Abstract
Background
Cardiovascular mortality is increased in patients with ankylosing spondylitis. A possible explanation might be a more prevalent atherogenic lipid profile in patients with ankylosing spondylitis than in the general population. It has been postulated that inflammation deteriorates the lipid profile, thereby increasing cardiovascular risk.
Objective
To explore the association between disease activity and lipid profile in patients with ankylosing spondylitis.
Methods
Disease activity parameters for ankylosing spondylitis and lipid levels (total cholesterol, high‐density lipoprotein cholesterol (HDLc) and triglycerides) were measured in 45 patients with ankylosing spondylitis for 6 months after starting treatment with leflunomide or placebo. Findings in this treatment group were compared with those in 10 patients with ankylosing spondylitis treated with etanercept. A specialised regression model, adjusting for repeated measurements, age and sex, was used to assess the influence of the disease activity variables on the lipid levels.
Results
Multilevel regression analyses showed significant associations between disease activity parameters and lipid levels—for instance, an increase of 30 mm at the end of the first hour in erythrocyte sedimentation rate was associated with a decrease of about 6% in total cholesterol level and a decrease of about 11% in HDLc levels. Similar significant associations were found between other disease activity parameters and lipid levels.
Conclusion
Increase in disease activity was associated with decreases in lipid levels. The decrease in HDLc levels tended to be almost twice as large as the decrease in total cholesterol levels, resulting in a more atherogenic lipid profile. Hence, effective treatment of disease activity in patients with ankylosing spondylitis may lower the cardiovascular risk by improving the lipid profile.
Ankylosing spondylitis is a chronic inflammatory disease that affects predominantly men, starts in young adulthood and results in immobility of the spine and sacroiliac joints. Although the number of studies investigating mortality in ankylosing spondylitis is limited, many of these show an increased total mortality in patients compared with the general population.1,2,3,4 Furthermore, this increased mortality seems to be predominantly caused by cardiovascular disease (CVD), with a twofold increased cardiovascular standard mortality.5
A possible explanation for this increased cardiovascular risk is a higher prevalence of conventional cardiovascular risk factors, such as a more atherogenic lipid profile.6 An atherogenic lipid profile is characterised by a reduced level of high‐density lipoprotein cholesterol (HDLc) and increased levels of total cholesterol, low‐density lipoprotein cholesterol and triglycerides. An important prognostic indicator for (future) CVD is the atherogenic index, which is the ratio of total cholesterol to HDLc.7,8,9,10
Whether or not an atherogenic lipid profile is present in patients with ankylosing spondylitis is presently unclear.11,12,13 Moreover, there is growing evidence that inflammation is associated with deterioration of the lipid profile,14,15 but so far data for patients with ankylosing spondylitis are lacking. Hence, we hypothesise that disease activity in inflammatory diseases, such as ankylosing spondylitis, worsens the lipid profile, thereby increasing the risk for (future) CVD. Consequently, lowering the disease activity in patients with ankylosing spondylitis may have a beneficial effect on the lipid profile.
Two recent studies,16,17 investigating the safety and efficacy of treatment with leflunomide and etanercept in patients with ankylosing spondylitis, gave us the opportunity to assess the relationship between disease activity and lipid profile in a total of 55 patients with ankylosing spondylitis, treated with leflunomide, etanercept or placebo.
Methods
Patients
All patients included in the study, aged 18–70 years, fulfilled the 1984 modified New York criteria for ankylosing spondylitis.18 The first population consisted of 45 consecutive patients with ankylosing spondylitis participating in a monocentre, randomised, double‐blind, placebo‐controlled phase II trial, in which the safety and efficacy of leflunomide were assessed.17 Thirty patients were treated with the active compound at a daily dose of 20 mg and 15 patients received placebo, for a duration of 24 weeks. During this period, other pharmacological treatments were maintained stable.
The findings of the aforementioned patients with ankylosing spondylitis were compared with those of a second population consisting of 10 patients with ankylosing spondylitis who were treated for 6 months with etanercept (25 mg twice weekly).18
Disease activity parameters
The Bath Ankylosing Spondylitis Global score,19 the Bath Ankylosing Spondylitis Functional Index20 and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI),21 all with a scale from 0 to 10, were determined by an experienced rheumatologist at baseline and weeks 4, 12 and 24. Furthermore, the erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) levels were measured at baseline and weeks 2, 4, 6, 9, 12, 16, 20 and 24. ESR was determined with local measurement techniques and expressed in millimetres per hour (in men <20 mm/h and in women <30 mm/h). CRP was measured using a turbidimetric assay supplied by Biomedical Diagnostics (Apeldoorn, The Netherlands) (<10 mg/l).
Lipids
Blood samples for lipid measurements were taken after an overnight fast at baseline and weeks 2, 4, 6, 9, 12, 16, 20 and 24. Serum total cholesterol (<5.0 mmol/l) and triglycerides (<2.2 mmol/l) were analysed by an enzymatic method using the appropriate assays supplied by Roche Diagnostics (Almere, The Netherlands) on a Hitachi 911 analyser (Roche), according to the instructions of the manufacturer. Polyethylene glycol‐modified enzymes were used for assessing the HDLc levels (in men >0.9 mmol/l and in women >1.1 mmol/l). The atherogenic index was calculated as the total cholesterol level divided by the HDLc level.
Statistics
Measuring lipid levels and disease activity parameters in a particular population at several time points, with variable time intervals, causes an important statistical problem—that is, “repeated measurements within subjects”. To adjust for repeated measurements within subjects, a multilevel linear regression analysis was used. This regression technique allows for both starting levels and progression over time to differ between patients. It calculates the regression coefficients of the progression over time of the various lipid levels, adjusted for repeated measurements within subjects and variable time intervals.22
Multilevel regression analysis is a longitudinal linear regression analysis. It combines many cross‐sectional linear regression models into one model of one variable over time. In this study, we measured the lipid levels over time and investigated the influence of the various other variables, such as disease activity parameters, on these lipid levels. Multilevel regression analysis quantifies this influence, or rather the association between the two variables, and tests it for statistical significance. Furthermore, the observed association between the two variables—that is, lipid levels and disease activity parameters—was also corrected for age and sex.
One advantage of combining the cross‐sectional data of the various time points into one association is that this increases the statistical power. Another important advantage of this method is that it holds into account and corrects for the possibility that one or a few patients with aberrant values distort the association between lipid levels and disease activity—that is, the above‐mentioned repeated measurements within subjects. If one or a few patients have, for example, an extreme level of a certain lipid, it will not distort the relationship this lipid has with disease activity, because the multilevel regression analysis will look only at this relationship and correct for the aberrant starting points.
As the primary goal of this investigation was to study the relationship between disease activity and lipid levels, it was not relevant whether the antirheumatic treatment altered disease activity; hence the data of placebo‐treated and leflunomide‐treated patients were put together. The findings were compared with those of a group of 10 patients with ankylosing spondylitis treated with etanercept, for whom similar analyses were conducted.
As the HDLc levels, the atherogenic index and triglyceride levels were not normally distributed, data were analysed with the natural logarithms of these values. For clarity, the regression coefficients for these lipids were retransformed to geometric means. The multilevel analyses were carried out with the statistical program MLwiN.23 A p value of ⩽0.05 was considered significant.
Results
Patients
The first group of 45 patients with ankylosing spondylitis (13 women and 32 men), with a mean age of 42 (standard deviation (SD) 11; range 21–66) years, was followed for 24 weeks. Nine patients had hypertension; eight of them used antihypertensive agents. Two patients were treated for hypercholesterolaemia. Non‐steroidal anti‐inflammatory drugs were used by 39 patients and prednisone was given to one.
The findings of 10 patients with ankylosing spondylitis (1 woman and 9 men), with a mean age of 41 (SD 10; range 28–60) years, starting treatment with etanercept, were compared with those of the first population. One patient was treated for type 2 diabetes mellitus, one for hypertension and one for hypercholesterolaemia. Nine patients used non‐steroidal anti‐inflammatory drugs.
All pharmacological treatments remained unchanged during the entire observation period. Table 1 shows the baseline characteristics, including demographic and clinical data.
Table 1 Baseline characteristics of participants.
| Leflunomide or placebo (n = 45) | Etanercept (n = 10) | |
|---|---|---|
| Demographic features | ||
| Age (years) | 42 (11) | 41 (10) |
| Sex (male:female) | 32:13 | 9:1 |
| Disease activity parameters | ||
| BASG | 6.4 (1.8) | 6.7 (1.6) |
| BASFI | 5.3 (1.8) | 4.4 (1.9) |
| BASDAI | 5.5 (1.4) | 4.9 (1.2) |
| ESR (mm/h) | 15 (10–31)* | 37 (13–49)* |
| CRP (mg/l) | 13 (4–40)* | 11 (4.4–29.4)* |
| Lipids | ||
| TC (mmol/l) | 4.9 (1.2) | 4.4 (0.8) |
| HDLc (mmol/l) | 1.1 (0.9–1.4)* | 1.3 (0.3) |
| Atherogenic index | 4.3 (3.1–5.5)* | 3.7 (1.1) |
| Triglycerides (mmol/l) | 1.2 (0.8–1.8)* | 1.1 (0.7–1.6)* |
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASG, Bath Ankylosing Spondylitis Global; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HDLc, high‐density lipoprotein cholesterol; TC, total cholesterol.
*Values are mean (SD) or median (interquartile range), as applicable.
Lipid levels and disease activity parameters
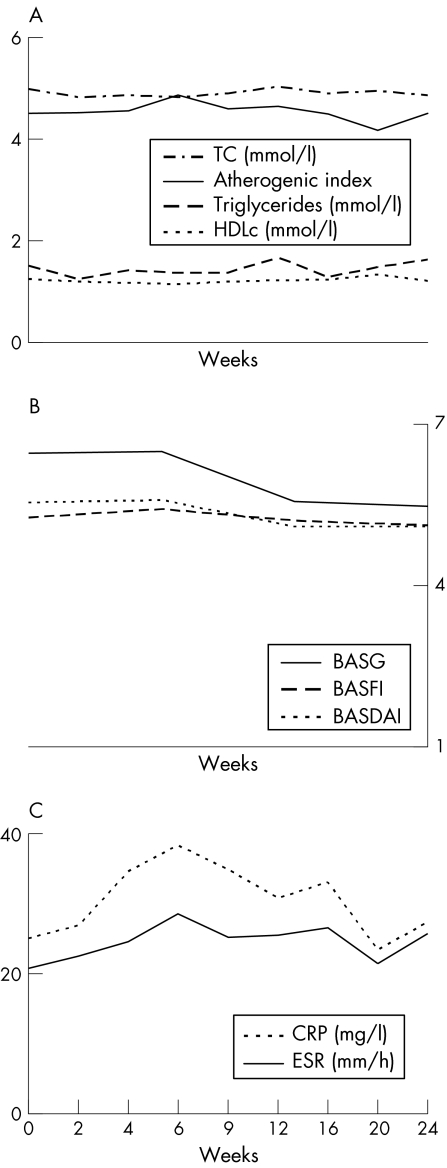
Figure 1 shows the lipid levels and the disease activity parameters in patients with ankylosing spondylitis, treated with placebo or leflunomide (n = 45). We found no significant changes in these variables during the 24‐week observation period. The multilevel regression analyses yielded several significant associations between lipid level progression over time and disease activity parameters; the height of the disease activity had a significant influence on the height of the lipid levels. Higher ESR and CRP levels were significantly (p<0.001) associated with lower total cholesterol levels, with regression coefficients of −0.01 and −0.01, respectively. The ln‐triglyceride levels also had an inverse relationship with ESR and CRP, with regression coefficients of −0.005 and −0.004, respectively. A similar relationship was observed between ln‐HDLc and ESR and CRP levels (regression coefficients of −0.004 and −0.002, respectively). Moreover, the disease activity parameters tended to have a linear relationship with the atherogenic index (p = 0.09).
Figure 1 (A–C) Mean lipid levels and disease activity variables during the 24 weeks of placebo treatment or lefunomide treatment. Antherogenic index, TC/HDLc; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASG, Bath Ankylosing Spondylitis Global; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HDLc, high‐density lipoprotein cholesterol; TC, total cholesterol.
The relationship between disease activity parameters and lipid levels, or the influence of disease activity on the lipid levels, is shown in table 2 as absolute values and percentages. The influence of ESR and CRP on HDLc levels was almost twice as large as the effect on total cholesterol levels, resulting in a higher atherogenic index (p<0.001).
Table 2 Influence of disease activity on the lipid levels.
| Increase in disease activity parameter | Lipids | Absolute change (mmol/l) | Relative decrease (%) | p Value |
|---|---|---|---|---|
| 30 mm/h ESR | TC | −0.29 | −6.0 | 0.001* |
| HDLc | −0.13 | −11.4 | 0.01* | |
| Atherogenic index | 0.29 | 6.8 | 0.09 | |
| Triglycerides | −0.15 | −12.6 | 0.04* | |
| 30 mg/l CRP | TC | −0.18 | −3.8 | 0.001* |
| HDLc | −0.06 | −5.7 | 0.001* | |
| Atherogenic index | 0.10 | 2.4 | 0.16 | |
| Triglycerides | −0.13 | −10.9 | 0.001* | |
| 1‐point BASG | TC | 0.01 | 0.1 | 0.79 |
| HDLc | −0.01 | −0.7 | 0.44 | |
| Atherogenic index | 0.03 | 0.6 | 0.47 | |
| Triglycerides | −0.05 | −4.4 | 0.01* | |
| 1‐point BASFI | TC | 0.02 | 0.4 | 0.62 |
| HDLc | −0.02 | −1.7 | 0.25 | |
| Atherogenic index | 0.08 | 2.0 | 0.19 | |
| Triglycerides | −0.06 | −4.5 | 0.01* | |
| 1‐point BASDAI | TC | −0.01 | −0.3 | 0.66 |
| HDLc | −0.00 | −0.4 | 0.71 | |
| Atherogenic index | 0.01 | 0.2 | 0.87 | |
| Triglycerides | −0.07 | −5.8 | 0.01* |
Atherogenic index, ratio of total cholesterol (TC) to high‐density lipoprotein cholesterol (HDLc); BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASG, Bath Ankylosing Spondylitis Global Score; CRP, C reactive protein; ESR, erythrocyte sedimentation rate.
Influence of disease activity parameters on lipid levels, calculated using multilevel regression analyses correcting for age, sex and repeated measurements within subjects.
*Indicates significant associations.
The multilevel analyses of patients treated with etanercept showed that an increased ESR, CRP, Bath Ankylosing Spondylitis Global score, Bath Ankylosing Spondylitis Functional Index and BASDAI significantly (p⩽0.02) decreased the total cholesterol levels (regression coefficients of −0.01, −0.02, −0.07, −0.12 and −0.13, respectively). Higher ESR and CRP levels were associated with lower HDLc and total cholesterol levels, albeit that the decrease in HDLc was (again) twice as large as the decrease in total cholesterol levels, resulting in a higher atherogenic index. Moreover, increase in other disease activity parameters was also associated with an increase in the atherogenic index; this association did not reach significance (p>0.08).
Discussion
Our investigation on patients with ankylosing spondylitis shows that higher disease activity (parameters) is associated with lower lipid levels and vice versa. This was observed for total cholesterol, HDLc and triglyceride levels, which were significantly associated with disease activity parameters as the ESR, CRP and BASDAI. Moreover, an increase in disease activity was associated with a decrease in total cholesterol levels and a more pronounced decrease in HDLc levels, subsequently resulting in a more atherogenic lipid profile.
The magnitude in which disease activity influences the lipid levels was limited, which questions its clinical relevance. However, although the observed influence is small, it should be noted that ankylosing spondylitis is a chronic inflammatory disease, which means that this small (detrimental) influence has a potential clinically relevant effect on the cardiovascular risk for many years. The importance of small differences in lipid levels over a prolonged period is best illustrated by several studies in which cardiovascular risk reduction was found by lowering lipid levels slightly but over a prolonged period. Firstly, the follow‐up of the Framingham cohort showed that a lower HDLc level of just 0.25 mmol/l was associated with a 50% higher risk for future vascular events.24 Secondly, a landmark study on fibrates showed a 22% reduction in the risk for CVD in the group receiving the active compound versus placebo. The group receiving the active compound showed only small changes in lipid levels—that is, 4% decrease in total cholesterol, 6% increase in HDLc and 31% decrease in triglyceride levels.25 Although these figures cannot be directly extrapolated to this study, they do indicate the clinical relevance of small changes in lipid levels.
Growing evidence suggests that inflammation has an important role in the pathogenesis of CVD, particularly in atherosclerosis.26 In addition to a postulated direct effect of inflammation on endothelial cells, mounting evidence suggests that inflammation can also increase the cardiovascular risk by deterioration of the lipid profile, which is supported by the findings of a study showing a decrease in HDLc and apolipoprotein A I levels and an increase in triglyceride and apolipoprotein B levels during an acute‐phase response.27 Other investigators found an association between an increase in lipids as oxidised low‐density lipoprotein cholesterol and proinflammatory cytokines as CRP, interleukin 6 and tumour necrosis factor α.28 The findings of the present study confirm these effects of inflammation on the various lipid concentrations.
High disease activity is characterised by increased cytokine expression and this could directly lead to altered lipid levels through effects on the liver or adipose tissue. Moreover, there may also be an indirect way through various intermediate factors—for example, metabolic or dietary factors. Patients with ankylosing spondylitis with high disease activity might be in a metabolic state comparable to those with rheumatoid cachexia.29 Decreasing disease activity would improve their general well‐being and physical function, with subsequent changes in lipid levels. The results of the present study support this hypothesis, as lower disease activity (parameters) was associated with a more favourable lipid profile.
This investigation is in line with the accumulating evidence of the intriguing interactions between dyslipidaemia, atherosclerosis and inflammation, showing a worsening of the lipid profile during increased disease activity. Obviously, more prospective investigations with cardiovascular end points are needed to further unravel these relationships.
Acknowledgements
We thank Professor Dr YS Smulders and Dr D van Schaardenburg for their advice and critical reading of the manuscript.
Abbreviations
BASDAI - Bath Ankylosing Spondylitis Disease Activity Index
CRP - C reactive protein
CVD - cardiovascular disease
ESR - erythrocyte sedimentation rate
HDLc - high‐density lipoprotein cholesterol
Footnotes
Competing interests: None.
Ethical approval: The local ethics committee approved the study.
References
- 1.Lehtinen K. Mortality and causes of death in 198 patients admitted to hospital with ankylosing spondylitis. Ann Rheum Dis 199352174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radford E P, Doll R, Smith P G. Mortality among patients with ankylosing spondylitis not given X‐ray therapy. N Engl J Med 197711572–576. [DOI] [PubMed] [Google Scholar]
- 3.Kaprove R E, Little A H, Graham D C. Ankylosing spondylitis: survival in men with and without radiotherapy. Arthritis Rheum 19802357–61. [DOI] [PubMed] [Google Scholar]
- 4.Smith P G, Doll R. Mortality among patients with ankylosing spondylitis after a single treatment course with X‐rays. BMJ 198213449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters M J, van der Horst‐Bruinsma I E, Dijkmans B A, Nurmohamed M T. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum 200434585–592. [DOI] [PubMed] [Google Scholar]
- 6.Divecha H, Sattar N, Rumley A, Cherry L, Lowe G D, Sturrock R. Cardiovascular risk parameters in men with ankylosing spondylitis in comparison with non‐inflammatory control subjects: relevance of systemic inflammation. Clin Sci (London) 2005109171–176. [DOI] [PubMed] [Google Scholar]
- 7.Kwiterovich P O. Detection and treatment of elevated blood lipids and other risk factors for coronary artery disease in youth. Ann N Y Acad Sci 1995748313–330. [DOI] [PubMed] [Google Scholar]
- 8.Rader D J. High density lipoproteins and atherosclerosis. Am J Cardiol 200290(Suppl)i62–i70. [DOI] [PubMed] [Google Scholar]
- 9.Lazarevic M B, Vitic J, Mladenovic V, Myones B L, Skosey J L, Swedler W I. Dyslipoproteinemia in the course of active rheumatoid arthritis. Semin Arthritis Rheum 199222172–178. [DOI] [PubMed] [Google Scholar]
- 10.Sharett A R, Ballantyne C M, Coady S A, Heiss G, Sorlie P D, Catellier D.et al Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A‐1 and B, and HDL density subfractions: the atherosclerosis risk in communities (ARIC) study. Circulation 20011041108–1113. [DOI] [PubMed] [Google Scholar]
- 11.Masi A T, Aldag J C, Mohan P C, Murugan T S R. Determinants of significantly lower serum total cholesterol levels in ankylosing spondylitis patients than age‐, gender‐, and medical service‐matched control patients: results of multivariate analysis. Arthritis Rheum 199942(Suppl)S300 [Google Scholar]
- 12.Jones S M, Harris C P D, Lloyd J, Stirling C A, Reckless J P D, Mc Hugh N J. Lipoproteins and their subfractions in psoriatic arthritis: identification of an atherogenic profile with active joint disease. Ann Rheum Dis 200059(11)904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masi A T, Aldag J C, Mohan P C, Murugan T S R. Significantly lower serum triglyceride levels in ankylosing spondylitis patients than age‐, gender‐, and medical service‐matched controls: results of multivariate analysis. Arthritis Rheum 200043(Suppl)S104 [Google Scholar]
- 14.Cabana V G, Siegel J N, Sabesin S M. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res 19893039–49. [PubMed] [Google Scholar]
- 15.Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR study). Arterioscler Thromb Vasc Biol 2002221162–1167. [DOI] [PubMed] [Google Scholar]
- 16.van Denderen J C, van der Paardt M, Nurmohamed M T, De Ryck Y M, Dijkmans B A, van der Horst‐Bruinsma I E. Double‐blind, randomised, placebo‐controlled study of leflunomide in the treatment of active ankylosing spondylitis. Ann Rheum Dis 2005641761–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calin A, Dijkmans B A, Emery P, Hakala M, Kalden J, Leirisalo‐Repo M.et al Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004631594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Linden S, Valkenburg H A, Cats A. Evaluation of the diagnostic criteria for ankylosing spondylitis; a proposal for the modification of the New York criteria. Arthritis Rheum 198427361–368. [DOI] [PubMed] [Google Scholar]
- 19.Jones S D, Steiner A, Garrett S L, Calin A. The Bath Ankylosing Spondylitis Patient Global Score (BAS‐G). Br J Rheumatol 19963566–71. [DOI] [PubMed] [Google Scholar]
- 20.Calin A, Garrett S, Whitelock H, Kennedy L G, O'Hea J, Mallorie P.et al A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994212281–2285. [PubMed] [Google Scholar]
- 21.Garrett S, Jenkinson T, Kennedy L G, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994212286–2291. [PubMed] [Google Scholar]
- 22.Twisk J W R.Applied longitudinal data analysis for epidemiology. A practical guide. Cambridge, UK: Cambridge University Press, 2003
- 23.Goldstein H, Browne W, Rasbash Multilevel modelling of medical data. JStat Med2002213291–3315. [DOI] [PubMed] [Google Scholar]
- 24.Sprecher D L, Watkins T R, Behar S, Brown W V, Rubins H B, Schaefer E J. Importance of high‐density lipoprotein cholesterol and triglyceride levels in coronary heart disease. Am J Cardiol 200391575–580. [DOI] [PubMed] [Google Scholar]
- 25.Rubins H B, Robins S J, Collins D, Fye C L, Anderson J W, Elam M B.et al Veterans Affairs High‐Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high‐density lipoprotein cholesterol. N Engl J Med 1999341410–418. [DOI] [PubMed] [Google Scholar]
- 26.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999340115–126. [DOI] [PubMed] [Google Scholar]
- 27.Burger D, Dayer J M. High‐density lipoprotein‐associated apolipoprotein A‐1: the missing link between infection and chronic inflammation? Autoimmun Rev 20021111–117. [DOI] [PubMed] [Google Scholar]
- 28.Hyka N, Dayer J M, Modoux C, Kohno T, Edwards C K, Roux‐Lombard P.et al Apolipoprotein A‐1 inhibits the production of interleukin‐1β and tumour necrosis factor‐α by blocking contact‐mediated activation of monocytes by T‐lymphocytes. Blood 2001972381–2389. [DOI] [PubMed] [Google Scholar]
- 29.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol 20028588–99. [DOI] [PubMed] [Google Scholar]