the total peripheral blood mononuclear cells. Discrepancies reported by different studies might be due, at least partly, to technical difficulties in the phenotypic characterisation of Treg cells. As the IL2 receptor α chain (CD25) is transiently up regulated in T cells after activation, circulating CD4+ CD25+ T cells are a heterogeneous population that includes a mixture of cells with effector, regulator and other functions. Moreover, in contrast with rodents, human CD25+ and CD25− CD4+ T cell subsets cannot be clearly defined by flow cytometry, and it has been suggested that only CD4+ CD25high cells possess a suppressive capacity.19 This handicap is even more relevant in the context of an inflammatory autoimmune disease, such as SLE, with a probable increase in the number of circulating activated T lymphocytes.20 Therefore, for Treg cells to be correctly characterised in patients with SLE, the expression levels of activation and differentiation markers among the CD4+ CD25+ T cells expressing low and high levels of CD25 antigen must be examined in patients and controls. Our study investigated the presence and phenotypic characteristics of CD4+ CD25low and CD4+ CD25high T lymphocytes in the peripheral blood of patients with SLE, and their possible association with treatment or with clinical manifestations.
Patients and methods
Patients
The regional ethics committee for clinical investigation approved this study. All patients (n = 110) were recruited from the Asturian Register (Asociacion Lúpicos de Asturias, Oviedo, Spain) of SLE.21 Only those patients who fulfilled at least four of the American College of Rheumatology criteria for SLE were included.22 Information on clinical manifestations (age at diagnosis, disease duration, malar rash, discoid lesions, photosensitivity, oral ulcers, arthritis, serositis, and renal, neurological or haematological disorder) was obtained after a detailed review of clinical history. In addition, at the time of sampling, patients were asked precise questions regarding the treatment received during the past 3 months. Anti‐double‐stranded DNA (dsDNA) antibodies at the time of sampling were quantified by ELISA (ELiA dsDNA, Pharmacia, Freiburg, Germany). Table 1 shows the demographic and clinical characteristics of the patients. Matched healthy controls (n = 56) were recruited from the Asturias Blood Transfusion Centre, Oviedo, Spain. Consent was obtained from patients and controls before their participation in the study.
Table 1 Characteristics and disease parameters of patients with systemic lupus erythematosus.
| Total patients with SLE | 110 |
| Women:men | 104:6 |
| Age at diagnosis (mean (SD)), years | 30.01 (12.13) |
| Clinical manifestations, n (%) | |
| Malar rash | 68 (61.8) |
| Discoid lesions | 21 (21.8) |
| Photosensitivity | 66 (60.0) |
| Oral ulcers | 51 (46.4) |
| Arthritis | 84 (76.4) |
| Serositis | 23 (20.9) |
| Renal disorder | 33 (30.0) |
| Neurological disorder | 9 (8.2) |
| Haematological disorder | 59 (53.6) |
| Presence of anti‐dsDNA, n (%) | 38 (34.5) |
| Median (range) level (IU/ml) | 54.45 (20–600) |
| Treatment, n (%) | |
| None or NSAIDs | 19 (17.3) |
| Malaria drugs | 58 (52.7) |
| Glucocorticoids | 56 (50.9) |
| Median (range) dose (mg/day) | 5 (1–40) |
| Immunosuppressive drugs | 18 (16.4) |
| Methotrexate | 7 (6.4) |
| Azathioprine | 6 (5.5) |
| Cyclophosphamide | 2 (1.8) |
| Ciclosporin A | 2 (1.8) |
| Mycophenolate mofetil | 1 (0.9) |
dsDNA, double‐stranded DNA; NSAID, non‐steroidal anti‐inflammatory drug; SLE, systemic lupus erythematosus.
Monoclonal antibodies
Phycoerythrin‐labelled monoclonal anti‐CD154, anti‐CD45RO, anti‐CD25 and anti‐CD152 (CTLA4), fluorescein isothiocyanate (FITC)‐labelled monoclonal anti‐CD69, anti‐CD25 and anti‐CD30, peridin‐chlorophyll protein (PerCP)‐labelled monoclonal anti‐CD4, and isotype‐matched and fluorochrome‐matched control antibodies were purchased from Becton Dickinson Pharmingen (San Diego, California, USA). Monoclonal anti‐GITR FITC antibodies were obtained from R&D Systems Europe (Abingdon, UK).
Phenotype analysis
Peripheral blood samples for immunophenotyping were collected in EDTA anticoagulant. For characterisation of CD4+ CD25+ lymphocytes, whole peripheral whole blood cells from controls were stained with anti‐CD4 PerCP and anti‐CD25 FITC or their respective isotype control antibodies. The lymphocyte population was gated according to forward‐and side‐scattered properties, and CD4+ T cells were gated using anti‐CD4 PerCP antibodies. Isotype‐matched and fluorochrome‐matched controls were used to set up quadrants. According to the intensity of CD25 expression, CD4+ CD25+ T cells were subdivided into CD25low (median fluorescence intensity (MFI)<25) and CD25high (MFI >25) populations. These two cellular subsets were further characterised by multiparametric phenotypic analysis using three‐colour flow cytometry in 18 healthy people and 60 patients with SLE. For intracellular measurement of CTLA4, cells were fixed and permeabilised after staining for CD4 and CD25 and incubated with anti‐CD152 phycoerythrin‐labelled monoclonal antibodies according to the manufacturer's instructions (Fix & Perm Kit; Caltag Laboratories, Burlingame, California, USA). The percentage of CD25low and CD25high T cells in the total CD4+ lymphocyte population was determined in 110 patients with SLE and 56 controls. Peripheral whole blood samples were stained with anti‐CD4 PerCP and either anti‐CD25 FITC or the isotype control. In all, 10 000 CD4+ cells were acquired after gating the lymphocyte population according to forward and side‐scattered properties. Analyses were carried out on a FACScan flow cytometer with CellQuest software (Becton Dickinson Pharmingen). Results are expressed as the percentage of cells or as the MFI of the gated population.
Statistical analysis
Differences in the percentage of CD4+ CD25+ T cells and the expression of activation and differentiation markers (MFI) between controls and patients with SLE were analysed using the non‐parametric Mann–Whitney U test. Correlations between the percentage of CD4+ CD25+ T cells and clinical parameters were carried out using Spearman's rank correlation test. Association between the use of corticosteroids and the presence of increased amounts of CD4+ CD25high T cells in patients was determined by binary logistic regression modelling, using the percentage of CD4+ CD25high T cells as the dichotomous dependent variable and patients without corticosteroid treatment as reference. SPSS V.12.0 was used for all calculations.
Results
Phenotypic characteristics and frequency of CD4+ CD25high T lymphocytes in patients with SLE
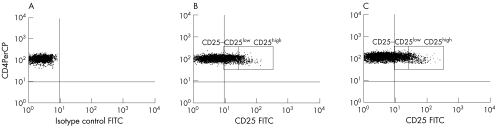
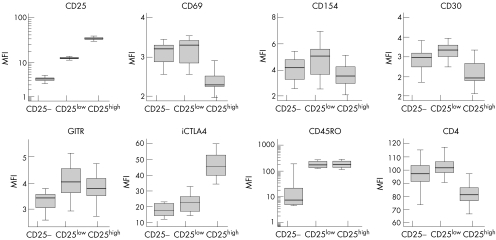
To determine the size of the Treg population in patients with SLE and controls, we analysed the percentage of gated CD4+ T lymphocytes expressing CD25 according to the regions shown in fig 1. CD25 is a marker for Treg cells, but it is also transiently up regulated on T cells on activation. However, it has been previously shown that only those cells expressing high levels of CD25 (CD25high) efficiently suppress proliferative responses, thus being considered true regulatory T cells.19 To verify that gated CD4+ CD25high T cells corresponded with Treg cells, we compared the expression of activation and differentiation molecules among CD25−, CD25low and CD25high CD4+ T cells in 18 controls. Figure 2 shows that CD25high lymphocytes were phenotypically distinguishable from CD25− and CD25low cells by the low expression of the activation markers CD69, CD154 and CD30, decreased levels of CD4 and increased expression of intracellular CTLA4, which are all characteristics attributable to Treg cells. CD25low cells, on the other hand, presented the highest MFI of activation markers. Thus, phenotypic analysis suggests that CD25low cells corresponded with recently activated effector T cells, whereas natural regulatory cells were included in the cell population expressing high levels of CD25.
Figure 1 Flow cytometric analysis of CD25 expression in CD4+ T cells. Peripheral blood samples were stained with CD25‐fluorescein isothiocyanate (FITC) and CD4‐peridin‐chlorophyll protein (CD4PerCP). Cells were gated first in the lymphocyte population according to forward and side‐scattered properties, and then in the CD4+/− population. In each patient and control sample, 10 000 CD4+ lymphocytes were acquired for analysis. Quadrant labels were set up according to isotype control (mouse immunoglobulin G2a‐FITC) (A), determining CD25− cells. CD25low and CD25high lymphocytes were measured in controls (B) and patients (C) as indicated.
Figure 2 Phenotypic characterisation of CD25−, CD25low and CD25high CD4+ T cell populations in controls. Box plots represent the median fluorescence intensity (MFI) of the indicated activation and differentiation markers obtained after flow cytometric analysis of 18 blood samples from controls. GITR, glucocorticoid‐induced tumour necrosis factor receptor; iCTLA4, intracellular CTLA4.
To ascertain whether the CD4+ CD25high cell population in patients with SLE contains those cells with regulatory functions, as in controls, we analysed the phenotypic profiles of gated CD25high and CD25low cells in 60 patients and compared them with those of controls (table 2). Results indicate that phenotypic characteristics of CD4+ CD25high lymphocytes in patients were similar to those in controls and corresponded to those of regulatory cells. The slight rise in the expression of various activation markers in both CD25low and CD25high cells in patients compared with that in controls indicated an activated status of CD4+ T lymphocytes in patients with SLE. Nonetheless, the phenotypic profile of CD4+ CD25high population in patients with SLE (high expression of GITR and intracellular CTLA4) fits that of regulatory T lymphocytes rather than that of effector‐activated lymphocytes.
Table 2 CD4+ CD25low and CD4+ CD25high T cell phenotypes in patients with systemic lupus erythematosus and in controls.
| Controls | Patients | ||||
|---|---|---|---|---|---|
| CD4+ CD25low | CD4+ CD25high | CD4+CD25low | CD4+CD25high | ||
| CD25 MFI | 12.81 (0.72) | 35.40 (2.43) | 13.41 (0.77)* | 35.35 (3.57) | |
| CD4 MFI | 99.83 (11.99) | 80.54 (9.73) | 99.03 (14.88) | 83.93 (12.03) | |
| CD69 | MFI | 3.16 (0.33) | 2.38 (0.25) | 3.63 (0.88)† | 2.93 (0.79)‡ |
| % | 1.30 (0.74) | 1.97 (0.96) | 2.73 (5.56)§ | 3.21 (3.43) | |
| CD154 | MFI | 5.02 (2.02) | 3.93 (1.83) | 6.22 (8.02) | 5.32 (7.90)¶ |
| % | 7.32 (12.79) | 6.31 (12.06) | 8.75 (16.05) | 8.87 (16.02) | |
| CD30 | MFI | 3.15 (0.34) | 2.58 (0.30) | 3.36 (0.56) | 2.94 (0.68)** |
| % | 0.41 (0.32) | 2.17 (1.79) | 0.83 (1.03) | 2.32 (2.84) | |
| iCTLA‐4 | MFI | 22.40 (5.71) | 46.32 (7.34) | 23.32 (7.14) | 47.73 (29.17) |
| % | 15.90 (6.43) | 58.70 (10.75) | 18.85 (17.42) | 53.25 (17.24) | |
| GITR | MFI | 4.08 (0.66) | 4.03 (0.95) | 4.57 (1.47) | 4.79 (2.00) |
| % | 8.26 (4.29) | 14.00 (9.01) | 12.36 (11.72) | 20.34 (13.87)†† | |
| CD45RO | MFI | 189.45 (59.09) | 191.53 (63.86) | 131.14 (78.84) | 142.43 (69.90) |
| % | 89.54 (4.46) | 84.64 (5.60) | 82.80 (11.83) | 85.33 (9.91) | |
GITR, glucocorticoid‐induced tumour necrosis factor receptor; iCTLA4, intracellular cytotoxin T lymphocyte‐associated antigen 4; MFI, median fluorescence intensity; SLE, systemic lupus erythematosus; %, percentage of positive cells in each region.
Values are mean (SD).
Differences between patients and controls were evaluated using the Mann–Whitney U test.
*p<0.001; †p = 0.025; ‡p = 0.001; §p = 0.022; ¶p = 0.022; **p = 0.045; ††p = 0.037.
Using the same settings, we subsequently determined the percentage of CD4+ CD25low and CD4+ CD25high T cells in 110 patients with SLE and 56 controls. Table 3 shows the substantial increase in the percentage of CD4+ CD25high cells in patients with SLE compared with controls. We also observed significantly raised frequency of CD4+ CD25low T cells in patients compared with controls.
Table 3 Percentage of CD25low and CD25high CD4+ T cells in controls and in patients with systemic lupus erythematosus.
| Controls (n = 56) | Patients (n = 110) | p Value | |
|---|---|---|---|
| CD4+ CD25low | 24.48 (6.95) | 28.97 (10.14) | 0.005 |
| CD4+ CD25high | 5.47 (2.43) | 8.34 (7.04) | 0.001 |
Values are mean (SD).
Differences were evaluated using the Mann–Whitney U test.
Association of CD4+ CD25high cells with clinical manifestations and treatment
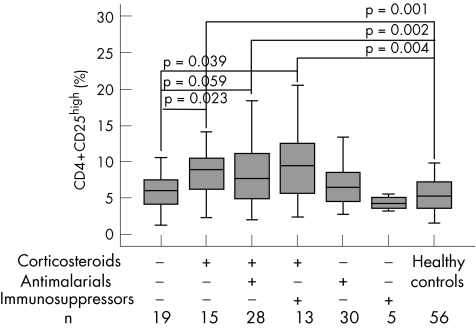
Given that the patients with SLE included in the study were unselected, possible putative correlations between the percentages of CD4+ CD25high or CD4+ CD25low T cells and clinical manifestations of the disease were considered. Spearman's rank correlation test did not show any notable relationship between age at diagnosis, malar rash, discoid lesions, photosensitivity, oral ulcers, arthritis, serositis, renal, neurological or haematological disorders, levels of anti‐dsDNA antibodies, and the size of the CD25high or CD25low population. Similarly, we determined possible correlations with the treatment followed during the 3 months before sampling. No associations were detected with the usage of malaria drugs or immunosuppressive drugs; however, a significantly higher percentage of CD4+ T cells at the CD25high region was found in patients taking glucocorticoids than in those not taking glucocorticoids (Spearman's r = 0.296, p = 0.002). No significant correlation was found between the use of glucocorticoids and the size of the CD25low population (r = 0.168, p = 0.079). Thus, to assess the influence of this treatment, we compared the percentages of CD4+ CD25high cells among healthy people, untreated patients and those undergoing single or combined treatment with glucocorticoids, malaria drugs and immunosuppressive drugs (fig 3). Interestingly, no significant differences were found in the mean (SD) percentage of CD25high cells between controls (5.46 (2.43)) and untreated patients (5.98 (2.39)). The largest percentage in this cellular population was observed among patients receiving glucocorticoid treatment, either alone (12.03 (14.07)) or combined with malaria drugs (9.34 (7.20)) or with immunosuppressive drugs (9.79 (5.01)), the differences being significant when compared with controls and with untreated patients (fig 3). Thus, as shown in table 4, only patients with SLE receiving (single or combined) glucocorticoids had significantly higher levels of CD4+ CD25high cells than controls. Moreover, although correlation between glucocorticoid dose and percentage of CD4+ CD25high cells was not significant (r = 0.253, p = 0.079), the effect seemed to be related to the dose of glucocorticoids, because patients treated with <5 mg/day (n = 9) had a percentage of CD4+ CD25high cells (5.52 (2.45)) similar to that in controls and in non‐steroid‐treated patients.
Figure 3 Size of the CD4+ CD25high T cell population in patients with systemic lupus erythematosus (SLE) stratified by treatment. Treatment received during the 3 months before sampling was registered at the time of sampling. The percentage of CD25high cells of the total CD4+ lymphocyte subset, as defined in fig 1, was determined in 110 patients with SLE and 56 controls after flow cytometric analysis of 10 000 CD4+‐acquired lymphocytes. Data are shown as box plots, where the lines in the boxes represent the median, the boxes represent the 25th to 75th centiles, and the lines outside the boxes represent the 10th and 90th centiles. Differences were evaluated using the Mann–Whitney U test.
Table 4 Relationship between percentage of CD4+ CD25high T cells and treatment of patients.
| n | CD4+ CD25high (%) | p Value | |
|---|---|---|---|
| Controls | 56 | 5.46 (2.43) | |
| Patient treatment | |||
| None or NSAIDs | 19 | 5.98 (2.39) | 0.377 |
| Glucocorticoids | 56 | 10.17 (9.11) | 0.001 |
| Others* | 35 | 6.67 (3.17) | 0.108 |
NSAID, non‐steroidal anti‐inflammatory drug.
Values are mean (SD).
Differences between each patient group and controls were evaluated using the Mann–Whitney U test.
*Malarial drugs or immunosuppressive drugs without corticosteroids.
On the basis of these results, we wished to ascertain the association between the use of glucocorticoids and the presence of increased amounts of CD4+ CD25high cells. Two groups were established on the basis of the mean value of CD4+ CD25high cells in patients (<8.4% and ⩾8.4%), and the relationship between the size of the Treg population and glucocorticoid treatment was determined by logistic regression modelling (table 5). Results indicated that glucocorticoid treatment was strongly associated with the presence of a high proportion of CD4+ CD25high cells; this association was sustained in the multivariate analysis after adjusting for clinical manifestations and level of anti‐dsDNA antibodies.
Table 5 Association between size of the CD4+ CD25high population and use of corticosteroids.
| CD4+ CD25high (%) | Corticosteroids | Univariate analysis, OR (95% CI), p value* | Multivariate analysis†, OR (95% CI), p value* | |
|---|---|---|---|---|
| Non–users, n (%) | Users, n (%) | |||
| Low (<8.4) | 43 (58.9) | 30 (41.1) | ||
| High (⩾8.4) | 11 (29.7) | 26 (70.3) | 3.39 (1.45 to 7.88) 0.005 | 3.59 (1.35 to 9.51) 0.010 |
*Calculated by unconditional binary logistic regression modelling, using the percentage of CD4+ CD25high T cells as the dependent variable and patients without glucocorticoid treatment as reference.
†Adjusted for clinical parameters: malar rash, discoid lesions, photosensitivity, oral ulcers, arthritis, serositis, renal, neurological or haematological disorder, and level of anti‐double‐stranded DNA antibodies at the time of sampling.
Discussion
We have characterised and quantified the CD4+ CD25low and CD4+ CD25high T cell populations in controls and in patients with SLE. CD4+ T cells expressing the IL2 receptor α chain do not constitute a homogeneous population and may include activated cells as well as lymphocytes with suppressor activities (Treg cells), especially in the context of a disease characterised by a chronic autoimmune inflammatory component. Thus, to identify Treg cells, we analysed the phenotypic expression of specific activation and differentiation markers on CD25−, CD25low and CD25high T cell subsets in patients and controls. About 5% of CD4+ T cells in controls showed high expression of CD25 (CD25high). These cells expressed low levels of CD69 and CD154 activation markers, but also expressed GITR and CD45RO and high levels of intracellular CTLA4, as well as decreased expression of CD4 antigen. These phenotypic features are distinctive of natural Treg cells according to previous reports.1,2,3,19 CD25low cells, in contrast, expressed the highest levels of activation markers and seemed to represent recently activated effector cells expressing low levels of the CD25 antigen.
When CD4+ T cells from patients were characterised according to the level of CD25 expression, the CD25low and CD25high cell populations showed, as in controls, phenotypic characteristics of effector and regulatory cells, respectively, although both populations showed signs of cellular activation. This suggests that the level of expression of CD25 may be a useful marker to identify regulatory cells in patients with SLE. Accordingly, we quantified the percentage of CD25high cell population in 110 unselected patients with SLE and compared it with that in controls, and found a considerable increase in the percentage of regulatory cells in the entire patient group. No marked correlation was observed between the size of the CD25high cell population and clinical manifestations of the disease (age at diagnosis, malar rash, discoid lesions, photosensitivity, oral ulcers, arthritis, serositis and renal, neurological or haematological disorder) and levels of anti‐dsDNA antibodies. Interestingly, however, we found an important positive correlation between the usage of glucocorticoids and the percentage of CD25high cells among the total CD4+ population. In fact, when patients were stratified according to treatment, only those patients receiving continuous glucocorticoids during the 3 months (⩾5 mg/day) before sampling had an increased percentage of CD4+ CD25high T cells. Treg cell counts in untreated patients or in patients receiving malaria drugs or immunosuppressive treatment were not considerably different from those in controls. These results indicate that the initial increased number of CD4+ CD25high cells that we observed in patients with SLE compared with controls may be secondary to glucocorticoid treatment, and suggest that corticosteroids may induce the expansion or the generation of Treg cells. However, it may also be possible that the increased frequency of CD4+ CD25high cells in glucocorticoid‐treated patients was due to a “contamination” of Treg cells with effector‐activated T cells in patients with a worse disease course, who consequently needed glucocorticoid treatment. Nevertheless, we do not think that this is the case in our study, as steroid treatment did not markedly modify the size of the true effector CD25low cell population. Although we observed an increased MFI or percentage of CD69+, CD154+ and CD30+ cells in the CD25high cell population in patients compared with that in controls, these figures did not vary considerably in the different groups of patients with SLE according to treatment.
An increased number of CD4+ CD25+ T cells has been reported in the peripheral blood of pregnant women23,24 and in patients with asthma after systemic treatment with glucocorticoids25; this supports a role of steroids in the expansion of Treg cells. It has also been reported that dexamethasone and IL7 added to in vitro cultures of human peripheral blood lymphocytes augmented the number of CD4+ CD25+ T cells and the number of CD25 molecules on the cell surface.26 In rodents, treatment of mice with oestrogens increased CD4+ CD25+ T cell number and forkhead box p3 expression level,27 and treatment with dexamethasone raised the proportion of CD4+ CD25+ Treg cells in lymphoid tissues.28 However, the way in which glucocorticoids may increase Treg cell population is completely unknown. It may be the consequence of a direct effect on precursor or mature Treg cells, promoting their induction or expansion, or, alternatively, steroids may induce a selective apoptosis in CD4+ CD25− T cells, as previously reported,28 thus indirectly increasing the frequency of CD4+ CD25high T cells. It has been also reported that T cell receptor‐mediated GITR expression in combination with other unidentified factors protects T cells from glucocorticoid‐mediated apoptosis.29 Given the biological relevance of our finding, new experiments need to be designed to clarify this issue.
Two previous studies have analysed Treg cells in patients with SLE, reporting inconsistent results. Crispin et al17 found a decreased percentage of CD25high CD4+ cells in 10 active untreated patients with SLE, accompanied by a higher frequency of in vivo activated CD69+ T cells. The discrepancy with the present results is probably due to differences in the selection of patients. Whereas only a few active untreated patients were included in the aforementioned study, a large number of unselected patients were analysed in our research. The other study18 also reported a decreased amount of CD4+ CD25+ T cells in the peripheral blood of patients with SLE, although, according to the authors' comments, counts were similar to those in controls as regards the total CD4+ T lymphocyte count. In summary, our results suggest the absence of quantitative defects in the population of CD4+ CD25high T cells in untreated or non‐glucocorticoid‐treated patients with SLE, whereas patients receiving glucocorticoids showed considerably higher percentages of this cellular population than controls or non‐steroid‐treated patients.
Acknowledgements
We thank the Asociación Lúpicos de Asturias (ALAS) for its continuous encouragement.
Abbreviations
CTLA4 - cytotoxin T lymphocyte‐associated antigen 4
dsDNA - double‐stranded DNA
FITC - fluorescein isothiocyanate
GITR - glucocorticoid‐induced tumour necrosis factor receptor
MFI - median fluorescence intensity
PerCP - peridin‐chlorophyll protein
SLE - systemic lupus erythematosus
TNF - tumour necrosis factor
Treg cells - natural regulatory T cells
Footnotes
Funding: This work was supported by grants SV‐04‐FMM‐01 from the Fundación Médica Mutua Madrileña and PI052409 from the Fondo de Investigación Sanitaria.
Competing interests: None.
Ethical approval: The Regional Ethics Committee for Clinical Investigation (Hospital Universitario Central de Asturias, Oviedo, Spain) gave ethical approval for this study.
References
- 1.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 20011931303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk A H. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med 20011931285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng W F, Duggan P J, Ponchel F, Matarese G, Lombardi G, Edwards A D.et al Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood 2001982736–2744. [DOI] [PubMed] [Google Scholar]
- 4.Gottenberg J E, Lavie F, Abbed K, Gasnault J, Le Nevot E, Delfraissy J F.et al CD4 CD25high regulatory T cells are not impaired in patients with primary Sjogren's syndrome. J Autoimmun 200524235–242. [DOI] [PubMed] [Google Scholar]
- 5.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther 20046R335–R346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Amelsfort J M, Jacobs K M, Bijlsma J W, Lafeber F P, Taams L S. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum 2004502775–2785. [DOI] [PubMed] [Google Scholar]
- 7.Cao D, Malmstrom V, Baecher‐Allan C, Hafler D, Klareskog L, Trollmo C.et al Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol 200333215–223. [DOI] [PubMed] [Google Scholar]
- 8.De Berardinis P, Londei M, Kahan M, Balsano F, Kontiainen S, Gale E A.et al The majority of the activated T cells in the blood of insulin‐dependent diabetes mellitus (IDDM) patients are CD4+. Clin Exp Immunol 198873255–259. [PMC free article] [PubMed] [Google Scholar]
- 9.Gessl A, Waldhausl W. Increased CD69 and human leukocyte antigen‐DR expression on T lymphocytes in insulin‐dependent diabetes mellitus of long standing. J Clin Endocrinol Metab 1998832204–2209. [DOI] [PubMed] [Google Scholar]
- 10.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin‐Su K.et al Multiple immuno‐regulatory defects in type‐1 diabetes. J Clin Invest 2002109131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindley S, Dayan C M, Bishop A, Roep B O, Peakman M, Tree T I. Defective suppressor function in CD4(+)CD25(+) T‐cells from patients with type 1 diabetes. Diabetes 20055492–99. [DOI] [PubMed] [Google Scholar]
- 12.Putheti P, Pettersson A, Soderstrom M, Link H, Huang Y M. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease‐modulating drugs. J Clin Immunol 200424155–161. [DOI] [PubMed] [Google Scholar]
- 13.Viglietta V, Baecher‐Allan C, Weiner H L, Hafler D A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 2004199971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriegel M A, Lohmann T, Gabler C, Blank N, Kalden J R, Lorenz H M. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med 20041991285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y M, Pirskanen R, Giscombe R, Link H, Lefvert A K. Circulating CD4+CD25+ and CD4+CD25+ T cells in myasthenia gravis and in relation to thymectomy. Scand J Immunol 200459408–414. [DOI] [PubMed] [Google Scholar]
- 16.Longhi M S, Ma Y, Bogdanos D P, Cheeseman P, Mieli‐Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T‐cells in autoimmune liver disease. J Hepatol 20044131–37. [DOI] [PubMed] [Google Scholar]
- 17.Crispin J C, Martinez A, Alcocer‐Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 200321273–276. [DOI] [PubMed] [Google Scholar]
- 18.Liu M F, Wang C R, Fung L L, Wu C R. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol 200459198–202. [DOI] [PubMed] [Google Scholar]
- 19.Baecher‐Allan C, Brown J A, Freeman G J, Hafler D A. CD4+CD25high regulatory cells in human peripheral blood. J Immunol 20011671245–1253. [DOI] [PubMed] [Google Scholar]
- 20.Su C C, Shau W Y, Wang C R, Chuang C Y, Chen C Y. CD69 to CD3 ratio of peripheral blood mononuclear cells as a marker to monitor systemic lupus erythematosus disease activity. Lupus 19976449–454. [DOI] [PubMed] [Google Scholar]
- 21.Lopez P, Mozo L, Gutierrez C, Suarez A. Epidemiology of systemic lupus erythematosus in a northern Spanish population: gender and age influence on immunological features. Lupus 200312860–865. [DOI] [PubMed] [Google Scholar]
- 22.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez‐Ramon S, Navarro A J, Aristimuno C, Rodriguez‐Mahou M, Bellon J M, Fernandez‐Cruz E.et al Pregnancy‐induced expansion of regulatory Tlymphocytes may mediate protection to multiple sclerosis activity. Immunol Lett 200596195–201. [DOI] [PubMed] [Google Scholar]
- 24.Somerset D A, Zheng Y, Kilby M D, Sansom D M, Drayson M T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T‐cell subset. Immunology 200411238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karagiannidis C, Akdis M, Holopainen P, Woolley N J, Hense G, Ruckert B.et al Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol 20041141425–1433. [DOI] [PubMed] [Google Scholar]
- 26.Chung I Y, Dong H F, Zhang X, Hassanein N M, Howard O M, Oppenheim J J.et al Effects of IL‐7 and dexamethasone: induction of CD25, the high affinity IL‐2 receptor, on human CD4+ cells. Cell Immunol 200423257–63. [DOI] [PubMed] [Google Scholar]
- 27.Polanczyk M J, Carson B D, Subramanian S, Afentoulis M, Vandenbark A A, Ziegler S F.et al Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol 20041732227–2230. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Murakami T, Oppenheim J J, Howard O M. Differential response of murine CD4+CD25+ and CD4+. Eur J Immunol 200434859–869. [DOI] [PubMed] [Google Scholar]
- 29.Zhan Y, Funda D P, Every A L, Fundova P, Purton J F, Liddicoat D R.et al TCR‐mediated activation promotes GITR upregulation in T cells and resistance to glucocorticoid‐induced death. Int Immunol 2004161315–1321. [DOI] [PubMed] [Google Scholar]