Abstract
IL‐32 is the name given to the NK4 transcript first reported in IL‐2 activated T lymphocytes and natural killer cells 13 years ago without known function. The novel cytokine has six isoforms. In an study to isolate a soluble form of the IL‐32 receptor from human urine, IL‐32α bound proteinase‐3 with high affinity and was not affected by enzyme inhibition. IL‐32α/IL‐32γ were expressed as recombinant molecules. The cytokine exhibits properties characteristic of proinflammatory cytokines and also induces the degradation of inhibitory κB and phosphorylation of mitogen activated protein p38. Monoclonal antibodies to IL‐32 identify its presence in a variety of human tissues from diseases states. Epithelial cells from healthy subjects express low levels of the cytokine, but in disease conditions such as chronic obstructive pulmonary disease, Crohn's disease and psoriasis, the expression increases markedly. IL‐32 is a major transcript in gene array studies in epithelial cells stimulated with IFNγ in vitro. In rheumatoid arthritis, synovial tissues reveals increased content of IL‐32, which correlates with severity of disease. A highly significant correlation has been observed between the number of synovial and macrophagic cells positive for IL‐32 and the level of erythrocytes sedimentation, IL‐1β, tumour necrosis factor α, and IL‐18. Thus, IL‐32 exhibits many properties of proinflammatory cytokines and associations with disease severity.
Keywords: interleukin, IL‐32, cytokine, rheumatoid arthritis
Interleukin (IL)‐32 is a proinflammatory cytokine originally described as a transcript termed NK4 found in activated natural killer (NK) cells and T lymphocytes.1 In a search for IL‐18 inducible genes independent of IL‐12 or IL‐15 costimulation, IL‐18 induced several expected proinflammatory genes in the human lung epithelial cell line A549. However, there was a high level of expression in a transcript termed NK4 with no known function. On expression of the recombinant form of the NK4 transcript, it became clear that NK4 encoded for a protein with many of the characteristics of proinflammatory cytokines.2 For these reasons, the name was changed to IL‐32. Although IL‐32 was first reported as transcript in IL‐2 activated NK and T cells, it appears that epithelial cells are a dominant and widespread source. In fact, the A549 cell line is a human lung carcinoma cell line. Others have reported the presence of mRNA for IL‐32 in Epstein–Barr virus infected lymphoma cells,3 neuroblastoma cells,4 and haematopoietic progenitor cells.5 Primary human B cells, even when stimulated with IgM or anti‐CD40, do not express significant IL‐32.6 However, the cytokine is highly expressed in activated primary human T cells following stimulation with anti‐CD3 or the combination of phorbol myristate acetate and ionomycin.6 Northern blot analysis of various human tissues from healthy subjects reveal low constitutive expression of steady state levels of mRNA in the prostate, moderate in the thymus, small intestine, and colon, but high in the spleen and peripheral blood leucocytes.2
IL‐32 in arthritis
In culture, synovial cells isolated from human biopsies take on a specialised stellate fibroblast‐like form first reported by Krane and Dayer.7 These fibroblast‐like synoviocytes (FLS) can be cultured from tissues of patients with rheumatoid arthritis (RA) as well as osteoarthritis. In general, steady state mRNA in FLS isolated from these two distinct arthritides express similar levels cytokines, chemokines, and their respective receptors when cultured in the absence of exogenous stimulation.8 However, constitutive expression is differentially observed between third passage FLS from RA compared with FLS from biopsies of osteoarthritis.8 In that study, synovial biopsies were obtained from eight patients with RA and nine patients with osteoarthritis. Gene array was performed with over 54 000 transcripts. The mean differential expression of IL‐32 in RA compared with osteoarthritis was 3.85‐fold greater (p = 0.0073).8 Another differentially expressed gene was monocyte chemoattractant protein (MCP)‐1 (also termed CCL2). MCP‐1 was expressed 2.5‐fold greater in FLS from RA compared with osteoarthritis (p = 0.02).8 The authors argue that because the level of expression of inflammatory genes from FLS is similar in RA and osteoarthritis, the differential increase in IL‐32 may implicate a role for this cytokine in RA.8 A similar argument was proposed for the high degree of expression of MCP‐1. Since recombinant IL‐32 stimulates chemokines from macrophagic cell in vitro,2 the finding of both IL‐32 and MCP‐1 may be more than coincidental.
Although the studies by Cagnard and coworkers provide an important observation in differential gene expression of IL‐32,8 direct evidence for IL‐32 in RA was reported by Joosten and colleagues.9 In their study, IL‐32 was shown to be expressed in the synovium of patients with RA, and associations with disease severity and the presence of other cytokines was made using immunohistochemistry with a monoclonal antibody to human IL‐32. Immunohistochemistry revealed that IL‐32 is highly expressed in RA synovial tissue biopsies, whereas IL‐32 was not observed in synovial tissues from patients with osteoarthritis. Moreover, in synovial biopsies from 29 RA patients with active disease, the level of IL‐32 staining correlated with erythrocyte sedimentation rate, a marker of systemic inflammation (r = 0.63, p<0.0003). Synovial staining of IL‐32 also correlated with indices of synovial inflammation (r = 0.80, p<0.0001) as well as synovial presence of tumour necrosis factor α (TNFα) (r = 0.68, p<0.004), IL‐1β (r = 0.79, p<0.0001), and IL‐18 (r = 0.82 p<0.001). When incubated with mouse macrophages deficient in toll‐like receptor 4 (TLR4), recombinant human IL‐32γ stimulated the production of TNFα, IL‐1β, and macrophage inflammatory protein‐2. IL‐32 was a potent inducer of prostaglandin E2 release in mouse macrophages and in human blood monocytes, an important property for inflammation. Following the injection of human IL‐32γ into the knee joints of naive mice, joint swelling, with pronounced influx of inflammatory cells and cartilage damage was observed. However, in TNFα deficient mice, IL‐32 driven joint swelling was absent and cell influx was markedly reduced, but loss of proteoglycan was unaffected, suggesting that IL‐32 activity is, in part, TNFα dependent. The loss of proteoglycan following the injection of IL‐32γ may be due to IL‐1β.
IL‐32 staining was observed in 25 of the 29 synovial biopsies; marked staining was predominantly found in the lining layer of the synovium. The cells that were the most positive for IL‐32 staining were macrophage‐like cells. The percentage of RA patients with IL‐32 positive biopsies was lower among the group showing little clinical arthritis compared with those with moderate or severe knee inflammation. Assessment scores for IL‐32 in the lining were highly correlated with those for microscopic inflammation on routinely stained tissue sections (r = 0.80, p<0.0001) and also with the acute phase reaction as measured by the erythrocyte sedimentation rate (r = 0.71, p<0.0001). TNFα was detectable in only 50% of patients with RA. In contrast IL‐1β staining was observed in most synovial biopsies (90% of RA patients) whereas IL‐18 was detectable in 79% of the same synovial tissue samples. The levels of IL‐32 and TNFα expression in the same biopsies were strongly correlated (r = 0.68, p<0.004 for lining). However, a greater association was found for IL‐32 presence in the lining layers with the expression of IL‐1β and IL‐18 in the same biopsies (r = 0.79, p<0.0001 and r = 0.82, p<0.0001, respectively).
IL‐32 in Crohn's disease
Activation of non‐specific inflammatory responses (innate immunity) plays an essential role in host defence against invading organisms. In general, these responses include the induction of proinflammatory cytokines, which assist the host in eliminating the infection by non‐immune mechanisms such as emigration of phagocytic cells and the production of toxic products to destroy the organisms. Indeed, bacterial products induce cytokines via pattern‐recognition receptors for several bacterial products. The two most clinically relevant families of microbial receptors are the cell‐surface TLRs and the intracellular nuclear oligomerisation domain (NOD) receptor family. IL‐32 acts in a synergistic manner with the NOD1 and NOD2 specific muropeptides of peptidoglycans for the release of IL‐1β and IL‐6 (3–10‐fold increase). In contrast, IL‐32 did not influence the cytokine production induced via TLR receptors. The synergistic effect of IL‐32 and the synthetic muramyl dipeptide (MDP) on cytokine production was absent in cells of patients with Crohn's disease bearing the NOD2 frame shift mutation 3020insC, demonstrating that the IL‐32/MDP synergism depends on NOD2. This in vitro synergism between IL‐32 and NOD2 ligands was consistent with a marked constitutive expression of IL‐32 in human colon epithelial tissue. In addition, the potentiating effect of IL‐32 on the cytokine production induced by the synthetic muropeptide FK‐156 was absent in NOD1‐deficient macrophages, supporting the interaction between IL‐32 and NOD1 pathways. Of importance, the synergism between IL‐32 and MDP/NOD2 for the induction of IL‐6 was dependent on the activation of caspase‐1 and the secretion of IL‐1β. Only additive effects of IL‐32 and muropeptides were observed for TNFα production. The modulation of intracellular NOD2 pathways by IL‐32, but not the cell‐surface TLRs, as well as the marked expression of IL‐32 in colon mucosa, suggest a role of IL‐32 in the pathogenesis of Crohn's disease.
Intracellular IL‐32
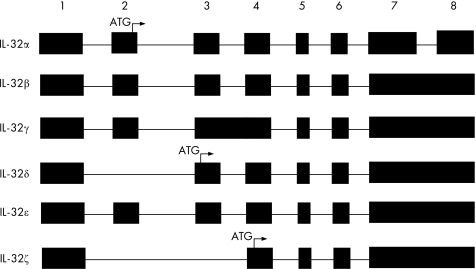
The amino acid sequence derived from the initial NK4 cDNA contained a signal peptide without a transmembrane domain.1 However, the transcript was never expressed as a recombinant protein and not sequenced. Therefore, it was assume that the protein coding for NK4 was a secreted protein. The NK4 transcript is presently termed IL‐32γ. It remains unknown if this isoform is, in fact, secreted. Primary human T cells stimulated with anti‐CD3 synthesise IL‐32 with a molecular weight of 25 kDa, which on western blot is found in lysates, not supernatants. Similar findings were reported for 293T cells transfected with either IL‐32γ or IL‐32β.6 In human peripheral blood mononuclear cells stimulated with ConA, most of the IL‐32 is found in the lysates.2 Overexpression of IL‐32α or IL‐32β in COS cells results in secreted IL‐32.2 Primary human blood monocytes secrete some IL‐32 into the supernatant. It remains unclear which of the IL‐32 isoforms (see fig 1) are secreted from a particular cell type and moreover, the type of stimuli that induce secretion. It is also clear that T cells activated by phorbol myristate acetate (PMA) and inomycin or NK cells activated by IL‐2 do not secrete IL‐32 or alternatively, the secreted IL‐32 is derived from “leaky” cells since there is co‐presence of GAPDH in the same supernatants. The authors concluded that IL‐32 is secreted only as a result of cell death.6 T cells are known not to secrete TNFα, but, nevertheless, membrane TNFα may account for a large component of the biological effects of TNFα.
Figure 1 Isoforms of human IL‐32. The numbers on the top represent the exon number. ATG indicates the first three codons for the N‐terminal amino acid of IL‐32. Isoforms α, β, γ, δ were reported in reference 2 and ε and ζ in reference 6.
Using HeLa cells overexpressing IL‐32β under the control of tetracycline removal undergo apoptosis.6 These results suggest that there is a role for intracellular IL‐32 and that role is one of cell death. Using the same HeLa cell line in which removal of tetracycline results in increased cell death, the co‐transfection short‐hairpin RNA coding for IL‐32 resulted in a 50% decrease in the expression of the IL‐32 protein intracellularly. Under conditions of increased expression of IL‐32, the RNA interference decreased cell death.6 These results also support the concept that high levels of IL‐32β intracellularly are inducing cell death. Whether such a phenomenon in HeLa cells overproducing IL‐32 is also a dominant property of IL‐32 in primary cells remains to be established.
The search for the IL‐32 receptor
In an attempt to isolate a putative IL‐32 receptor, we employed ligand affinity chromatography. This method has been used to isolate the soluble (extracellular) forms of cell‐bound receptors from highly concentrated human urine.10 Therefore, concentrated urine was applied to a column comprised of IL‐32α covalently immobilised to agarose beads. After extensive washing with neutral buffer, bound proteins were eluted at low pH. Aliquots of the various fractions were resolved by SDS‐PAGE under non‐reducing conditions and the protein bands were visualised by silver staining. We detected a broad band corresponding to a specific IL‐32α binding protein with an apparent molecular weight between 28 kDa and 32 kDa protein mainly in the acid‐eluted fraction.11 A band of this molecular weight was not observed in the wash fractions, which contain non‐binding urinary proteins. The purified elution fraction 3 was concentrated and subjected to SDS‐PAGE. Bands were visualised by silver staining and the 28–32 kDa protein band was excised and analysed by liquid‐chromatography and tandem mass spectrometry. The sequence of three tryptic peptides was unequivocally determined and revealed that the isolated IL‐32α binding protein was proteinase 3.
We measured the binding affinities of urinary or neutrophil‐derived proteinase 3 (PR3) to IL‐32α by surface plasmon resonance. The dissociation constant was determined to be 2.65×10−8 M.11 The experiment was repeated with PR3 that was rendered enzymatically inactive by pretreatment with phenylmethylsulphonyl fluoride (PMSF). Unexpectedly, the dissociation constant did not change significantly (kD = 7.9×10−8 M). A similar analysis was performed with PR3 purified from neutrophils. The affinity was comparable with that of the urinary PR3 (kD = 1.2×10−8 M). As in the case of urinary PR3, the affinity of the neutrophil PR3 was not affected by pretreatment with PMSF (kD = 3.5×10−8 M). Thus, PR3 derived from urine and neutrophils has similar binding affinities to recombinant IL‐32α and binding of IL‐32α to PR3 is independent of its enzymatic activity.
Cumulative data indicate that PR3 can have direct effects on intracellular processes in the absence of proteolytic activity. For example, PR3 rendered enzymatically inactive by α1‐proteinase inhibitor has been shown to induce IL‐8, both at the transcriptional and translational level.12 Also, a secreted, inactive form of PR3 (a complex of PR3 and the serine proteinase inhibitor α1‐antitrypsin), as well as an enzymatically silent mutant of PR3 have been shown to down modulate DNA synthesis in normal haematopoietic progenitor cells.13 This effect was reversed by the presence of granulocyte‐macrophage colony stimulating factor, implying that PR3 can function as a counterbalance to regulators of proliferation.13 In addition, enzymatically inactive PR3 induce apoptosis of bovine pulmonary artery endothelial cells.14 PR3 fragments generated by deletion of the catalytic triad are enzymatically inactive but induce apoptosis in human umbilical vein endothelial cells.14 Therefore, PR3 seems to be a multifunctional protein influencing cell cycle, differentiation, and cell death.
We attempted to dissect the binding capability of PR3 from its enzymatic activity, the BIAcore binding was performed with PMSF treated PR3. PMSF, a serine protease inhibitor, inactivates PR3.11 No significant change in the binding affinity of either urinary‐ or neutrophil‐derived PR3 to IL‐32α was observed following inactivation by PMSF. The rapid turnover of substrates bound to the active site of enzymes exclude the possibility of isolating enzymes by binding to their immobilised substrates. The enzyme PR3 was isolated due to its binding to its “substrate” IL‐32α. Therefore, it is likely that the binding of PR3 to the immobilised IL‐32α represents the non‐enzymatic interaction of enzyme to substrate and supports the concept that binding of IL‐32α and the processing of IL‐32α are two separate properties of PR3. The non‐enzymatic role of PR3 as an inhibitor IL‐32α activity similar to other cytokine binding proteins16 is yet to be established.
A role for PR3 in cytokine mediated disease may be due to its enzymatic property by which PR3 activates the proteinase activated receptor (PAR)‐2. PAR‐2 is found in many tissues where it participates in proinflammatory and pathological roles. For example, in mice deficient for PAR‐2, surgical trauma induced, leucocyte mediated endothelial inflammation is reduced.17 PAR‐2 appears to play an essential role in models of arthritis. Inflammatory arthritis is significantly decreased in PAR‐2 deficient mice and PAR‐2 agonists induce joint inflammation.18 PAR‐2 specific activating peptides induce colonic granulocyte infiltration and elevated T helper type 1 cytokines but not in PAR‐2 deficient mice.19 Other investigators have reported a significant participation of PAR‐2 in airway secretion and inflammation20 and in infectious colitis.21 As its name connotes, PAR‐2 is activated by proteases; trypsin, mast cell chymase, and PR3 all activate PAR‐2 and result in downstream inflammation.
Cytokine induced inflammation is also linked to PR3 and PAR‐2 activation. For example, in cells activated by agonistic anti‐PR3 antibodies, chemokines IL‐8 and MCP‐1 are readily produced but transfection with small interfering RNA specific for PAR‐2 markedly reduced the production of these inflammatory chemokines.22 In addition to agonist anti‐PR3, several proinflammatory cytokines such as IL‐1 and TNF increase both membrane as well as soluble PR3.22 The cytokine‐induction of PR3 results in cleavage and activation of PAR‐2 with its downstream proinflammatory effects. Interferon γ (IFNγ) is a particularly potent inducer of PR3 activity in epithelial cells22 and IFNγ induced epithelial cell PR3 also cleaves the inactive IL‐18 precursor into an active cytokine.23 Therefore, it is possible that the induction of IL‐32 in IFNγ stimulated epithelial cells2 includes the induction of active PR322 with subsequent limited proteolysis resulting in increased IL‐32 activity.
As most of the IFNγ induced PR3 is membrane bound,22 it is an attractive hypothesis that cleavage and increased activity of IL‐32 described in this report take place on the cell membrane. It is likely that IL‐32 is first inserted into the plasma membrane via three putative myristoylated side groups2 followed by PR3‐mediated cleavage. Lacking signal peptides, myristoylated cytokines such as IL‐1α and TNFα are found as membrane inserted proteins where they are biologically active.24,25,26 Membrane IL‐1α is cleaved by a calcium activated calpain27 and membrane TNFα is cleaved by serine proteases, both resulting in the release of the cytokines into the extracellular space. In the present study, limited proteolysis of soluble recombinant IL‐32α resulted in the formation of two peptides of 16 kDa and 13 kDa. When these fragments of IL‐32 were assessed for biological activity, there was an increase in the induction of macrophage inflammatory protein‐2 and IL‐8 from mouse and human monocytes, respectively. Extended cleavage of IL‐32 by PR3 destroyed the cytokine. It is possible that membrane IL‐32 is oriented so that membrane PR3 cleaves the cytokine by limited proteolysis. A similar limited proteolysis by serine proteases of membrane TNFα also exists.28
Acknowledgements
The authors thank D Novick, M Rubinstein, D‐Y Yoon, M Netea, L Joosten, W van den Berg, T Azam, E C Lewis, C K Edwards, L Y Chang, J Crapo, E Chan, and M Saavedra for their contributions to these studies.
Abbreviations
FLS - fibroblast‐like synoviocytes
IL - interleukin
MCP - monocyte chemoattractant protein
NOD - nuclear oligomerisation domain
NK - natural killer
PAR - proteinase activated receptor
PMSF - phenylmethylsulphonyl fluoride
PR3 - proteinase 3
RA - rheumatoid arthritis
TNF - tumour necrosis factor
TLR - toll‐like receptor
Footnotes
These studies are supported by NIH Grants AI‐15614 (CAD), HL‐68743 (CAD), and CA‐04 6934 (CAD), and Amgen, Inc.
Competing interests: none declared
References
- 1.Dahl C A, Schall R P, He H L, Cairns J S. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol 1992148597–603. [PubMed] [Google Scholar]
- 2.Kim S H, Han S Y, Azam T, Yoon D Y, Dinarello C A. Interleukin‐32: a cytokine and inducer of TNFalpha. Immunity 200522131–142. [DOI] [PubMed] [Google Scholar]
- 3.Carter K L, Cahir‐McFarland E, Kieff E. Epstein‐Barr virus‐induced changes in B‐lymphocyte gene expression. J Virol 20027610427–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park G H, Choe J, Choo H J, Park Y G, Sohn J, Kim M K. Genome‐wide expression profiling of 8‐chloroadenosine‐ and 8‐chloro‐cAMP‐treated human neuroblastoma cells using radioactive human cDNA microarray. Exp Mol Med 200234184–193. [DOI] [PubMed] [Google Scholar]
- 5.Brown J, Matutes E, Singleton A, Price C, Molgaard H, Buttle D.et al Lymphopain, a cytotoxic T and natural killer cell‐associated cysteine proteinase. Leukemia 1998121771–1781. [DOI] [PubMed] [Google Scholar]
- 6.Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S.et al Involvement of IL‐32 in activation‐induced cell death in T cells. Int Immunol 200618233–240. [DOI] [PubMed] [Google Scholar]
- 7.Dayer J M, Graham R, Russell G, Krane S M. Collagenase production by rheumatoid synovial cells: stimulation by a human lymphocyte factor. Science 1977195181–183. [DOI] [PubMed] [Google Scholar]
- 8.Cagnard N, Letourneur F, Essabbani A, Devauchelle V, Mistou S, Rapinat A.et al Interleukin‐32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast‐like synoviocytes. Eur Cytokine Netw 200516289–292. [PubMed] [Google Scholar]
- 9.Joosten L A, Netea M G, Kim S H, Yoon D Y, Oppers‐Walgreen B, Radstake T R.et al IL‐32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A 20061033298–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novick D, Engelmann H, Wallach D, Leitner O, Revel M, Rubinstein M. Purification of soluble cytokine receptors from normal human urine by ligand‐affinity and immunoaffinity chromatography. J Chromatogr 1990510331–337. [DOI] [PubMed] [Google Scholar]
- 11.Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello C A, Kim S H. Proteinase 3 is an IL‐32 binding protein. Proc Natl Acad Sci U S A 20061033316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger S P, Seelen M A, Hiemstra P S, Gerritsma J S, Heemskerk E, van der Woude F J.et al Proteinase 3, the major autoantigen of Wegener's granulomatosis, enhances IL‐8 production by endothelial cells in vitro. J Am Soc Nephrol 19967694–701. [DOI] [PubMed] [Google Scholar]
- 13.Skold S, Rosberg B, Gullberg U, Olofsson T. A secreted proform of neutrophil proteinase 3 regulates the proliferation of granulopoietic progenitor cells. Blood 199993849–856. [PubMed] [Google Scholar]
- 14.Yang J J, Preston G A, Pendergraft W F, Segelmark M, Heeringa P, Hogan S L.et al Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants. Am J Pathol 2001158581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato T, Sakamoto E, Kutsuna H, Kimura‐Eto A, Hato F, Kitagawa S. Proteolytic conversion of STAT3alpha to STAT3gamma in human neutrophils: role of granule‐derived serine proteases. J Biol Chem 200427931076–31080. [DOI] [PubMed] [Google Scholar]
- 16.Novick D, Kim S ‐ H, Fantuzzi G, Reznikov L, Dinarello C A, Rubinstein M. Interleukin‐18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 199910127–136. [DOI] [PubMed] [Google Scholar]
- 17.Lindner J R, Kahn M L, Coughlin S R, Sambrano G R, Schauble E, Bernstein D.et al Delayed onset of inflammation in protease‐activated receptor‐2‐deficient mice. J Immunol 20001656504–6510. [DOI] [PubMed] [Google Scholar]
- 18.Ferrell W R, Lockhart J C, Kelso E B, Dunning L, Plevin R, Meek S E.et al Essential role for proteinase‐activated receptor‐2 in arthritis. J Clin Invest 200311135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cenac N, Coelho A M, Nguyen C, Compton S, Andrade‐Gordon P, MacNaughton W K.et al Induction of intestinal inflammation in mouse by activation of proteinase‐activated receptor‐2. Am J Pathol 20021611903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunzelmann K, Sun J, Markovich D, Konig J, Murle B, Mall M.et al Control of ion transport in mammalian airways by protease activated receptors type 2 (PAR‐2). FASEB J 200519969–970. [DOI] [PubMed] [Google Scholar]
- 21.Hansen K K, Sherman P M, Cellars L, Andrade‐Gordon P, Pan Z, Baruch A.et al A major role for proteolytic activity and proteinase‐activated receptor‐2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci U S A 20051028363–8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uehara A, Sugawara Y, Sasano T, Takada H, Sugawara S. Proinflammatory cytokines induce proteinase 3 as membrane‐bound and secretory forms in human oral epithelial cells and antibodies to proteinase 3 activate the cells through protease‐activated receptor‐2. J Immunol 20041734179–4189. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A.et al Neutrophil proteinase 3‐mediated induction of bioactive IL‐18 secretion by human oral epithelial cells. J Immunol 20011676568–6575. [DOI] [PubMed] [Google Scholar]
- 24.Kurt‐Jones E A, Beller D I, Mizel S B, Unanue E R. Identification of a membrane‐associated interleukin‐1 in macrophages. Proc Natl Acad Sci U S A 1985821204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplanski G, Farnarier C, Kaplanski S, Porat R, Shapiro L, Bongrand P.et al Interleukin‐1 induces interleukin‐8 from endothelial cells by a juxacrine mechanism. Blood 1994844242–4248. [PubMed] [Google Scholar]
- 26.Kriegler M, Perez C, DeFay K, Albert I, Lu S D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell 19885345–53. [DOI] [PubMed] [Google Scholar]
- 27.Miller A C, Schattenberg D G, Malkinson A M, Ross D. Decreased content of the IL‐1α processing enzyme calpain in murine bone marrow‐derived macrophages after treatment with the bezene metabolite hydroquinone. Tox Lett 199474177–184. [DOI] [PubMed] [Google Scholar]
- 28.Robache‐Gallea S, Morand V, Bruneau J M, Schoot B, Tagat E, Realo E.et al In vitro processing of human tumor necrosis factor‐alpha. J Biol Chem 199527023688–23692. [DOI] [PubMed] [Google Scholar]