Abstract
The significance of immune responses to certain heat shock proteins (HSPs) that develop in virtually all inflammatory diseases is only now becoming clear. In experimental models, HSPs prevent or arrest inflammatory damage, and initial clinical trials in chronic inflammatory disease have shown HSP peptides to promote production of anti‐inflammatory cytokines—indicating immunoregulatory potential. HSPs are ubiquitous self‐antigens that are highly expressed in inflamed tissues. The prokaryotic homologous proteins, present in every bacterial species, are dominantly immunogenic. This is striking, especially as these proteins have large areas of sequence homologies with the host (mammalian) counterparts. In several experimental models of autoimmune diseases, immunisation with bacterial HSPs inhibited disease development, as did oral/nasal administration. Based on the experimental evidence so far, it is tempting to speculate that: firstly, exposure to homologues of these self‐antigens, as present in, for instance, the bacterial intestinal flora, has a decisive impact on the regulation of self‐tolerance at the level of T cells; and secondly, such proteins or their derivative peptides may have a role in an antigen specific immunotherapy approach involving modulation of relevant T cells, without the immediate necessity of defining disease specific autoantigens. Recent findings in experimental asthma and atherosclerosis have indicated that the field of application of such immunotherapy can be broader than just autoimmunity.
Keywords: heat shock proteins, T cells, regulation, chronic inflammation
Heat shock proteins (HSPs) were originally discovered as proteins induced by heat stress and were named accordingly. Nevertheless, other stressful stimuli, such as hypoxia, toxic chemicals, and inflammation, also induce HSP expression.1,2 Several distinct families of HSPs can be distinguished, based on their molecular weight: HSP10, HSP40, HSP60, HSP70, HSP90, and HSP100. All the members of the HSP families play an important role in cell survival under both physiological and stress conditions due to their function in chaperoning other intracellular proteins during (re)folding and assembly.3,4
HSPs are evolutionary highly conserved proteins and are abundantly expressed in both prokaryotic and eukaryotic organisms. Despite their evolutionary sequence conservation, even between microbes and their host self‐homologues, the microbial proteins are highly immunogenic and have been implicated in the control of autoimmune inflammation due to a cross‐reactive immune response. HSP immunisations have been shown to inhibit autoimmune disorders such as diabetes and arthritis both in animal models and in initial clinical trials in patients with chronic inflammatory disease.5,6,7,8,9,10,11,12
Over the past years, several research groups have shown that HSP reactive T cells might play a role in this regulatory function and in the induction of anti‐inflammatory cytokines in chronic inflammation.
Immune suppression
The observation that eukaryotic and prokaryotic HSPs have high sequence homology prompted the hypothesis that HSPs might be potential candidates for molecular mimicry and could act as potentially dangerous autoantigens. Studies showing that elevated HSP levels and anti‐HSP antibodies are present in autoimmune inflammatory responses, such as arthritis, multiple sclerosis, and diabetes, seemed to support this hypothesis. Intriguingly though, initial studies in adjuvant arthritis, a Mycobacterium tuberculosis induced arthritis, demonstrated that immunisation with the dominant mycobacterial antigen, HSP60, abrogated subsequently induced disease.13
This protective effect is not exclusive for adjuvant arthritis and has been detected in an array of chronic inflammatory animal models, such as experimental autoimmune encephalomyelitis, collagen induced arthritis, and diabetes.5,14,15,16 As the latter models do not depend on immunisation with M. tuberculosis, the suppressive effect of mycobacterial HSP60 seems independent of the subsequent disease inducing antigen. In addition, experiments exploring the suppressive capacity of mycobacterial HSP60 in more detail have illustrated that only the self‐HSP cross‐reactive peptides induced protection against adjuvant arthritis via the induction of self‐HSP‐reactive regulatory T cells.17
Innate immunity
Besides modulating inflammatory responses via the induction of HSP‐reactive regulatory T cells, HSPs can directly activate the immune system through surface receptors such as toll‐like receptor (TLR)2, TLR4, CD91, CD40 and CD14.18,19 However, such reports have been questioned by studies indicating that proinflammatory activity of HSP via such receptors was solely due to bacterial contaminants.20,21,22 Even though this cannot be entirely excluded, because regular tests to exclude that contamination by lipopolysaccharide (LPS) is responsible for the observed effects (such as polymyxin B preincubation and/or protein denaturing by boiling HSP), might not affect all other bacterial compounds, several reports show that highly purified HSP can still activate dendritic cells and macrophages. For example, HSP derived from murine liver and kidney has been shown to be able to activate dendritic cells and macrophages.23 Moreover, cells that were triggered to upregulate cell‐surface HSP70 could induce TLR2 and TLR4 signalling in macrophages.24,25
In addition to these reports of highly purified HSP, other papers have described distinct activation of TLR signalling after HSP binding. HSP induced signalling via TLR is dependent on CD14 expression, whereas LPS signalling is still present in the absence of the coreceptor, though signalling is enhanced in the presence of CD14. Furthermore HSP70 induces a Ca2+ flux in monocytes after TLR binding, which is absent after LPS triggering.25,26 Moreover, HSP60 induced TLR signalling has been shown to be dependent on endocytosis, in contrast with LPS, which signals at the cell surface.27
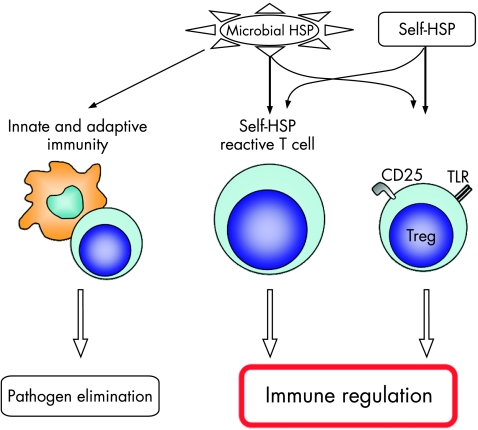
Although extensive data support a role for HSP in the activation of both innate and adaptive immune cells (fig 1), it is apparent from the above mentioned data that knowledge of the origin and purity of the HSP proteins used in experiments is important.
Figure 1 The complexity of heat shock protein (HSP)–immune‐system interactions. Microbial HSPs trigger both innate and adaptive immune reactions leading to pathogen elimination in infection. Self‐HSPs are involved in immune reactions leading to immunoregulation. Conserved epitopes of microbial HSP trigger self‐HSP‐reactive T cells, which lead to immunoregulation. By this, in the kinetics of microbial HSP immunity, proinflammatory responses are followed by inflammation dampening immunoregulation. In addition, self‐HSPs activate through the direct triggering of innate receptors on regulatory T (Treg) cells. TLR, toll‐like receptor.
Mechanisms
Initial reports showed that immunisation with mycobacterial HSP60 protected animals against adjuvant arthritis. Characterisation of the protective mycobacterial HSP60 epitopes showed that only conserved epitopes that induced a T cell response to self‐HSP60 abrogated arthritis induction.17 T cell lines specific for this self‐HSP peptide could transfer protection against the induction of adjuvant arthritis in Lewis rats.
Since upregulation of HSPs is part of an inflammatory response, self‐HSP‐reactive T cells with a regulatory phenotype might be part of the physiological termination of the inflammatory response. Several studies in which bacterial HSP60 or HSP70 pre‐immunisations were used to protect against subsequently induced inflammatory diseases have shown induction of interleukin (IL)‐10 producing T cells on restimulation with both bacterial and self‐HSPs.9,17,28,29 Also in a murine model of proteoglycan induced arthritis (PGIA), mycobacterial HSP70 protected against disease induction. Analysis of the lymphoid cells obtained from the HSP70 protected mice revealed the presence of IL‐10 producing cells on restimulation with HSP70 as well as with the arthritis inducing PG. In other words, HSP immunisation has led to the production of a regulatory phenotype in both HSP specific cells and cells specific for the disease associated PG antigen. This indicates that HSP immunisation might spread regulatory activity in cases where the autoantigen is unknown to potentially proinflammatory T cells and still can induce antigen specific regulation (Berlo et al, unpublished results, 2006). Recently we have developed a new TCR‐Tg mouse that expresses T cells specific for arthritis inducing human cartilage PG.30
To explore the mechanism of immune regulation by antigen specific IL‐10 producing T cell in an arthritis model in more depth, we have retrovirally transduced these PG specific cells with the murine IL‐10 gene and green fluorescent protein (GFP) as selection marker. Transfer of these antigen specific IL‐10+ cells not only suppressed the induction of arthritis in acceptor mice compared with cells transduced with GFP alone, but also induced IL‐10 producing proteoglycan specific T cells within these mice, thereby spreading their regulatory function. Moreover, transfer of T cells with irrelevant TCR specificity did not abolish the development of PGIA when transduced with the same construct, emphasising the importance of antigen specificity of the regulatory T cells (Guichelaar et al, unpublished results, 2006). These data suggest that spreading of the regulatory capacity is both dependent on IL‐10 production and TCR specificity. However, it is attractive to assume that comparable mechanisms play a role in the spreading of tolerance after HSP70 immunisation in PGIA.
Self‐HSP‐reactive T cells are not only part of the adult T cell repertoire, but also are considered to play an important role in maintaining immune homoeostasis. Studies using transgenic animals overexpressing HSP60 still displayed self‐HSP‐reactive T cell responses, underlining that the lack of central deletion is not because of the absence of HSP expression in the thymus.31 This suggests that during T cell development in the thymus central deletion is either not occurring or not complete, allowing the development of self‐HSP‐reactive T cells. Under homoeostatic conditions, these self‐reactive T cells do not induce autoimmune inflammatory responses. Therefore, these self‐HSP‐specific T cells must be kept in a tolerant or regulatory mode. This can be achieved by the regular exposure to regulatory cytokines such as IL‐10. IL‐10 is known to be associated with stress (stressed cells do produce IL‐10) and therefore exposure to HSP. Expansion of HSP‐reactive T cells usually occurs in the presence of IL‐10. In addition, HSP expression is upregulated in all cells at the site of inflammation, which includes non‐professional antigen presenting cells lacking costimulatory molecules. These cells will induce an anergic state in these HSP specific T cells. Finally, high levels of commensal HSPs are present at the normal mucosa, and since mucosal antigen exposure induces regulatory T cells32,33,34 exposure to such HSPs might favour the induction of HSP specific mucosal regulatory T cells. This is supported by studies showing that mucosal administration of HSP60 indeed induces regulatory T cells that mirror the phenotype of mucosally induced regulatory T cells to previously novel antigens in IL‐10 production.35 Furthermore, changes in the gut microflora have been shown to change the susceptibility to subsequently induced arthritis.36,37,38,39
Recent reports in the literature further support the role of HSP induced regulatory activity. Besides direct induction of HSP‐specific regulatory T cells due to presentation at tolerance inducing mucosal sites, one can speculate that HSP might aid in the expansion of existing regulatory T cells via antigen non‐specific responses.
As described above HSPs have the ability to directly interact with different cells of the immune system by triggering TLR. HSP60 induced TLR signalling on T cells, independent of LPS, enhanced GATA3 expression, suggesting the induction of a T helper 2 phenotype.39,40 Also, HSP70 has been shown to induce anti‐inflammatory cytokines in both peripheral blood and synovial derived monocytes from patients with arthritis. In addition, HSP70 has been shown to enhance IL‐10 production by mouse bone marrow dendritic cells.41 These data suggest that HSPs might induce a microenvironment that is favourable for the induction of regulation.
In addition to direct signalling of HSP via TLR on antigen resenting cells, HSP60 has been shown to specifically activate T cells via TLR2 and regulate migration and adhesion.40 Intriguingly, TLR2 signalling on regulatory T cells has been shown to expand existing CD25+ regulatory T cells.42,43 In line with these findings, recently a fascinating study showed that HSP60 and HSP60‐derived peptide triggering of TLR2 on CD25+ regulatory T cells enhanced their suppressive function in in vitro assays.44 This direct enhancement of regulatory T cell number and function could significantly enhance the regulatory capacity of HSP immunisations.
Protective effects of bacterial HSP immunisations could be due to the high homology of the protein. Since only cross‐reactive peptides seem to induce a regulatory T cell response involving the induction of IL‐10, self‐HSP‐reactive responses might be regulatory, whereas bacterial HSP epitopes, which are uniquely present in microbial HSP, might induce proinflammatory responses. Which factors contribute to the selection and dominance of such self‐reactive epitopes on bacterial HSP immunisations are unclear, but such knowledge might contribute to the therapeutic potential of HSPs.
Data from animal experiments strongly point toward a regulatory role for HSP in arthritis. In addition, human trials have confirmed the immunomodulatory role of HSP. For example, in patients with juvenile idiopathic arthritis, HSP60 reactivity correlated with beneficial outcome of disease, and HSP60 specific T cells from patients had a regulatory phenotype, producing IL‐10 and transforming growth factor β.7,45 Moreover, among patients with rheumatoid arthritis, those with HSP60‐reactive synovial T cells acquired the regulatory phenotype on in vitro restimulation with self‐HSP60 and suppressed production of tumour necrosis factor by autologous blood T cells.46 Initial clinical trials using HSPs and HSP‐derived peptides showed promising results in dampening the clinical disease score in ongoing disease.8,11,12
In conclusion, it is clear that HSP proteins and peptides have an immunomodulatory role. Immune modulation is essential for an organism for prevention of excessive inflammation and subsequent organ damage, but inflammatory reactions are a prerequisite to eradicate harmful pathogens. Therefore, the immune system has developed several tightly regulated mechanisms, which, depending on time, location, and intensity of the inflammatory response, will be able to regulate the immune response. HSPs might play an important role in such an educated regulatory mechanism and might provide a strategic target for developing therapies in various inflammatory disorders, including rheumatoid arthritis.
Abbreviations
HSP - heat shock protein
IL - interleukin
PG - proteoglycan
PGIA - proteoglycan induced arthritis
TLR - toll‐like receptor
Footnotes
Competing interests: none declared
References
- 1.Lindquist S. The heat‐shock response. Annu Rev Biochem 1986551151–1191. [DOI] [PubMed] [Google Scholar]
- 2.Ellis R J. The molecular chaperone concept. Semin Cell Biol 199011–9. [PubMed] [Google Scholar]
- 3.Fink A L. Chaperone‐mediated protein folding. Physiol Rev 199979425–449. [DOI] [PubMed] [Google Scholar]
- 4.Craig E A. Chaperones: helpers along the pathways to protein folding. Science 19932601902–1903. [DOI] [PubMed] [Google Scholar]
- 5.Birk O S, Elias D, Weiss A S, Rosen A, van‐der Zee R, Walker M D.et al NOD mouse diabetes: the ubiquitous mouse hsp60 is a beta‐cell target antigen of autoimmune T cells. J Autoimmun 19969159–166. [DOI] [PubMed] [Google Scholar]
- 6.Elias D, Markovits D, Reshef T, van der Zee R, Cohen I R. Induction and therapy of autoimmune diabetes in the non‐obese diabetic (NOD/Lt) mouse by a 65‐kDa heat shock protein. Proc Natl Acad Sci U S A 1990871576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakken A B, van Eden W, Rijkers G T, Kuis W, Toebes E A, de Graeff‐Meeder E R.et al Autoreactivity to human heat‐shock protein 60 predicts disease remission in oligoarticular juvenile rheumatoid arthritis. Arthritis Rheum 1996391826–1832. [DOI] [PubMed] [Google Scholar]
- 8.Prakken B J, Samodal R, Le T D, Giannoni F, Yung G P, Scavulli J.et al Epitope‐specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A 20041014228–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL‐10‐producing T cells that cross‐react with the mammalian self‐hsp70 homologue. J Immunol 20001642711–2717. [DOI] [PubMed] [Google Scholar]
- 10.van Eden W, Hauet‐Broere F, Berlo S, Paul L, van der Zee R, de Kleer I.et al Stress proteins as inducers and targets of regulatory T cells in arthritis. Int Rev Immunol 200524(3–4)181–197. [DOI] [PubMed] [Google Scholar]
- 11.Albani S, Keystone E C, Nelson J L, Ollier W E, La Cava A, Montemayor A C.et al Positive selection in autoimmunity: abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nat Med 19951448–452. [DOI] [PubMed] [Google Scholar]
- 12.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen I R. Beta‐cell function in new‐onset type 1 diabetes and immunomodulation with a heat‐shock protein peptide (DiaPep277): a randomised, double‐blind, phase II trial. Lancet 20013581749–1753. [DOI] [PubMed] [Google Scholar]
- 13.van Eden W, Thole J E, van der Zee R, Noordzij A, van Embden J D, Hensen E J.et al Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 1988331171–173. [DOI] [PubMed] [Google Scholar]
- 14.Birnbaum G, Kotilinek L, Miller S D, Raine C S, Gao Y L, Lehmann P V.et al Heat shock proteins and experimental autoimmune encephalomyelitis. II: environmental infection and extra‐neuraxial inflammation alter the course of chronic relapsing encephalomyelitis, J Neuroimmunol 199890149–161. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen C, Gedon E, Jaquet C, Sany J. Gastric administration of recombinant 65 kDa heat shock protein delays the severity of type II collagen induced arthritis in mice. J Rheumatol 199825763–767. [PubMed] [Google Scholar]
- 16.Thompson S J, Butcher P D, Patel V K, Rook G A, Stanford J, van der Zee R.et al Modulation of pristane‐induced arthritis by mycobacterial antigens. Autoimmunity 19911135–43. [DOI] [PubMed] [Google Scholar]
- 17.Anderton S M, van der Zee R, Prakken B, Noordzij A, van Eden W. Activation of T cells recognizing self 60‐kD heat shock protein can protect against experimental arthritis. J Exp Med 1995181943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintana F J, Cohen I R. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol 20051752777–2782. [DOI] [PubMed] [Google Scholar]
- 19.Pockley A G. Heat shock proteins as regulators of the immune response. Lancet 2003362469–476. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Gao B, Tsan M F. Induction of cytokines by heat shock proteins and concanavalin A in murine splenocytes. Cytokine 200532149–154. [DOI] [PubMed] [Google Scholar]
- 21.Gao B, Tsan M F. Induction of cytokines by heat shock proteins and endotoxin in murine macrophages. Biochem Biophys Res Commun 20043171149–1154. [DOI] [PubMed] [Google Scholar]
- 22.Gao B, Tsan M F. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem 200327822523–22529. [DOI] [PubMed] [Google Scholar]
- 23.Panjwani N N, Popova L, Srivastava P K. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J Immunol 20021682997–3003. [DOI] [PubMed] [Google Scholar]
- 24.Korbelik M, Sun J, Cecic I. Photodynamic therapy‐induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res 2005651018–1026. [PubMed] [Google Scholar]
- 25.Asea A, Rehli M, Kabingu E, Boch J A, Bare O, Auron P E.et al Novel signal transduction pathway utilized by extracellular HSP70: role of toll‐like receptor (TLR) 2 and TLR4. J Biol Chem 200227715028–15034. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Whittall T, McGowan E, Younson J, Kelly C, Bergmeier L A.et al Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol 20051743306–3316. [DOI] [PubMed] [Google Scholar]
- 27.Vabulas R M, Ahmad‐Nejad P, da Costa C, Miethke T, Kirschning C J, Hacker H.et al Endocytosed HSP60s use toll‐like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin‐1 receptor signaling pathway in innate immune cells. J Biol Chem 200127631332–31339. [DOI] [PubMed] [Google Scholar]
- 28.Quintana F J, Carmi P, Mor F, Cohen I R. Inhibition of adjuvant‐induced arthritis by DNA vaccination with the 70‐kd or the 90‐kd human heat‐shock protein: immune cross‐regulation with the 60‐kd heat‐shock protein. Arthritis Rheum 2004503712–3720. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka S, Kimura Y, Mitani A, Yamamoto G, Nishimura H, Spallek R.et al Activation of T cells recognizing an epitope of heat‐shock protein 70 can protect against rat adjuvant arthritis. J Immunol 19991635560–5565. [PubMed] [Google Scholar]
- 30.Berlo S E, Guichelaar T, Ten Brink C B, van Kooten P J, Hauet‐Broeren F, Ludanyi K.et al Increased arthritis. Susceptibility in cartilage proteoglycan‐specific T cell receptor‐transgenic mice. Arthrtis Rheum 2006542423–2433. [DOI] [PubMed] [Google Scholar]
- 31.Anderson M S, Venanzi E S, Klein L, Chen Z, Berzins S P, Turley S J.et al Projection of an immunological self shadow within the thymus by the aire protein. Science 20022981395–1401. [DOI] [PubMed] [Google Scholar]
- 32.Strober W, Kelsall B, Marth T. Oral tolerance. J Clin Immunol 1998181–30. [DOI] [PubMed] [Google Scholar]
- 33.Samsom J N, Hauet‐Broere F, Unger W W, VAN Berkel L A, Kraal G. Early events in antigen‐specific regulatory T cell induction via nasal and oral mucosa. Ann N Y Acad Sci 20041029385–389. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Kuchroo V K, Inobe J, Hafler D A, Weiner H L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 19942651237–1240. [DOI] [PubMed] [Google Scholar]
- 35.Samsom J N. Regulation of antigen‐specific regulatory T‐cell induction via nasal and oral mucosa. Crit Rev Immunol 200424157–177. [DOI] [PubMed] [Google Scholar]
- 36.Kohashi O, Kuwata J, Umehara K, Uemura F, Takahashi T, Ozawa A. Susceptibility to adjuvant‐induced arthritis among germfree, specific‐pathogen‐free, and conventional rats. Infect Immun 197926791–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breban M A, Moreau M C, Fournier C, Ducluzeau R, Kahn M F. Influence of the bacterial flora on collagen‐induced arthritis in susceptible and resistant strains of rats. Clin Exp Rheumatol 19931161–64. [PubMed] [Google Scholar]
- 38.Thompson S J, Elson C J. Susceptibility to pristane‐induced arthritis is altered with changes in bowel flora. Immunol Lett 199336227–231. [DOI] [PubMed] [Google Scholar]
- 39.Zanin‐Zhorov A, Bruck R, Tal G, Oren S, Aeed H, Hershkoviz R.et al Heat shock protein 60 inhibits Th1‐mediated hepatitis model via innate regulation of Th1/Th2 transcription factors and cytokines. J Immunol 20051743227–3236. [DOI] [PubMed] [Google Scholar]
- 40.Zanin‐Zhorov A, Nussbaum G, Franitza S, Cohen I R, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. Faseb J 2003171567–1569. [DOI] [PubMed] [Google Scholar]
- 41.Detanico T, Rodrigues L, Sabritto A C, Keisermann M, Bauer M E, Zwickey H.et al Mycobacterial heat shock protein 70 induces interleukin‐10 production: immunomodulation of synovial cell cytokine profile and dendritic cell maturation. Clin Exp Immunol 2004135336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutmuller R P, den Brok M H, Kramer M, Bennink E J, Toonen L W, Kullberg B J.et al Toll‐like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest 2006116485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H, Komai‐Koma M, Xu D, Liew F Y. Toll‐like receptor 2 signaling modulates the functions of CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A 20061037048–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanin‐Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen I R. Heat shock protein 60 enhances CD+CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest 20061162022–2032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.de Kleer I M, Kamphuis S M, Rijkers G T, Scholtens L, Gordon G, De Jager W.et al The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T cells directed to human heat‐shock protein 60 capable of producing the regulatory cytokine interleukin‐10. Arthritis Rheum 2003482001–2010. [DOI] [PubMed] [Google Scholar]
- 46.van Roon J A, van Eden W, van Roy J L, Lafeber F J, Bijlsma J W. Stimulation of suppressive T cell responses by human but not bacterial 60‐kD heat‐shock protein in synovial fluid of patients with rheumatoid arthritis. J Clin Invest 1997100459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]