Abstract
The dengue virus molecular typing method described by Lanciotti and coworkers (R. S. Lanciotti, C. H. Calisher, D. J. Gubler, G. J. Chang, and A. Vance-Vorndam, J. Clin. Microbiol. 30:545-551, 1992) is used worldwide for diagnosis and surveillance. However, it failed to detect DENV-1 variants in Cambodia due to a point mutation. We describe an improvement of the method that allows the detection of additional DENV-1 strains, including potential variants.
Dengue virus (Flaviviridae, genus Flavivirus) serotypes 1 to 4 are transmitted to humans mainly by Aedes aegypti mosquitoes. Human infection leads to a clinical picture ranging from asymptomatic infection or mild dengue fever to severe dengue hemorrhagic fever (DHF) or dengue shock syndrome. Dengue is a major public health problem of increasing concern, as evidenced by the estimate of 50 million infections annually, including 400,000 DHF cases, and 2.5 billion individuals at risk (16). Southeast Asia, including Cambodia, is a an area of dengue hyperendemicity, where the four serotypes are circulating simultaneously and DHF cases are reported each year. Since no vaccine is available to prevent the disease, its control relies mainly on surveillance, vector control, and case management (16).
Laboratory diagnosis of dengue infection includes molecular techniques such as reverse transcription (RT)-PCR, for which more than 16 different protocols have been published (9). One protocol, developed by Lanciotti et al. (10), a seminested RT-PCR, targets the C and pre-M region. It uses universal outer dengue primers D1 and D2, followed by a subsequent serotype-specific seminested PCR combining primer D1 with one of the following internal primers: TS1, TS2, TS3, or TS4. This assay is known to exceed the sensitivity of virus isolation when aliquots of the same sample are used (8) and is widely used for diagnosis and surveillance of dengue, particularly in Southeast Asian countries (2-5, 7, 13, 15).
In Cambodia, our laboratory surveillance of dengue utilizes hemagglutination inhibition and immunoglobulin M capture enzyme-linked immunosorbent assays, virus isolation on AP61 and Vero E6 cells (12), and seminested RT-PCR by the method of Lanciotti et al. (10) with slight modifications. Briefly, 100 μl of serum was extracted by using Trizol LS reagent (Life Technologies, Gaithersburg, Md.), according to the manufacturer's instructions. The resulting RNA pellet was resuspended in 30 μl of RNase-free water and incubated for 10 min at 55°C, and then 5 μl was used in an RT reaction in a 15-μl mixture containing 500 μM deoxynucleoside triphosphate (dNTP), 0.25 μM D2 primer, 20 U of RNasin (Promega, Madison, Wis.), 5 U of avian myeloblastosis virus reverse transcriptase (Promega), 5× RT buffer, and RNase-free water. RT was carried out at 42°C for 1 h. The first-round PCR was carried out in a 50-μl volume containing 2.5 mM MgCl2, 0.2 mM (each) dNTPs (Boehringer Mannheim, Mannheim, Germany), 0.2 μM (each) D1 and D2 outer primers, 1× Taq buffer, 2 U of Taq polymerase (Promega), 5 μl of the RT product, and PCR-grade water. The PCR program started with 5 min of denaturation at 95°C, followed by 35 cycles consisting of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and ended with a 72°C final extension step for 10 min. The second-round PCR was performed in a 50-μl reaction mixture containing 2.5 mM MgCl2, 0.2 mM (each) dNTPs, 0.2 μM (each) D1, TS1, TS2, TS3, and TS4 primers, 10× Taq buffer, 2 U of Taq polymerase, and 5 μl of the diluted first-round PCR product (1:100 in PCR-grade water). The cycling program started with a 5-min denaturation step at 94°C, followed by 25 cycles each at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and ended with a 72°C final extension step for 10 min. Positive (dengue viral RNA) and negative (no template) controls were included for each step of the process (extraction, RT, and first- and second-round PCR). In our hands, the minimum detection level of the nested assay is 100 50% tissue culture infective doses (TCID50) per milliliter.
In 2001, discordant results between virus isolation and seminested RT-PCR were observed for eight patients. Indeed, DENV-1 was isolated from patients' sera whereas the seminested RT-PCR detected in the first round a correctly sized DNA product (511 bp) but failed to detect DENV-1 in the second round. Given that (i) virological tests were performed successfully within the same week on aliquots of the same sample stored at −80°C, ruling out the degradation of viral RNA due to conservation conditions, (ii) positive controls yielded PCR products of the expected size, (iii) a second-round PCR performed with D1 and TS1 primers only (to avoid primer interactions during multiplex PCR) or with diluted positive first-round PCR products (to rule out potential inhibitor effects) also yielded negative results, we hypothesized that the amplification failure of the second-round PCR with primer TS1 might be related to the presence of a DENV-1 variant strain in the sample, resulting in a primer-template mismatch.
To test our hypothesis, nine first-round PCR products obtained from samples which were positive for DENV-1 isolation but for which second-round PCR results were negative and positive for six and three samples, respectively, were sequenced by using the dye termination cycle sequencing technique and an ABI Prism 373 automated sequencer. Sequences were aligned by using the ClustalW program (14), and the alignment of the relevant region is shown in Fig. 1. The sequences revealed that isolates successfully amplified by second-round PCR using D1 and TS1 (L0904278, L1108177, and L117016) exhibited a C at position 568 (corresponding to the nucleotide complementary to the 3′-most nucleotide of primer TS1) while the isolates that failed to be amplified (L0810173, L0814160, L1123235, L0725239, L0806336, and L0926212) had a T at the same position. This observation indicated that the amplification failed because of a primer-template mismatch at that 3′ position of the primer. Therefore, a new primer, TS1bis (5′-CGT CTC AGT GAT CCG GGG RC-3′) was designed. TS1bis is degenerate at position 568 (R = A or G) and has one extra nucleotide (C) designed to anneal to position 567. This primer allowed us to amplify DENV-1 isolates from Cambodia that are amplifiable or not amplifiable by using the original TS1 primer from Lanciotti's protocol. The PCR sensitivity with TS1bis was equivalent to that with TS1 (100 TCID50/ml). Moreover, the use of TS1bis did not alter the specificity of the technique, since genomes of DENV-2, DENV-3, DENV-4, and other related flaviviruses (yellow fever, West Nile, Japanese encephalitis, and Langat viruses) were not amplified (data not shown).
FIG. 1.
Alignment of nine sequences of dengue 1 viruses isolated in Cambodia, with the DENV-1 Nauru/74 strain sequence included as a reference (10). Identities at nucleotide positions are shown by dots. The sequence numbering is based on the genomic Nauru/74 strain sequence. The target region of the TS1 and TS1bis primers is shown in the gray box, and primer sense is indicated by the horizontal arrow. The vertical arrow shows nucleotide position 568 (letters in bold), where a TS1 primer mismatch occurs.
This genetic variant with the mismatch to primer TS1 was quite frequent among the samples tested in our laboratory. Indeed, in addition to the eight patients who were DENV-1 isolation positive and first-round-PCR positive but D1-TS1 second-round-PCR negative, 12 of 38 other patients with comparable PCR results were identified. In addition, we used seminested RT-PCR with TS1bis to screen additional acute-phase samples collected within the provinces where DENV-1 variants were detected in 2001. Although these samples yielded negative results with other methods (virus isolation, flavivirus serology, and a modification of the technique of Lanciotti et al. with TS1 primer), we detected the DENV-1 TS1 mismatch genetic variant in 1 sample out of 38, demonstrating that some dengue cases can be missed if the TS1 primer is relied on.
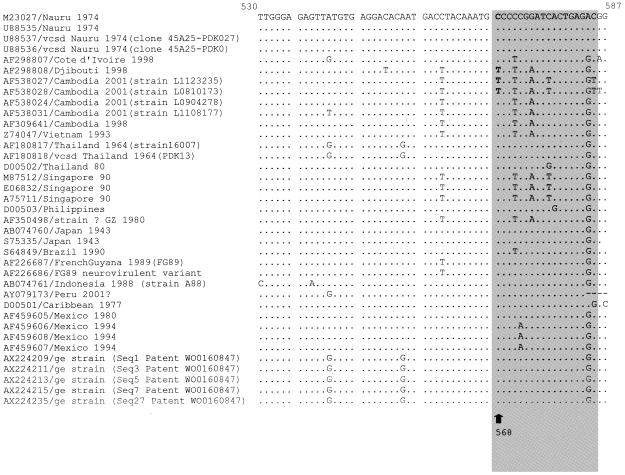
To investigate whether such TS1 mismatch variants existed elsewhere, we used the Blast program (1) to search the GenBank/EMBL database for other DENV-1 examples of the mismatch mutation at nucleotide position 568. Thirty-three sequences encompassing the target sequence for TS1 and TS1bis in the pre-M region were found at the time of the search (July 2002). Their alignment with our sequences using ClustalW software (14) showed that only one other strain (Djibouti, 1998) had the same mismatch mutation (Fig. 2).
FIG. 2.
Alignment of the four sequence patterns representative of the nine sequences of dengue 1 viruses isolated in Cambodia, along with other dengue 1 virus sequences available from the GenBank/EMBL databases. Identities at nucleotide positions are shown by dots. The sequence numbering is based on the Nauru/74 sequence. The target region of the TS1 and TS1bis primers is shown in the gray box, and primer sense is indicated by the horizontal arrow. The vertical arrow shows position 568 (letters in bold), where the TS1 primer mismatch occurs. Strains are designated as follows: accession number/geographical origin, year of isolation (isolate). vcsd, vaccine candidate strain derived; ge, genetically engineered.
DENV-1 variant strains that escape diagnosis by virus isolation and identification with the type-specific monoclonal antibody 15F3-1-15 (reacting with NS1 protein) instead of the monoclonal antibody D2-1F1-3 (reacting with the E protein) (6, 11) have been described To our knowledge, ours is the first description of false-negative results due to a genetic variant. We demonstrated the association of a mutation at position 568 of DENV-1 strains with an RT-PCR amplification failure using the seminested protocol of Lanciotti et al. (10). Although Lanciotti et al. reported the absence of amplification with the seminested RT-PCR of one DENV-1 isolate from Southeast Asia, they suspected a low viral titer or presence of a PCR inhibitor in the sample as the cause of the amplification failure.
Only 33 sequences including the target sequence of the TS1 primer in the pre-M region were found in GenBank. None of the Southeast Asian sequences exhibited the mismatch mutation that we have described. Nevertheless, the presence of such variant strains in neighboring countries where the method of Lanciotti et al. (10) is used should be assessed. The presence of the mismatch mutation in a strain isolated in Djibouti demonstrates that the variant could be widespread. Therefore, when the method described by Lanciotti et al. is used, we recommend the use of TS1bis where TS1 fails for DENV-1 typing. Finally, our study emphasized the need for the combined use of virus isolation and molecular techniques in reference laboratories involved in dengue virus surveillance and typing.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cam, B. V., L. Fonsmark, N. B. Hue, N. T. Phuong, A. Poulsen, and E. D. Heegaard. 2001. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 65:848-851. [DOI] [PubMed] [Google Scholar]
- 3.Chokephaibulkit, K., P. Kankirawatana, S. Apintanapong, V. Pongthapisit, S. Yoksan, U. Kositanont, and P. Puthavathana. 2001. Viral etiologies of encephalitis in Thai children. Pediatr. Infect. Dis. J. 20:216-218. [DOI] [PubMed] [Google Scholar]
- 4.Corwin, A. L., R. P. Larasati, M. J. Bangs, S. Wuryadi, S. Arjoso, N. Sukri, E. Listyaningsih, S. Hartati, R. Namursa, Z. Anwar, S. Chandra, B. Loho, H. Ahmad, J. R. Campbell, and K. R. Porter. 2001. Epidemic dengue transmission in southern Sumatra, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 95:257-265. [DOI] [PubMed] [Google Scholar]
- 5.Deubel, V. 1997. The contribution of molecular techniques to the diagnosis of dengue infection, p. 335-365. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Oxon, United Kingdom.
- 6.Gubler, D.J. 1987. Application of serotype-specific monoclonal antibodies for identification of dengue viruses, p. 3-14. In C. Yunker (ed.), Arboviruses in arthropod cells in vitro. CRC Press, Boca Raton, Fla.
- 7.Huyen, N. N., T. V. Be, and G. E. Morris. 1997. Nucleotide sequences from the capsid and pre-protein regions of dengue viruses from Vietnam. Biochem. Soc. Trans. 25:54S. [DOI] [PubMed]
- 8.Innis, B. L. 1995. Dengue and dengue hemorrhagic fever, p. 103-146. In J. S. Porterfield (ed.), Exotic viral infections. Chapman & Hall Medical, London, United Kingdom.
- 9.Kuno, G. 1998. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 72:27-41. [DOI] [PubMed] [Google Scholar]
- 10.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and A Vance-Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannekarn, N., K. Morita, M. Tanaka, A. Igarashi, W. Usawattanakul, V. Sirisanthana, B. L. Innis, N. Sittisombut, A. Nisalak, and S. Nimmanitya. 1993. Application of polymerase chain reaction for identification of dengue viruses isolated from patients sera. Microbiol. Immunol. 37:41-47. [DOI] [PubMed] [Google Scholar]
- 12.Reynes, J. M., A. Laurent, V. Deubel, E. Telliam, and J. P. Moreau. 1994. The first epidemic of dengue hemorrhagic fever in French Guiana. Am. J. Trop. Med. Hyg. 51:545-553. [PubMed] [Google Scholar]
- 13.Salomon, T., N. M. Dung, D. W. Vaughn, R. Kneen, L. T. T. Thao, B. Raengsakulrach, H. T. Loan, N. P. J. Day, J. Farrar, K. S. A. Myint, M. J. Warrell, W. S. James, A. Nisalak, and N. J. White. 2000. Neurological manifestations of dengue infection. Lancet 355:1053-1059. [DOI] [PubMed] [Google Scholar]
- 14.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 11:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannityta, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. 2000. Communicable diseases 2000: highlights of activities in 1999 and major challenges for the future. WHO/CDS/2000.1, p. 102. World Health Organization, Geneva, Switzerland.