Abstract
Current data suggest that as many as 1 in 1000 treated individuals may develop progressive multifocal leucoencephalopathy (PML) in concert with the use of natalizumab. Natalizumab was withdrawn in early 2005. The present paper provides a comprehensive description of PML and reviews the role of natalizumab in the pathogenesis of PML. It is likely that use of drugs which cause specific perturbations of the immune system will be accompanied by similar rare infections. Thus researchers should be on the alert when using such agents in clinical trials.
Keywords: natalizumab, progressive multifocal leucoencephalopathy, demyelination, multiple sclerosis, monoclonal antibodies
On 28 February 2005, natalizumab (Tysabri), a promising drug for relapsing and remitting multiple sclerosis (MS)1,2 was withdrawn from clinical studies and the broader market. This followed the observation of three cases of progressive multifocal leucoencephalopathy (PML), a rare demyelinating central nervous system caused by the polyoma virus, JC virus (JCV). Two patients developed PML in the SENTINEL study3,4 which evaluated the value of natalizumab and interferon beta (Avonex) in the treatment of relapsing remitting MS. The third patient received natalizumab in a clinical trial for Crohn's disease.5 Approximately 3000 individuals had been treated with natalizumab at that time suggesting that as many as 1 in 1000 treated individuals may develop PML in concert with the use of natalizumab.6 From the data currently available, the incidence of other infectious complications of the central nervous system (CNS) with natalizumab did not significantly exceed that of the placebo groups. This unique association of natalizumab and PML was entirely unforeseen. Not only does this association serve as window into the biology of this rare infectious disorder, but it also provides a wake‐up call in the use of monoclonal antibodies that perturb specific components of the immune system.
Progressive multifocal leucoencephalopathy
PML is a rare disease that almost always occurs in the setting of immunosuppression. Typically, the abnormality is one of cell‐mediated immunity, more specifically, a general impairment of the T helper (Th) 1‐type Th cell function.7 A detailed review of the English literature from 1958, the year in which this disease was initially described,8 to 1984 identified only 230 cases.9 At that time, lymphoproliferative disorders were the most common predisposing illnesses9 accounting for more than 60%. Other predisposing illnesses in that series included myeloproliferative diseases (6.5%), carcinoma (2.2%), granulomatous and inflammatory diseases such as tuberculosis and sarcoidosis (7.4%), and other immune deficiency states (16.1%).9 Although acquired immune deficiency syndrome (AIDS) was included in the latter category, it accounted for 2.1% with but five recognised cases of PML occurring with the then recently described immunodeficiency disorder.10,11,12 However, the AIDS pandemic soon changed the demography of PML dramatically, dwarfing all other predisposing conditions. Indeed, before the adoption in 1996 of highly active antiretroviral therapy (HAART) for the treatment of AIDS, approximately 5% of all human immunodeficiency virus (HIV)‐infected persons ultimately developed PML.13,14 Statistics from the US Centers for Disease Control indicate that 88% of all PML is associated with immunosuppression due to HIV/AIDS15 and in some populations it is even higher.16
No other disorder predisposes to the development of PML with the same degree of frequency. An incidence of PML of 1 in 1000 for those treated with natalizumab would likely equal or exceed that of other predisposing conditions and appropriately raised concern. However, natalizumab is not the only monoclonal antibody associated with PML. Isolated case reports have been reported in patients receiving another monoclonal antibody, rituximab,17,18,19,20 a chimeric anti‐CD20 monoclonal antibody used in the treatment of B cell lymphoma.21 In contrast with natalizumab, the patients treated with rituximab had had underlying lymphoproliferative disorders and were treated with aggressive chemotherapeutic regimens, both well established risk factors for the development of PML. Indeed, a review of 46 non‐HIV infected patients with PML in the setting of lymphoproliferative disorders suggested that rituximab had actually delayed the onset of PML.17
Clinical and radiographic manifestations
As its name implies, PML involves the white matter of the CNS typically resulting in a multifocal disturbance, but isolated lesions may be seen. The clinical manifestations simply reflect the sites of nervous system involvement. The most common abnormalities are weakness, cognitive disturbances, and visual impairment.9,22 Other symptoms include gait disorders, sensory loss, speech and language disturbances, headache, and seizures. The visual impairment is chiefly a visual field loss due to involvement of the retrochiasmal visual pathways. Unlike MS, optic nerve disease does not occur with PML. Likewise, although the lesions of PML may be observed rarely at autopsy in the spinal cord,23 clinical myelopathy secondary to PML is essentially unheard of. Differences in frequency may exist regarding the symptoms and signs of PML in patients with AIDS versus those with other predisposing conditions, particularly with respect to seizures and headaches, which appear to be more common in the former, and visual abnormalities, which may be more common in the latter.
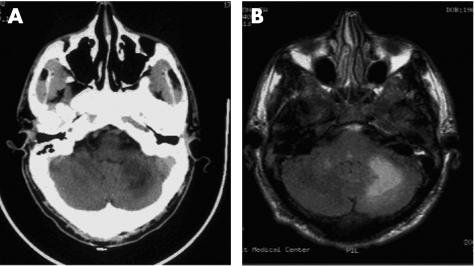
Neuroimaging is critical to establishing the diagnosis. The computed tomography scan (fig 1A) reveals hypodense lesions in the white matter of the brain without mass effect.24 Faint contrast enhancement at the borders of the lesions occurs in less than 10% of cases.24 Magnetic resonance imaging of the brain reveals hyperintense signal abnormalities on T2‐weighted and fluid attenuated inversion recovery images (FLAIR) (fig 1B). These lesions are typically hypointense on T1‐weighted images.24 A faint peripheral enhancement with gadolinium is observed on occasion.24 Lesions tend to predominate in the frontal and parieto‐occipital lobes, but may occur in virtually any region, including the basal ganglia, brainstem, and cerebellum.24 Mass effect is unanticipated; however, both mass effect and florid contrast enhancement may be seen with the immune reconstitution inflammatory syndrome (IRIS), a disorder that attends the effective recovery of the immune system that typically follows the introduction of an antiretroviral regimen in treatment‐naive AIDS patients.25,26
Figure 1 Neuroimaging of progressive multifocal leucoencephalopathy. (A) Computed tomography scan showing a large hypodense lesion in the right cerebellar hemisphere. (B) Magnetic resonance (FLAIR) image showing a hyperintense signal abnormality in the right cerebellar hemisphere.
Diagnosis
The gold standard for diagnosis of PML remains the demonstration of a classic histopathological triad, namely, demyelination (fig 2), enlarged oligodendroglial nuclei, and bizarre astrocytes, coupled with the immunocytochemical or electron microscopic demonstration of the causative JC virus. In the absence of brain biopsy, a compatible clinical and radiographic presentation associated with the demonstration of JC virus in the cerebrospinal fluid (CSF) by polymerase chain reaction is virtually diagnostic of the disorder. CSF polymerase chain reaction can detect 10 copies per 10 μl27,28 and is rarely, if ever, falsely positive (EO Major, personal communication, 1 October 2005), although sensitivity varies widely.
Figure 2 Pathology of progressive multifocal leucoencephalopathy. (A) Gross specimen shows a moth‐eaten appearance in the region of demyelination in the subcortex of the right frontal lobe. (B) Histopathological specimen (haematoxylin and eosin, Luxol fast blue stain) shows the demyelination in the affected subcortical region.
Prognosis and treatment
The prognosis of PML is poor. In the pre‐HAART era, the median survival of PML complicating AIDS was 3.5 months.16,29 In one study of 154 patients with AIDS‐associated PML, more than 50% of patients were dead within one month of the time of diagnosis.16 Survival in this subset of patients does not appear to be significantly different from that occurring with any other immunosuppressive condition. Restoration of the immune system as occurs following the removal of immunosuppressive regimens30 or the institution of HAART in AIDS patients may result in longer survival and stabilisation or recovery of the neurological disorder. Even before the availability of HAART, survival exceeding 12 months had been observed in as many as 10% of patients with AIDS‐associated PML.16,31 Following the introduction of HAART, as many as 50% of patients with AIDS‐associated PML demonstrated long term survival (>12 months).32,33,34,35,36,37,38 The benefit of HAART in AIDS‐associated PML has not been universally observed39 and appears to be most evident in treatment‐naive patients.40
A tight correlation exists between the cellular immune response against JCV and a favourable clinical outcome.41,42,43 JCV specific cytotoxic T lymphocytes in these patients are likely associated with inflammatory infiltrates that result in alterations of the blood–brain barrier and the contrast enhancement seen on imaging studies. These JCV specific cytotoxic T lymphocytes likely prevent further disease progression and decrease CSF JC viral load by eliminating infected oligodendrocytes. The lack of recurrence of PML in some of the patients exhibiting long term survival and recovery reflects clearance of JCV from the CSF.38 Recovery of an effective immune response to JCV, as may occur following the introduction of HAART, occasionally results in IRIS. This syndrome is characterised by new or worsening neurological deficits, an increased number or size of lesions observed by neuroimaging, contrast enhancement of these lesions, and brain oedema.25,44,45,46,47,48 Fatal outcomes have been reported.25,49 Parenthetically, IRIS was seen three months after the discontinuation of natalizumab in one of the reported patients, the only currently surviving individual with natalizumab associated PML.4
There are no proved therapies for PML. Nucleoside analogues have been employed because they impede the synthesis of DNA.50 In vitro studies51 have clearly demonstrated the ability of cytosine arabinoside (Cytarabine, ARA‐C), a cytosine analogue, to inhibit JCV replication. A large number of anecdotal reports and small case series have suggested the value of this therapy in PML52,53,54,55,56,57; however, a carefully conducted clinical trial of AIDS‐related PML failed to show any value of either intravenous or intrathecal administration of ARA‐C when compared with placebo.58 Among the suggested but unproved therapies are topotecan and camptothecin (topoisomerase I inhibitors),59,60,61 cidofovir,62,63 small interfering RNAs,64 interleukin (IL)‐2,65,66 and interferons.56,67,68 The improved understanding of the mechanisms of JCV entry into the cell69,70,71 may lead to new forms of therapy.
Biology of PML and JCV
On the basis of the electron microscopic appearance, ZuRhein in 1965 suggested that PML was the consequence of a polyoma virus.72 Her impression was confirmed when a polyoma, labelled JC virus, was isolated in human fetal glial cells.73 To date, JCV is the only virus confirmed to be responsible for the disorder.74 JCV belongs to the Polyomavirus genus in the Papovaviridae family. It is a double stranded DNA virus with icosahedral symmetry that codes for six regulatory and structural proteins including the non‐structural but multifunctional protein (T) and three capsid proteins (VP1, VP2, VP3). The T protein has been demonstrated to be associated with malignant transformation of astroglial and other tissues.75
Seroepidemiological studies indicate that this virus is ubiquitous with a worldwide distribution that plateaus by age 20 years.76 As many as 80–90% of some populations has been exposed to JCV and in some urban areas, this number may exceed 95%,76 although lower rates of exposure occur in isolated populations.77 Following infection, the virus becomes latent in some tissues. Whether latency universally follows infection remains uncertain. Tissues that are latently infected include bone marrow, tonsils, spleen, and kidney.78,79,80,81 The virus uses the 5‐hydroxytryptamine 2a receptor69 and enters the infected cell through clathrin dependent endocytosis.70,71 The genotype of JCV capable of infecting the brain exhibits a 98 base pair tandem repeat in its promoter region which is not evident in JCV isolated from the kidney or urine82 indicating that the genome of the promoter region is important for cellular tropism. The oligodendrocyte, the glial cell responsible for the production of CNS myelin, supports the lytic cycle of JCV infection with cell death resulting from necrosis leading to demyelination.83 JCV infection of astrocytes is typically non‐productive84 though the infection may be either lytic or abortive.83
JC virus DNA can be demonstrated in circulating B lymphocytes. It is present in the blood of ⩾90%85 of persons with PML. In immunosuppressed populations, JCV has been detected by polymerase chain reaction in the peripheral blood mononuclear cells with variable frequencies ranging from 18%86 to 38%,85 and its presence appears to correlate inversely with CD4 lymphocyte counts.86 The detection of JCV in otherwise healthy individuals is rare.6,87
The development of PML is likely a stochastic occurrence in which a series of events must occur in order for clinical disease to occur. Firstly, the individual must be infected with JCV. Secondly, the virus must establish latency. Studies of antibody to JCV in patients with PML demonstrate IgG rather than IgM implying that the illness results from a recrudescence of a latent infection rather than a recently acquired infection.88 Thirdly, JCV must have the appropriate gene arrangement in the promoter region to support neurotropism. Gene rearrangement may be necessary for this to develop in some individuals. Fourthly, JCV needs to be reactivated and, once reactivated, travel into the brain either as cell free virus or, perhaps more likely, as cell associated virus. Lastly, the virus must establish productive infection of the oligodendrocytes and avoid clearance by JCV specific cytotoxic T lymphocytes, the predominant cellular immunosurveillance mechanism.
AIDS and natalizumab in the pathogenesis of PML
HIV infection is clearly unique in predisposing to PML. Prior to the introduction of HAART, 1 in 20 HIV‐infected individuals ultimately developed PML.89 This percentage overwhelms that of all other underlying illnesses combined, including B cell malignancies. Among potential explanations for the increased frequency of PML in the setting of HIV infection are the following89:
differences in the degree and duration of the cellular immunosuppression in HIV infection compared with other immunosuppressive conditions
reactivation of latent JCV in B lymphocytes (possibly secondary to immune signals leading to polyclonal B cell activation)
facilitation of the entry into the brain of JCV infected B lymphocytes by alterations in the blood–brain barrier due to HIV, and as a consequence of upregulation of adhesion molecules on the brain vascular endothelium due to HIV infection
transactivation of JCV by the HIV tat protein and by cytokines and chemokines induced by HIV infection
Any or all of these may be responsible for the observed increase.
Natalizumab and PML
The current estimate of the incidence of PML with natalizumab use is roughly 1 in 1000 for patients90; however, this estimate is based on relatively small numbers. If the hypothesis regarding the pathogenesis of PML is correct, the longer an individual is immunosuppressed, the greater the likelihood of developing the disorder. A higher frequency may pertain for patients treated with longer courses of natalizumab, as the two MS patients were treated for 244 and 31 months3 before developing the disorder. The patient in the Crohn's trial had a shorter course of therapy, but had been on other immunosuppressive agents and was lymphopenic when natalizumab was reinitiated.5 Additional surveillance will be required to ascertain the precise risk for the development of PML and whether duration of therapy influences the appearance of the disorder. Although larger numbers of patients were treated when the drug became commercially available in November 2004, it was removed from the market in February 2005; therefore, those individuals received a very short course of therapy, estimated at one to three courses of treatment.90 A review of 3417 patients who had been exposed to natalizumab in clinical trials failed to reveal additional cases of PML. Also, no JCV was detected in the CSF from 329 patients receiving natalizumab in MS trials or 67 in trials for Crohn's disease or rheumatoid arthritis.90 To date, it appears that PML was unique in that there were no other opportunistic infections that occurred with a significantly increased frequency in the natalizumab treated population. The estimated incidence suggests that, although substantially lower than its occurrence in AIDS, natalizumab predispose individuals to the development of PML at a rate that may exceed that of most other underlying disorders.
Natalizumab, a monoclonal antibody to α4 integrin that prevents entry of inflammatory cells into brain and other tissues that use α4 integrin (VLA 4) to bind with vascular cell adhesion molecule (VCAM),91 effectively prevents the entry of inflammatory cells into the brain. One month after the administration of the drug, 80% of binding sites remain occupied.91 Based on its effect on gadolinium enhancing lesions, its biological activity persists for at least three months.92 In natalizumab treated MS patients, CD4+ and CD8+ T cells, CD 19+ B cells, and CD138+ plasma cells were significantly reduced in the CSF compared with controls and CD4/CD8 ratios in the CSF mirrored those seen in HIV‐infected patients.93 Although CSF CD4/CD8 ratios normalised six months after cessation of therapy, low CSF lymphocyte counts persisted.93
Why natalizumab appears to uniquely predispose to the development of PML remains unexplained. Two mechanisms immediately come to mind. Firstly, natalizumab is likely to have an important effect on the cellular immune response to JCV infection. JCV specific cytotoxic T lymphocytes are critical for containing PML.42,43 These cells may be important not only in the brain, but also at peripheral sites of latency containing JCV at the time of reactivation where they may control reactivation of JCV in the bone marrow, spleen, and possibly tonsillar tissues. The administration of natalizumab would be anticipated to prevent the entry of these cells into the brain and perhaps to sites of viral latency precluding it from clearing or suppressing the viral infection.
Secondly, α4β1 VCAM mediates the homing and retention of lymphocytes in the bone marrow and spleen.94 Blocking α4β1 integrin could result in a release of B cells from the bone marrow and spleen,94 the very sites of JCV latency. Coupled with diminished immunosurveillance for JCV at these sites, this might increase the load of JCV in the peripheral blood. Supporting the premise that B cells are mobilised by natalizumab are reports of increased numbers of circulating lymphocytes95 following the administration of natalizumab. Whether this mechanism is operative will require additional study. A study of 214 patients enrolled in natalizumab studies, from whom stored plasma was available showed that 5 (2.3%) had JCV detectable by polymerase chain reaction95; however, assaying JCV titres in plasma may underestimate its actual load.
The coadministration of Avonex (interferon beta1a) with natalizumab in both MS patients developing PML suggests the possibility that this drug served as a cofactor for the development of PML. The absence of reported cases of PML in MS patients treated with interferon beta and the development of PML in the Crohn's patient5 who had not received interferon beta implies that natalizumab alone predisposes to PML. As with many of the questions surrounding the relation between PML and natalizumab, additional surveillance in an expanded population should answer this and other questions.
Conclusion
The occurrence of PML with natalizumab was unanticipated. Almost certainly, similar rare infections will accompany the use of this new class of drug, which results in highly specific perturbations of the immune system. A high state of vigilance will be required for these agents as we obtain increased experience with their use. Rational protocols developed from our increased understanding of disease pathogenesis will likely assist in lowering the risk of these complications. A scientifically rigorous risk–benefit analysis will need to be performed in every instance to rationally inform treating physicians and the patients for whom they are responsible.
Abbreviations
AIDS - acquired immune deficiency syndrome
CNS - central nervous system
CSF - cerebrospinal fluid
HAART - highly active antiretroviral therapy
HIV - human immunodeficiency virus
JCV - JC virus
MS - multiple sclerosis
PML - progressive multifocal leucoencephalopathy
Footnotes
Competing interests: none declared
References
- 1.Miller D H, Khan O A, Sheremata W A, Blumhardt L D, Rice G P, Libonati M A.et al A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 200334815–23. [DOI] [PubMed] [Google Scholar]
- 2.O'Connor P W, Goodman A, Willmer‐Hulme A J, Libonati M A, Metz L, Murray R S.et al Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology 2004622038–2043. [DOI] [PubMed] [Google Scholar]
- 3.Kleinschmidt‐DeMasters B K, Tyler K L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta‐1a for multiple sclerosis. N Engl J Med 2005353369–374. [DOI] [PubMed] [Google Scholar]
- 4.Langer‐Gould A, Atlas S W, Green A J, Bollen A W, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005353375–381. [DOI] [PubMed] [Google Scholar]
- 5.Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M.et al Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med 2005353362–368. [DOI] [PubMed] [Google Scholar]
- 6.Berger J R, Koralnik I J. Progressive multifocal leukoencephalopathy and natalizumab—unforeseen consequences. N Engl J Med 2005353414–416. [DOI] [PubMed] [Google Scholar]
- 7.Weber F, Goldmann C, Kramer M, Kaup F J, Pickhardt M, Young P.et al Cellular and humoral immune response in progressive multifocal leukoencephalopathy. Ann Neurol 200149636–642. [PubMed] [Google Scholar]
- 8.Astrom K E, Mancall E L, Richardson E P., Jr Progressive multifocal leuko‐encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain 19588193–111. [DOI] [PubMed] [Google Scholar]
- 9.Brooks B R, Walker D L. Progressive multifocal leukoencephalopathy. Neurol Clin 19842299–313. [PubMed] [Google Scholar]
- 10.Bernick C, Gregorios J B. Progressive multifocal leukoencephalopathy in a patient with acquired immune deficiency syndrome. Arch Neurol 198441780–782. [DOI] [PubMed] [Google Scholar]
- 11.Miller J R, Barrett R E, Britton C B, Tapper M L, Bahr G S, Bruno P J. Progressive multifocal leukoencephalopathy in a male homosexual with T‐ cell immune deficiency. N Engl J Med 19823071436–1438. [DOI] [PubMed] [Google Scholar]
- 12.Snider W D, Simpson D M, Nielsen S, Gold J W, Metroka C E, Posner J B. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol 198314403–418. [DOI] [PubMed] [Google Scholar]
- 13.Berger J R, Kaszovitz B, Post M J, Dickinson G. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann Intern Med 198710778–87. [DOI] [PubMed] [Google Scholar]
- 14.Kure K, Llena J F, Lyman W D, Soeiro R, Weidenheim K M, Hirano A.et al Human immunodeficiency virus‐1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Hum Pathol 199122700–710. [DOI] [PubMed] [Google Scholar]
- 15.Selik R M, Karon J M, Ward J W. Effect of the human immunodeficiency virus epidemic on mortality from opportunistic infections in the United States in 1993. J Infect Dis 1997176632–636. [DOI] [PubMed] [Google Scholar]
- 16.Berger J R, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol 1998459–68. [DOI] [PubMed] [Google Scholar]
- 17.Garcia‐Suarez J, de Miguel D, Krsnik I, Banas H, Arribas I, Burgaleta C.et al Changes in the natural history of progressive multifocal leukoencephalopathy in HIV‐negative lymphoproliferative disorders: impact of novel therapies. Am J Hematol 200580271–281. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg S L, Pecora A L, Alter R S, Kroll M S, Rowley S D, Waintraub S E.et al Unusual viral infections (progressive multifocal leukoencephalopathy and cytomegalovirus disease) after high‐dose chemotherapy with autologous blood stem cell rescue and peritransplantation rituximab. Blood 2002991486–1488. [DOI] [PubMed] [Google Scholar]
- 19.Matteucci P, Magni M, Di Nicola M, Carlo‐Stella C, Uberti C, Gianni A M. Leukoencephalopathy and papovavirus infection after treatment with chemotherapy and anti‐CD20 monoclonal antibody. Blood 20021001104–1105. [DOI] [PubMed] [Google Scholar]
- 20.Steurer M, Clausen J, Gotwald T, Gunsilius E, Stockhammer G, Gastl G, Nachbaur D. Progressive multifocal leukoencephalopathy after allogeneic stem cell transplantation and posttransplantation rituximab. Transplantation 200376435–436. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin P, White C A, Grillo‐Lopez A J, Maloney D G. Clinical status and optimal use of rituximab for B‐cell lymphomas. Oncology (Williston Park) 1998121763–9 discussion 176970, 17757. [PubMed] [Google Scholar]
- 22.Berger J R, Levy R M, Flomenhoft D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome‐associated progressive multifocal leukoencephalopathy. Ann Neurol 199844341–349. [DOI] [PubMed] [Google Scholar]
- 23.Henin D, Smith T W, De Girolami U, Sughayer M, Hauw J J. Neuropathology of the spinal cord in the acquired immunodeficiency syndrome. Hum Pathol 1992231106–1114. [DOI] [PubMed] [Google Scholar]
- 24.Whiteman M L, Post M J, Berger J R, Tate L G, Bell M D, Limonte L P. Progressive multifocal leukoencephalopathy in 47 HIV‐seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology 1993187233–240. [DOI] [PubMed] [Google Scholar]
- 25.Safdar A, Rubocki R J, Horvath J A, Narayan K K, Waldron R L. Fatal immune restoration disease in human immunodeficiency virus type 1‐infected patients with progressive multifocal leukoencephalopathy: impact of antiretroviral therapy‐associated immune reconstitution. Clin Infect Dis 2002351250–1257. [DOI] [PubMed] [Google Scholar]
- 26.Vendrely A, Bienvenu B, Gasnault J, Thiebault J B, Salmon D, Gray F. Fulminant inflammatory leukoencephalopathy associated with HAART‐induced immune restoration in AIDS‐related progressive multifocal leukoencephalopathy. Acta Neuropathol (Berl) 2005109449–455. [DOI] [PubMed] [Google Scholar]
- 27.Moret H, Brodard V, Barranger C, Jovenin N, Joannes M, Andreoletti L. New commercially available PCR and microplate hybridization assay for detection and differentiation of human polyomaviruses JC and BK in cerebrospinal fluid, serum, and urine samples. J Clin Microbiol 2006441305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber T, Klapper P E, Cleator G M, Bodemer M, Luke W, Knowles W.et al Polymerase chain reaction for detection of JC virus DNA in cerebrospinal fluid: a quality control study. European Union Concerted Action on Viral Meningitis and Encephalitis. J Virol Methods 199769(1–2)231–237. [DOI] [PubMed] [Google Scholar]
- 29.Tassie J M, Gasnault J, Bentata M, Deloumeaux J, Boue F, Billaud E.et al Survival improvement of AIDS‐related progressive multifocal leukoencephalopathy in the era of protease inhibitors. Clinical Epidemiology Group. French Hospital Database on HIV. AIDS 1999131881–1887. [DOI] [PubMed] [Google Scholar]
- 30.Price R W, Nielsen S, Horten B, Rubino M, Padgett B, Walker D. Progressive multifocal leukoencephalopathy: a burnt‐out case. Ann Neurol 198313485–490. [DOI] [PubMed] [Google Scholar]
- 31.Berger J R, Mucke L. Prolonged survival and partial recovery in AIDS‐associated progressive multifocal leukoencephalopathy. Neurology 1988381060–1065. [DOI] [PubMed] [Google Scholar]
- 32.Albrecht H, Hoffmann C, Degen O, Stoehr A, Plettenberg A, Mertenskotter T.et al Highly active antiretroviral therapy significantly improves the prognosis of patients with HIV‐associated progressive multifocal leukoencephalopathy. AIDS 1998121149–1154. [DOI] [PubMed] [Google Scholar]
- 33.Clifford D B, Yiannoutsos C, Glicksman M, Simpson D M, Singer E J, Piliero P J.et al HAART improves prognosis in HIV‐associated progressive multifocal leukoencephalopathy [see comments]. Neurology 199952623–625. [DOI] [PubMed] [Google Scholar]
- 34.Inui K, Miyagawa H, Sashihara J, Miyoshi H, Tanaka‐Taya K, Nishigaki T.et al Remission of progressive multifocal leukoencephalopathy following highly active antiretroviral therapy in a patient with HIV infection. Brain Dev 199921416–419. [DOI] [PubMed] [Google Scholar]
- 35.Miralles P, Berenguer J, Garcia de Viedma D, Padilla B, Cosin J, Lopez‐Bernaldo de Quiros J C.et al Treatment of AIDS‐associated progressive multifocal leukoencephalopathy with highly active antiretroviral therapy. Aids 1998122467–2472. [DOI] [PubMed] [Google Scholar]
- 36.Tantisiriwat W, Tebas P, Clifford D B, Powderly W G, Fichtenbaum C J. Progressive multifocal leukoencephalopathy in patients with AIDS receiving highly active antiretroviral therapy. Clin Infect Dis 1999281152–1154. [DOI] [PubMed] [Google Scholar]
- 37.Antinori A, Ammassari A, Giancola M L, Cingolani A, Grisetti S, Murri R.et al Epidemiology and prognosis of AIDS‐associated progressive multifocal leukoencephalopathy in the HAART era. J Neurovirol 20017323–328. [DOI] [PubMed] [Google Scholar]
- 38.De Luca A, Giancola M L, Ammassari A, Grisetti S, Paglia M G, Gentile M.et al The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS‐associated progressive multifocal leukoencephalopathy [in process citation]. J Infect Dis 20001821077–1083. [DOI] [PubMed] [Google Scholar]
- 39.De Luca A, Ammassari A, Cingolani A, Giancola M L, Antinori A. Disease progression and poor survival of AIDS‐associated progressive multifocal leukoencephalopathy despite highly active antiretroviral therapy [letter]. AIDS 1998121937–1938. [PubMed] [Google Scholar]
- 40.Wyen C, Hoffmann C, Schmeisser N, Wohrmann A, Qurishi N, Rockstroh J.et al Progressive multifocal leukencephalopathy in patients on highly active antiretroviral therapy: survival and risk factors of death. J Acquir Immune Defic Syndr 2004371263–1268. [DOI] [PubMed] [Google Scholar]
- 41.Du Pasquier R A, Smith P S, Joseph J T, Mazullo J M, De Girolami U.et al JCV‐specific cellular immune response correlates with a favorable clinical outcome in HIV‐infected individuals with progressive multifocal leukoencephalopathy. J Neurovirol 20017318–322. [DOI] [PubMed] [Google Scholar]
- 42.Koralnik I J. Overview of the cellular immunity against JC virus in progressive multifocal leukoencephalopathy. J Neurovirol 20028(suppl 2)59–65. [DOI] [PubMed] [Google Scholar]
- 43.Koralnik I J, Du Pasquier R A, Letvin N L. JC virus‐specific cytotoxic T lymphocytes in individuals with progressive multifocal leukoencephalopathy. J Virol 2001753483–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du Pasquier R A, Koralnik I J. Inflammatory reaction in progressive multifocal leukoencephalopathy: harmful or beneficial? J Neurovirol 20039(suppl 1)25–31. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann C, Hoffmann C, Horst H A, Albrecht H, Schlote W. Progressive multifocal leucoencephalopathy with unusual inflammatory response during antiretroviral treatment. J Neurol Neurosurg Psychiatry 2003741142–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cinque P, Bossolasco S, Brambilla A M, Boschini A, Mussini C, Pierotti C.et al The effect of highly active antiretroviral therapy‐induced immune reconstitution on development and outcome of progressive multifocal leukoencephalopathy: study of 43 cases with review of the literature. J Neurovirol 20039(suppl 1)73–80. [DOI] [PubMed] [Google Scholar]
- 47.Cinque P, Pierotti C, Vigano M G, Bestetti A, Fausti C, Bertelli D.et al The good and evil of HAART in HIV‐related progressive multifocal leukoencephalopathy. J Neurovirol 20017358–363. [DOI] [PubMed] [Google Scholar]
- 48.Thurnher M M, Post M J, Rieger A, Kleibl‐Popov C, Loewe C, Schindler E. Initial and follow‐up MR imaging findings in AIDS‐related progressive multifocal leukoencephalopathy treated with highly active antiretroviral therapy. AJNR Am J Neuroradiol 200122977–984. [PMC free article] [PubMed] [Google Scholar]
- 49.Di Giambenedetto S, Vago G, Pompucci A, Scoppettuolo G, Cingolani A, Marzocchetti A.et al Fatal inflammatory AIDS‐associated PML with high CD4 counts on HAART: a new clinical entity? Neurology 2004632452–2453. [DOI] [PubMed] [Google Scholar]
- 50.Goodman A G, Gilman A, Goodman L A.The Pharmacological Basis of Therapeutics. New York: MacMillan Publishing Co, 1985
- 51.Hou J, Major E O. The efficacy of nucleoside analogs against JC virus multiplication in a persistently infected human fetal brain cell line. J Neurovirol 19984451–456. [DOI] [PubMed] [Google Scholar]
- 52.Bauer W R, Turel A P, Jr, Johnson K P. Progressive multifocal leukoencephalopathy and cytarabine. Remission with treatment. JAMA 1973226174–176. [PubMed] [Google Scholar]
- 53.Conomy J, Beard N S, Matsumoto H, Roessmann U. Cytarabine treatment of progressive multifocal leukoencephalopathy. Clinical course and detection of virus‐like particles after antiviral chemotherapy. JAMA 19742291313–1316. [DOI] [PubMed] [Google Scholar]
- 54.Lidman C, Lindqvist L, Mathiesen T, Grane P. Progressive multifocal leukoencephalopathy in AIDS. AIDS 199151039–1041. [PubMed] [Google Scholar]
- 55.O'Riordan T, Daly P A, Hutchinson M, Shattock A G, Gardner S D. Progressive multifocal leukoencephalopathy—remission with cytarabine. J Infect Dis 19902051–54. [DOI] [PubMed] [Google Scholar]
- 56.Tashiro K, Doi S, Moriwaka F, Maruo Y, Nomura M. Progressive multifocal leucoencephalopathy with magnetic resonance imaging verification and therapeutic trials with interferon. J Neurol 1987234427–429. [DOI] [PubMed] [Google Scholar]
- 57.Van Horn G, Bastian F, Moake J. Progressive multifocal leukoencephalopathy: failure of response to transfer factor and cytarabine. Neurology 197828794–797. [DOI] [PubMed] [Google Scholar]
- 58.Hall C D, Dafni U, Simpson D, Clifford D, Wetherill P E, Cohen B.et al Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team [see comments]. N Engl J Med 19983381345–1351. [DOI] [PubMed] [Google Scholar]
- 59.Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I.et al Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology 199748836–845. [DOI] [PubMed] [Google Scholar]
- 60.Kerr D A, Chang C F, Gordon J, Bjornsti M A, Khalili K. Inhibition of human neurotropic virus (JCV) DNA replication in glial cells by camptothecin. Virology 1993196612–618. [DOI] [PubMed] [Google Scholar]
- 61.O'Reilly S. Efficacy of camptothecin in progressive multifocal leucoencephalopathy. Lancet 1997350291. [DOI] [PubMed] [Google Scholar]
- 62.Andrei G, Snoeck R, Vandeputte M, De Clercq E. Activities of various compounds against murine and primate polyomaviruses. Antimicrob Agents Chemother 199741587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marra C M, Rajicic N, Barker D E, Cohen B A, Clifford D, Donovan Post M J.et al A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. Aids 2002161791–1797. [DOI] [PubMed] [Google Scholar]
- 64.Radhakrishnan S, Gordon J, Del Valle L, Cui J, Khalili K. Intracellular approach for blocking JC virus gene expression by using RNA interference during viral infection. J Virol 2004787264–7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunschner L, Scott T F. Sustained recovery of progressive multifocal leukoencephalopathy after treatment with IL‐2. Neurology 2005651510. [DOI] [PubMed] [Google Scholar]
- 66.Przepiorka D, Jaeckle K A, Birdwell R R, Fuller G N, Kumar A J, Huh Y O.et al Successful treatment of progressive multifocal leukoencephalopathy with low‐dose interleukin‐2. Bone Marrow Transplant 199720983–987. [DOI] [PubMed] [Google Scholar]
- 67.Steiger M J, Tarnesby G, Gabe S, McLaughlin J, Schapira A H. Successful outcome of progressive multifocal leukoencephalopathy with cytarabine and interferon. Ann Neurol 199333407–411. [DOI] [PubMed] [Google Scholar]
- 68.Huang S S, Skolasky R L, Dal Pan G J, Royal W, 3rd, McArthur J C. urvival prolongation in HIV‐associated progressive multifocal leukoencephalopathy treated with alpha‐interferon: an observational study. J Neurovirol 19984324–332. [DOI] [PubMed] [Google Scholar]
- 69.Elphick G F, Querbes W, Jordan J A, Gee G V, Eash S, Manley K.et al The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 20043061380–1383. [DOI] [PubMed] [Google Scholar]
- 70.Pho M T, Ashok A, Atwood W J. JC virus enters human glial cells by clathrin‐dependent receptor‐mediated endocytosis. J Virol 2000742288–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Querbes W, Benmerah A, Tosoni D, Di Fiore P P, Atwood W J. A JC virus‐induced signal is required for infection of glial cells by a clathrin‐ and eps15‐dependent pathway. J Virol 200478250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ZuRhein G. Particles resembling papovavirions in human cerebral demyelinating disease. Science 19651481477–1479. [DOI] [PubMed] [Google Scholar]
- 73.Padgett B L, Walker D L, ZuRhein G M, Eckroade R J, Dessel B H. Cultivation of papova‐like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 197111257–1260. [DOI] [PubMed] [Google Scholar]
- 74.Major E O, Amemiya K, Tornatore C S, Houff S A, Berger J R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus‐induced demyelinating disease of the human brain. Clin Microbiol Rev 1992549–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khalili K, Del Valle L, Otte J, Weaver M, Gordon J. Human neurotropic polyomavirus, JCV, and its role in carcinogenesis. Oncogene 2003225181–5191. [DOI] [PubMed] [Google Scholar]
- 76.Walker D, Padgett B. The epidemiology of human polyomaviruses. In: Sever J, Madden D, eds.Polyomaviruses and Human Neurological Disease. New York: Alan R Liss, Inc, 198399–106. [PubMed]
- 77.Brown P, Tsai T, Gajdusek D C. Seroepidemiology of human papovaviruses. Discovery of virgin populations and some unusual patterns of antibody prevalence among remote peoples of the world. Am J Epidemiol 1975102331–340. [DOI] [PubMed] [Google Scholar]
- 78.Caldarelli‐Stefano R, Vago L, Omodeo‐Zorini E, Mediati M, Losciale L, Nebuloni M.et al Detection and typing of JC virus in autopsy brains and extraneural organs of AIDS patients and non‐immunocompromised individuals. J Neurovirol 19995125–133. [DOI] [PubMed] [Google Scholar]
- 79.Monaco M C, Atwood W J, Gravell M, Tornatore C S, Major E O. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol 1996707004–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sabath B F, Major E O. Traffic of JC virus from sites of initial infection to the brain: the path to progressive multifocal leukoencephalopathy. J Infect Dis 2002186(suppl 2)S180–S186. [DOI] [PubMed] [Google Scholar]
- 81.Monaco M C, Jensen P N, Hou J, Durham L C, Major E O. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol 1998729918–9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berger J, Major E. Progressive multifocal leukoencephalopathy. In: Merigan TJ, Bartlett J, Bolognesi D, eds. Textbook of AIDS Medicine. Baltimore: Williams & Wilkins, 1999403–420.
- 83.Watanabe I, Preskorn S H. Virus‐cell interaction in oligodendroglia, astroglia and phagocyte in progressive multifocal leukoencephalopathy. An electron microscopic study. Acta Neuropathol (Berl) 197636101–115. [DOI] [PubMed] [Google Scholar]
- 84.Mazlo M, Tariska I. Are astrocytes infected in progressive multifocal leukoencephalopathy (PML)? Acta Neuropathol(Berl) 19825645–51. [DOI] [PubMed] [Google Scholar]
- 85.Tornatore C, Berger J R, Houff S A, Curfman B, Meyers K, Winfield D.et al Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol 199231454–462. [DOI] [PubMed] [Google Scholar]
- 86.Andreoletti L, Lescieux A, Lambert V, Si‐Mohamed A, Matta M, Wattre P.et al Semiquantitative detection of JCV‐DNA in peripheral blood leukocytes from HIV‐1‐infected patients with or without progressive multifocal leukoencephalopathy. J Med Virol 2002661–7. [DOI] [PubMed] [Google Scholar]
- 87.Koralnik I J, Boden D, Mai V X, Lord C I, Letvin N L. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology 199952253–260. [DOI] [PubMed] [Google Scholar]
- 88.Weber T, Weber F, Petry H, Luke W. Immune response in progressive multifocal leukoencephalopathy: an overview. J Neurovirol 20017311–317. [DOI] [PubMed] [Google Scholar]
- 89.Berger J R. Progressive multifocal leukoencephalopathy in acquired immunodeficiency syndrome: explaining the high incidence and disproportionate frequency of the illness relative to other immunosuppressive conditions. J Neurovirol 20039(Suppl 1)38–41. [DOI] [PubMed] [Google Scholar]
- 90.Yousry T A, Major E O, Ryschkewitsch C, Fahle G, Fischer S, Hou J.et al Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006354924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rudick R A, Sandrock A. Natalizumab: alpha4‐integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother 20044571–580. [DOI] [PubMed] [Google Scholar]
- 92.Tubridy N, Behan P O, Capildeo R, Chaudhuri A, Forbes R, Hawkins C P.et al The effect of anti‐alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology 199953466–472. [DOI] [PubMed] [Google Scholar]
- 93.Stuve O, Marra C M, Jerome K R, Cook L, Cravens P D, Cepok S.et al Immune surveillance in multiple sclerosis patients treated with natalizumab. Neurology 200666(Suppl 2)A250. [DOI] [PubMed] [Google Scholar]
- 94.von Andrian U H, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med 200334868–72. [DOI] [PubMed] [Google Scholar]
- 95.Tyasbri package insert, Biogenidec
- 96.Major E O. JC virus in the CSF and plasma of natalizumab treated patients, European Committee for Treatment and Research in Multiple Sclerosis, 25 September–1 October 2005, Thessaloniki, Greece (abstract)