Abstract
Carotid intimal-medial thickening was observed in a 23-year-old woman with acute cytomegalovirus (CMV) infection. The thickening disappeared after her recovery from the infection. As endothelial cells are common targets of CMV, this thickening suggests that CMV infection causes vascular lesions, even in otherwise healthy individuals.
CASE REPORT
A 23-year-old Japanese woman suffered from infectious mononucleosis in August 1996. The patient was healthy and had no history of recurrent infection, vasculitis, thromboembolism, or injury to her neck. She smoked occasionally 10 cigarettes a day but was not an intravenous-drug abuser. She had not taken oral contraceptives. She was not pregnant when high fever and skin eruption were noted in mid-April 1997. Laboratory data showed liver dysfunction (aspartate aminotransferase, 128 IU/liter; alanine aminotransferase, 165 IU/liter; lactate dehydrogenase, 1,234 IU/liter), thrombocytopenia (12.5 × 104/μl), and a positive result for C-reactive protein (1.0 mg/dl). The patient's white cell count (7,200/μl) and red cell count (454 × 104/μl) were both within normal limits. A urine test was normal. Virological studies, including tests for human immunodeficiency virus, rubella virus, herpes simplex virus, measles virus, and human parvovirus B19, failed to detect abnormalities, except that she was positive for anticytomegalovirus (CMV) immunoglobulin M (IgM) antibody. Her fever subsided in early June 1997, but she complained of fatigue.
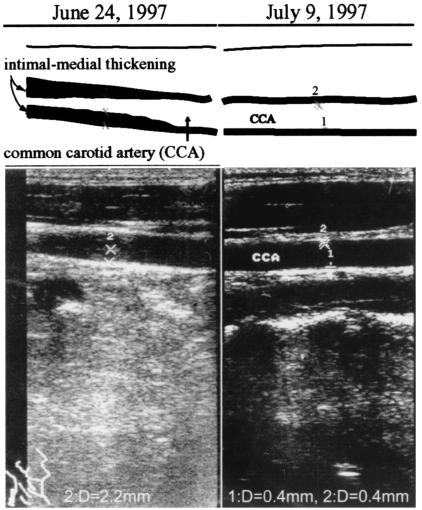
On 16 June 1997, she was admitted to our hospital to diagnose her illness. Physical examination at admission showed moderate splenomegaly and a systolic bruit over her right carotid artery but no neck pain. Laboratory findings showed slightly increased liver enzyme levels (aspartate aminotransferase, 59 IU/liter; alanine aminotransferase, 73 IU/liter; lactate dehydrogenase, 457 IU/liter) and thrombocytopenia (11.5 × 104/μl). The test for C-reactive protein was negative. Virological studies showed that an anti-CMV IgM antibody test was negative, whereas the anti-CMV IgG antibody titer was high. DNA encoding the immediate early gene of CMV was detected in her peripheral white blood cells by using nested PCR. The level of antinuclear antibody was a border value, and tests for autoantibodies, including anti-DNA, anti-RNP, anti-Sm, anti-SS-A/Ro, anti-SS-B/La, anticardiolipin, anti-cardiolipin-β2-glycoprotein I complex, and antineutrophil cytoplasmic antibody, were negative. Serological tests for syphilis and lupus anticoagulant were negative. The levels of activated partial thromboplastin time, antithrombin III, fibrinogen, thrombomodulin, and fibrinogen degradation products were 39.7 s (normal, 20 to 40 s), 95.9% (77.4 to 127.2%), 230 mg/dl (209.7 to 380.9 mg/dl), 2.8 U/ml (<4.0 U/ml), and 0.77 μg/ml (<8.9 μg/ml), respectively. The level of D-dimer were, however, slightly increased at 1.02 μg/ml (<0.5 μg/ml). Echocardiography showed no abnormalities, but ultrasonography showed intimal-medial thickening (2.2 mm in thickness) of the right common carotid artery (Fig. 1, left). Adherent thrombi may have been associated with the thickening, but distinguishing between adherent thrombi and the intimal layer by ultrasonography was difficult. In early July 1997, the thickened areas disappeared spontaneously and the intimal-medial layer was normal (0.4 mm thick) (Fig. 1, right). The patient subsequently recovered and left the hospital on 16 July 1997.
FIG. 1.
Ultrasonograph of the right carotid artery.
In January 2000, CMV DNA tests of peripheral white blood cells were negative and all laboratory findings were normal. Ultrasonography of the carotid artery failed to detect abnormalities, and no bruit was noted.
In healthy individuals, CMV infection is often asymptomatic. Occasionally, CMV causes various symptoms, such as fever, eruption, lymphadenitis, liver dysfunction, leukopenia, thrombocytopenia, or hepatosplenomegaly. In immunocompromised hosts, such as patients with AIDS or recipients of organ transplants, CMV infection sometimes causes endotheliitis and subsequent thrombosis, which may result in enteritis, gastrointestinal (GI) ulceration, meningoencephalitis, pneumonitis, retinitis, or skin ulcer (5). CMV vasculitis in the GI tract causes diarrhea, weight loss, GI bleeding, and abdominal pain. Clinical signs of CMV vasculitis in the central nervous system are also nonspecific and include encephalopathy, dementia, cranial nerve palsies, and myeloradiculopathy.
Our patient was healthy and had no predisposing risk factors for thrombosis except for smoking. CMV vasculitis may be rare in healthy individuals, but a 40-year-old woman in whom CMV infection was serologically confirmed developed a purpuric rash on both lower legs after 20 days of an influenza-like illness (3). A 50-year-old man with active CMV disease also developed extensive mesenteric arterial and venous thrombosis ever though he showed no risk of developing thrombosis (13).
In our patient, the carotid intimal-medial thickening occurred during an episode of acute CMV infection and disappeared about 3 weeks later when the patient recovered from the infection. This suggests a causative relationship between the CMV infection and the carotid vasculitis. Koskinen et al. (9) analyzed 762 endomyocardial biopsy specimens from 47 heart allograft recipients, who all received basic immunosuppressive treatment. Of these 47 patients, 28 patients developed CMV infection, as judged by their being positive for CMV antigenemia, 48 ± 43 (mean ± standard deviation) days after the operation. In 24 of the 28 CMV-infected recipients, the subendothelium of small intramyocardial arterioles was inflamed at the onset of CMV antigenemia. The subendothelial inflammation subsided slowly when the CMV antigenemia was over. Only 5 of 19 CMV-free recipients had an inflamed subendothelium. This study suggests that the subendothelium is inflamed during active CMV infection, a pattern to which the clinical course of our patient conformed.
Several mechanisms of CMV-mediated vascular changes have been proposed. Endothelial cells (ECs), vascular smooth muscle cells (VSMCs), and fibroblasts are major targets for CMV infection. CMV-infected ECs transform into cytomegalic inclusion cells, in which CMV propagates (6). EC-propagated CMV acquires an ability to infect ECs more efficiently and causes cytopathic change progressing to complete lysis of the infected cells (8). Adherent thrombi are easily formed at lesions where ECs are damaged in vivo. CD40 can be expressed at high levels on the surfaces of CMV-infected ECs, and an increased level of E-selectin can be induced on those cells after stimulation with CD40 ligand (11). CMV increases the levels of inflammatory cytokines, such as interleukin-1β, interleukin-6, or tumor necrosis factor alpha, and growth factors, such as platelet-derived growth factor or transforming growth factor β (1, 7, 14). Thus, infection of ECs by CMV may result in an augmented process of inflammation and in thrombogenesis. The main region of CMV-induced inflammation is in the tunica intima. Therefore, clinically, intimal thickening could be an indicator of CMV vasculitis.
Recent studies have shown that inflammation is associated with atherosclerosis (10). Therefore, the inflammation caused by CMV could be associated with atherogenesis. Levels of anti-CMV IgG antibodies are positively correlated with carotid intimal-medial thickness and atherosclerosis in immunocompetent hosts (2, 12). Reactivation of CMV infection may be responsible for the restenosis sometimes observed following successful angioplasty due to excessive accumulation of VSMCs (4). CMV accelerates VSMC proliferation by inhibiting the function of p53 (15). CMV is a suspect pathogen of inflammatory abdominal aortic aneurysm, which is characterized by chronic inflammation and severe atheromatous changes in the aneurysmal wall (16). However, it is still unclear whether atherosclerotic plaque develops as a result of intermittent reactivation of CMV.
It is worth noting that the carotid arterial thickening in the young healthy woman of this case produced no symptoms, suggesting that asymptomatic vascular injuries should be considered in cases of CMV infection. Further investigation is needed to clarify the relationship between CMV infection and atherosclerosis in healthy immunocompetent hosts.
REFERENCES
- 1.Alcami, J., T. Barzu, and S. Michelson. 1991. Induction of an endothelial cell growth factor by human cytomegalovirus infection of fibroblasts. J. Gen. Virol. 72:2765-2770. [DOI] [PubMed] [Google Scholar]
- 2.Borgia, M. C., C. Mandolini, C. Barresi, G. Battisti, F. Carletti, and M. R. Capobianchi. 2001. Further evidence against the implication of active cytomegalovirus infection in vascular atherosclerotic diseases. Atherosclerosis 157:457-462. [DOI] [PubMed] [Google Scholar]
- 3.Crowley, B., J. Dempsey, A. Olujohungbe, A. Khan, K. Mutton, and C. A. Hart. 2002. Unusual manifestations of primary cytomegalovirus infection in patients without HIV infection and without organ transplants. J. Med. Virol. 68:237-240. [DOI] [PubMed] [Google Scholar]
- 4.Epstein, S. E., E. Speir, Y. F. Zhou, E. Guetta, M. Leon, and T. Finkel. 1996. The role of infection in restenosis and atherosclerosis: focus on cytomegalovirus. Lancet 348:s13-s17. [DOI] [PubMed] [Google Scholar]
- 5.Golden, M. P., S. M. Hammer, C. A. Wanke, and M. A. Albrecht. 1994. Cytomegalovirus vasculitis. Medicine 73:246-255. [PubMed] [Google Scholar]
- 6.Grefte, A., M. Giessen, W. Son, and T. H. The. 1993. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J. Infect. Dis. 167:270-277. [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto, G. K., M. M. Monich, B. D. Clark, P. E. Auron, M. F. Stinski, and G. W. Hunninghake. 1990. Modulation of interleukin 1 beta gene expression by the immediate early genes of human cytomegalovirus. J. Clin. Investig. 85:1853-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahl, M., D. Siegel-Axel, S. Stenglein, G. Jahn, and C. Sinzger. 2000. Efficient lytic infection of human arterial endothelial cells by human cytomegalovirus strains. J. Virol. 74:7628-7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koskinen, P., K. Lemstrom, C. Bruggeman, I. Lautenschlager, and P. Häyry. 1994. Acute cytomegalovirus infection induces a subendothelial inflammation (endothelialitis) in the allograft vascular wall. Am. J. Pathol. 144:41-50. [PMC free article] [PubMed] [Google Scholar]
- 10.Libby, P. 2003. Vascular biology of atherosclerosis: overview and state of the art. Am. J. Cardiol. 91:3A-6A. [DOI] [PubMed] [Google Scholar]
- 11.Maisch, T., B. Kropff, C. Sinzger, and M. Mach. 2002. Upregulation of CD40 expression on endothelial cells infected with human cytomegalovirus. J. Virol. 76:12803-12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieto, F. J., E. Adam, P. Sorlie, H. Farzadegan, J. L. Melnick, G. W. Comstock, and M. Szklo. 1996. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation 94:922-927. [DOI] [PubMed] [Google Scholar]
- 13.Ofotokun, I., C. Carlson, S. D. Gitlin, G. Elta, T. P. Singleton, and D. M. Markovitz. 2001. Acute cytomegalovirus infection complicated by vascular thrombosis. Clin. Infect. Dis. 32:983-986. [DOI] [PubMed] [Google Scholar]
- 14.Smith, P. D., S. S. Saini, M. Raffeld, J. F. Manischewitz, and S. M. Wahl. 1992. Cytomegalovirus induction of tumor necrosis factor-alpha by human monocytes and mucosal macrophages. J. Clin. Investig. 90:1642-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speir, E., R. Modali, E. S. Huang, M. B. Leon, F. Shawl, T. Finkel, and S. E. Epstein. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 16.Yonemitsu, Y., K. Nakagawa, S. Tanaka, R. Mori, K. Sugimachi, and K. Sueishi. 1996. In situ detection of frequent and active infection of human cytomegalovirus in inflammatory abdominal aortic aneurysms: possible pathogenic role in sustained chronic inflammatory reaction. Lab. Investig. 74:723-736. [PubMed] [Google Scholar]