Abstract
Interleukin (IL)‐7 is a potent immunoregulatory cytokine that is detected in joints of patients with rheumatoid and juvenile idiopathic arthritis and which correlates with parameters of disease. Several synovial cell types that play an important role in inflammation and immunopathology, such as macrophages, dendritic cells, and fibroblasts, produce IL‐7. IL‐7 induces cytokines produced by arthritogenic T cells (for example, interferon γ (IFNγ), IL‐17), T cell differentiating factors (for example, IL‐12), chemokines capable of attracting inflammatory cells (for example, macrophage induced gene (MIG), macrophage inflammatory protein (MIP)‐1α) as well as molecules involved in cell adhesion, migration, and costimulation (for example, lymphocyte function associated antigen (LFA)‐1, CD40, CD80). In addition, IL‐7 can induce bone loss by stimulating osteoclastogenesis that is dependent on receptor activator of nuclear factor κB ligand (RANKL). IL‐7 induces tumour necrosis factor α (TNFα) secretion by T cells and by monocytes after T cell dependent monocyte/macrophage activation. Importantly, induction of both IL‐7 and IL‐7 induced effects seems to be able to operate independent of TNFα. Together this suggests that IL‐7 is an important cytokine in several rheumatic conditions, capable of inducing inflammation and immunopathology. Thus it may be an important target for immunotherapy.
Keywords: interleukin‐7, immunopathology, T cells, macrophages, rheumatoid arthritis
Rheumatoid arthritis (RA) is characterised by persistent inflammation of the joints, which results in progressive destruction of cartilage and bone.1 Several studies have revealed an important role for CD4+ T cells, B cells, and macrophages in the inflamed joints of patients with RA.2,3,4,5,6,7,8 Large numbers of these cells have been demonstrated in the synovial fluid and tissue of RA patients and their numbers and/or activity have been shown to correlate with clinical symptoms.3,4,5,7,9,10 Several “anti‐T cell”, anti‐B cell and “anti‐macrophage” therapies have resulted in good clinical responses8,11,12 in a substantial proportion of patients with RA. Despite this success, a large number of patients still do not respond to therapy or respond partially, and effects of therapy are transient requiring repeated drug administration. This implies that it is still worthwhile to counteract other mediators and mechanisms that have been indicated to play an important role in the immunopathology of RA. Recent findings imply that interleukin (IL)‐7, a potent immunoregulatory cytokine, could play a unique role in immunopathology of RA as well as other (rheumatic) inflammatory diseases.
Role of IL‐7 in immunity and inflammation
IL‐7 is a member of the IL‐2 family and signals through the IL‐7 receptor‐α chain (IL‐7Rα), in conjunction with the common gamma chain (γc). A related cytokine, the stromal cell derived lymphopoietin‐1 (TSLP‐1), also interacts with IL‐7Rα, but signals by formation of a heterodimer with a γc‐like chain, called the TSLP receptor.13,14,15
Epithelial cells in lymphopoietic tissues such as bone marrow, thymus, spleen, and the gut produce IL‐7. Apart from the sites of lymphopoiesis, many cell types throughout the body, such as keratinocytes, hepatocytes, endothelial cells as well as cells from the immune system including monocytes, and (follicular) dendritic cells, have the potential to produce IL‐7.16,17,18 Both IL‐7 increased thymic output and expansion of peripheral T cells have been shown to contribute to expansion and maintenance of the peripheral T cell pool.16,19 With progressing age and strong thymus atrophy20 the expansion and maintenance of the T cell pool becomes largely dependent on thymic independent pathways.16,21 IL‐7 plays an important role in T cell homoeostasis in humans and in mice by provision of signals for proliferation, growth, and survival of both developing and mature T cells.16,22,23
Apart from the stimulating mechanisms that regulate T cell numbers, IL‐7 has been shown to stimulate several effector functions of not only T cells but also other cells of both the acquired and innate immune systems. Although IL‐7 can stimulate IL‐4 production, human in vitro studies have shown that IL‐7 primarily induces T helper (Th) 1 cytokine secretion (IFNγ production) by both human CD4+ and CD8+ T cells in the absence of IL‐4 production.24 In mixed mononuclear cell cultures from peripheral blood of healthy controls IL‐7 induces IFNγ but no IL‐4 secretion (van Roon, unpublished data). Besides cytokine secretion, IL‐7 stimulates cytotoxic activity mediated by CD8+ T cells.25,26 In addition to affecting function of CD4+ and CD8+ T cells, IL‐7 also augments the function of natural killer (NK) cells.27 In humans, this activity seems to be restricted to function of mature NK cells, since disruption of the IL‐7 pathway in IL‐7R deficient individuals does not lead to impaired development of NK cells.28
The effects of IL‐7 on B cell activity differ significantly between mice and humans. Although clear effects on B cell development have been shown in IL‐7 knockout mice, and immature human B cells can respond to IL‐7,29 IL‐7Rα deficiency in humans does not affect B cell development.28,30 IL‐7Rα deficient individuals only have abnormalities in T cell development as measured by diminished CD3+ T cell numbers and reduced lymphocyte proliferation to mitogen and allogenic cells, whereas B cell numbers are normal.28,30 Despite the lack of direct effect of IL‐7 on B cell numbers, IL‐7Rα deficiency does influence B cell activity since Ig levels in IL‐7Rα deficient humans can be reduced.28 As the IL‐7Rα expression levels of mature human B cells are low to absent this observation might be largely related to T cell dependent effects on B cell activity.
In addition to the acquired immune system, IL‐7 has also been shown to activate cells from the innate immune system. IL‐7 induces secretion of IL‐1α, IL‐1β, IL‐6, IL‐8, macrophage inflammatory protein (MIP)‐1β and tumour necrosis factor α (TNFα) by human monocytes.25,31,32 However, it should be noticed that high, possibly supraphysiological, levels of IL‐7 (50–100 ng/ml) are required to induce this cytokine secretion, approximately 100–1000‐fold higher than observed for activation of T cells.25,33,34 This may be related to the much lower to absent expression of the IL‐7Rα protein levels on monocytes compared with T cell surfaces.35 In contrast with T cells and monocytes, IL‐7 does not have any direct effect on granulocyte activity, related to the absence of IL‐7Rα expression on these cells.36
In vivo administration of IL‐7 in mice has also shown strong effects on cells from the acquired and innate immune systems. Administration of IL‐7 resulted in increases in T cells, NK cells, and macrophages, and stimulation of B lymphocyte production.37 T cells from IL‐7‐treated mice have been shown to have enhanced proliferative responses to various stimuli in vitro, and these cells were able to potentiate cytotoxic T lymphocyte responses in vivo.37 In bone marrow transplant studies, in which mice were treated with IL‐7 after transplantation, lymphocyte regeneration was accelerated and T and B cell function improved.38 An in vivo mouse model of IL‐7 transfected glioma cells showed reduction of tumorigenicity that was reversed by injecting an anti‐IL‐7 antibody at the tumour site. In addition IL‐7 can promote delayed‐type hypersensitivity reactions in mice.39
Effects of IL‐7 in rodents and primates differ markedly in some aspects, as demonstrated by studies of IL‐7Rα deficient humans and mice.16,28,30 Thus analysis of IL‐7 induced effects in primates is also important. In baboons, IL‐7 increased virus specific IFNγ producing CD4+ T cell numbers.40 After treatment of baboons (after TBI and CD34 cell transplantation) and Indian rhesus macaques with IL‐7, CD4+ and CD8+ lymphocytes populations were increased and lymph nodes were enlarged compared with untreated animals.41,42 Furthermore, IL‐7 increased the ability of CD4+ and CD8+ T central memory and T effector memory cells to produce the proinflammatory cytokines TNFα and IFNγ.41 In contrast with IL‐7 stimulated T cell reconstitution on TBI followed by CD34 cell transplantation, IL‐7 did not increase B cell, monocyte, and NK cell counts in baboons.42
The above data indicate that IL‐7 is an important immunoregulatory cytokine, which stimulates immunity that could contribute to inflammation and inflammation induced immunopathology in RA as well as other chronic inflammatory (rheumatic) diseases.
Role of IL‐7 in inflammation in (rheumatoid) arthritis
Recent studies indicate IL‐7 to be a factor with many activities which could contribute to inflammation and tissue destruction in RA in a unique manner.
IL‐7/IL‐7 receptor expression in RA
Serum levels of IL‐7 in patients with RA have been shown to be higher than in healthy controls and correlate positively with markers of inflammation. Although a number of groups have reported increased serum levels of IL‐7 in patients with RA and juvenile idiopathic arthritis (JIA),33,43,44 there are conflicting data on serum levels.45 Such differences in IL‐7 levels may be due to heterogeneity in drug use between the studies as explained below. In support of a role of IL‐7 in RA synovitis is the observation that in RA synovial fluid levels of IL‐7 (up to 480 pg/ml) were strongly elevated compared with the levels in synovial fluid of patients with osteoarthritis (a joint disease with mild or no inflammation).34 Furthermore, in synovial tissue (biopsies) from RA patients high IL‐7 levels are expressed by macrophages, fibroblasts, and endothelial cells throughout the tissue. Numbers of IL‐7+ cells have been shown to strongly correlate with the presence of CD68+ macrophages in the lining and sub‐lining. Double staining has demonstrated that CD68+ macrophages are major producers of IL‐7.34 In addition, dendritic‐like cells in the lymphoid follicles have been found.34 Recently, we supported the latter observation by showing that in vitro GM‐CSF/IL‐4 generated dendritic cells from RA patients were indeed significant producers of IL‐7 (van Roon et al, unpublished data). In addition, IL‐7 production by dendritic cells from healthy controls has been previously shown by other groups.18,46
Both in RA peripheral blood and synovial fluid the receptor that is essential for IL‐7 signalling (IL‐7Rα) is primarily expressed on T cells, with the highest expression on CD4+ T cells. In addition NK T cells express considerable IL‐7Rα on their cell surface although at a lower level than on CD4+ T cells. In contrast, CD19+ B cells and monocytes in the circulation of patients with RA express no to very little IL‐7Rα on their surface. However, subpopulations of macrophages and CD19+ B cells from synovial fluid display increased surface IL‐7Rα levels (Hartgring et al, unpublished data). This might be due to the local inflammatory milieu because treatment of monocytes with cytokines and toll‐like receptor agonists has been shown to induce IL‐7Rα expression on these cells (van Roon et al, unpublished data). The IL‐7Rα distribution suggests that in RA patients T cells may be a primary target of IL‐7 and that initial IL‐7 driven effects may be primarily mediated by T cells. In vitro studies support this suggestion.
IL‐7 activity on human CD4+ T cells and monocytes in vitro
RA is characterised by a diverse autoreactive T cell response against numerous self‐antigens expressed in the inflamed joints (for example, collagen type II, heat‐shock proteins, aggrecan).47 However, although detectable, such T cells are present in a low frequency and are part of an oligo/polyclonal intra‐articular T cell response.48 Several groups have tried to explain this widespread T cell response, but a causal linkage has not been found. In fact, the contribution of T cells in RA may have been underestimated because, based on the use of T cell receptor (TCR) mediated mitogenic stimuli, hyporesponsiveness of intra‐articular T cells from RA patients to TCR driven activation has been suggested.49 This contrasts with the observation that in RA joints a large activated T cell pool is found. Until now, there is a limited number of factors that explain these (hyper)activated T cells.50,51
Our data have demonstrated that intra‐articular CD4+ T cells are hyperresponsive to IL‐7.34 In co‐cultures of monocytes/macrophages and CD4+ T cells, T cell activation was shown to require cell contact and was related to the IL‐7 induced expression of costimulatory molecules on macrophages from the synovial fluid, such as CD40, CD86, and, in particular, CD80.34 In addition, upregulation of costimulatory molecules such as lymphocyte function associated antigen (LFA)‐1 and CD69 has been observed and could play an important role in (CD4+) T cell activation. Recently we have shown that this IL‐7 induced contact dependent activation, which is associated with monocyte activation (measured by upregulation of CD80 and CD40), is also associated with TNFα production.35 IL‐7 (at similar concentrations) fails to induce TNFα secretion by isolated T cells or monocytes cultured separately. These data support our previous data that IL‐7 induces TNFα secretion by mononuclear cells from the synovial fluid of RA patients.33
Previously we have shown that IL‐7 promotes arthritogenic Th 1 cell activity in cultures of mononuclear cells from RA patients. IL‐7 primes T cells for IFNγ and TNFα production in contrast to IL‐4 production.33 IFNγ induction by IL‐7 is dependent on IL‐12 since blockade of IL‐12 markedly reduced IFNγ production. Interestingly, IL‐7 induced production of TNFα is not inhibited by IL‐12 blockade.33 This may be related to the induction of other regulatory cytokines. Using cytokine arrays we found that IL‐7 can not only stimulate Th 1 activity (IFNγ production) but possibly also Th 17 activity because IL‐17 production by RA mononuclear cells was increased by IL‐7. This was in the absence of induction of Th 2 activity (no IL‐4, IL‐5). Th 1 activity was associated with induction of Th 1 cell differentiating factors such as IL‐12 and small amounts of IL‐18. Interestingly, IL‐7 also induced chemokines (macrophage induced gene (MIG) and MIP‐1α) that can lead to chemotaxis of—in particular—Th 1 cells.
Persistence of IL‐7 activity and levels on anti‐TNFα treatment
Anti‐TNFα treatment is presently the treatment of choice in refractory RA. Although this treatment is effective in a significant number of patients, considerable numbers of patients do not respond.12 From a utilisation perspective (possible use of anti‐IL‐7 treatment) it has been of major importance to study IL‐7 in relation to TNFα and under anti‐TNFα conditions.
IL‐7 significantly enhances TNFα production by isolated naive and memory CD4+ T cells from RA patients that were costimulated with CD3/28.33,52 However, recently we have found that IL‐7 can also induce T cell dependent production of TNFα by monocytes.35,53 In addition, the levels of expression of TNFα and IL‐7 in the synovial fluid and synovial tissue correlated significantly. This relation indicates that in the synovial compartment IL‐7 could contribute to increased TNFα levels. Alternatively, TNFα inducing IL‐7 production could contribute to increased IL‐7 levels. TNFα has been shown to induce IL‐7 production by RA fibroblasts and bone marrow stromal cells.54,55 To test the latter possibility we measured the effect of TNFα blockade on circulating IL‐7 levels in RA patients. It was observed that in non‐responding patients serum levels of IL‐7 were not reduced but in fact slightly increased, whereas in anti‐TNFα responders IL‐7 levels were significantly decreased.35 After two weeks of therapy changes in disease activity scores correlated with changes in IL‐7 levels. The persistence of IL‐7 levels after TNFα blockade in a non‐responding subpopulation of patients suggests that, in particular in these patients, IL‐7 could persist to promote inflammatory responses (not regulated by TNFα). In addition, as IL‐7 in vitro has been shown to induce TNFα production, IL‐7 blockade could at least partially prevent TNFα induced inflammation. Furthermore, in vitro studies have shown that to a considerable extent IL‐7 induced proinflammatory responses cannot be abolished by TNFα blockade.35 Potentially, because of their mutual inductive capacities, synergistic immunosuppression may be achieved by blockade of TNFα together with blockade of IL‐7.
Role of IL‐7 in immunopathology in (rheumatoid) arthritis
IL‐7 is an inducer of TNFα, which has been shown to be a pivotal inducer of inflammation and joint destruction in a large proportion of RA patients. This implies that IL‐7 may also contribute besides IL‐7 induced TNFα dependent inflammation to TNFα dependent joint destruction. However, independent of TNFα IL‐7 could also promote joint destruction by the induction of other mediators—for example, IL‐17. In addition, by induction of many proinflammatory cytokines IL‐7 can promote inflammation and consequently inflammation induced destruction of joint tissues such as cartilage and bone.
Another mechanism contributing to joint destruction that IL‐7 might induce in particular is the activation of fibroblasts. Th 1 cells, either by cell contact or cytokine secretion (for example, IFNγ), have been shown to activate fibroblasts.56 Because IL‐7 induces Th 1 activation it is anticipated that IL‐7 might also induce fibroblast activation and possibly fibroblast induced destruction of cartilage and bone matrices. However, direct proof is needed. In this respect, the independence of IL‐7 from TNFα in this context remains to be demonstrated. The notion that TNFα, in contrast with IL‐7, does not induce but inhibits Th 1 development, points towards IL‐7 induced, TNFα independent, effects that could occur.57
Apart from fibroblast activation IL‐7 recently has been shown to play a pivotal role in osteoclastogenesis and bone loss. In mice IL‐7 induces bone loss through increased osteoclastogenesis, whereas it has been found that IL‐7Rα deficient mice show greatly increased femoral trabecular bone volume compared with wild‐type and heterozygous littermates.58 The IL‐7‐induced bone loss in mice is mediated by induction of receptor activator of nuclear factor κB ligand (RANKL) and TNFα production by T cells.59 These cytokines were found to induce osteoclasts from monocytes and bone marrow B cell precursors.59 Similarly, IL‐7 induced osteoclast formation from human monocytes in a T cell dependent way that was strongly (approximately 50%) dependent on RANKL.55 Together, this suggests that IL‐7 could contribute to bone destruction in RA by induction of T cell dependent osteoclastogenesis.
In addition to the production of IL‐7 by cells from the synovial tissue, increased expression of IL‐7 mRNA by articular cartilage chondrocytes from RA patients compared with chondrocytes from patients with osteoarthritis has been detected.60 We have recently demonstrated by immunohistochemistry IL‐7 protein expression in chondrocytes in the cartilage tissue from a patient with RA. The role of this IL‐7 remains to be elucidated, however, it does not seem to play a role in the metabolism of cartilage matrix components as cartilage proteoglycan turnover is not affected directly by IL‐7 (van Roon et al, unpublished observations).
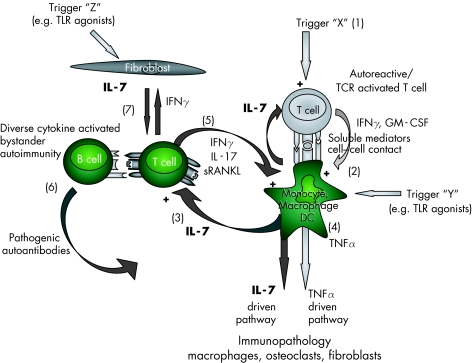
Based on data from our work and other groups we propose a concept in which IL‐7 is suggested to induce cell contact dependent spreading of the autoimmunity and immunopathology in RA via an alternative, primarily cytokine driven route (fig 1). This unique route could operate, at least in a subpopulation of patients or in certain stages of the disease, largely independent of TNFα induced immunopathology. This pathway may not only play a role in RA but also in other chronic inflammatory rheumatic diseases.
Figure 1 On the basis of our published data and preliminary work, and recent data from other groups, we propose the following concept: in autoimmune diseases such as rheumatoid arthritis (RA) an unknown trigger “X” (1) may cause (self)‐antigen specific T cell activation, resulting in cytokine production (for example, by interferon γ (IFNγ) and granulocyte macrophage‐colony stimulating factor (GM‐CSF)) and cell contact that lead to activation of cells such as monocytes, macrophages, and dendritic cells (2) which is associated with interleukin (IL)‐7 and tumour necrosis factor α (TNFα) production.18 Increased IL‐7 (not TNFα34,57) induces cell contact dependent,34,61 cytokine activated T cells (3) causing a spreading of T cell activation associated with autoantigenic recognition (possibly intermediate affinity self‐antigens).62 This can operate independent of TNFα (4). Such cytokine activated, bystander T cells in turn stimulate monocytic cells and possibly B cells (5).28,34 As a consequence, monocytes differentiate into macrophages and osteoclasts that mediate inflammation and joint destruction.34,55,59 Activated B cells in their turn are potent antigen presenting cells and can develop into plasma cells that secrete pathogenic autoantibodies (6).8 Finally, activated T cells could interact (via cell contact and cytokine production) with and activate synovial fibroblasts, which can be associated with further IL‐7 production (7).56 Stimulated IL‐7 production by both fibroblasts and macrophages (which could also be induced by alternative routes, triggers “Y” and “Z”—for example, via toll‐like receptor agonists) can further enhance IL‐7‐driven immunopathology. RANKL, receptor activator of nuclear factor κB ligand; TLR, toll‐like receptor.
IL‐7 in other chronic inflammatory (autoimmune) diseases
In patients with JIA, with active systemic disease, plasma levels of IL‐7 have been shown to be higher than in healthy children.44 Patients with JIA in remission had levels comparable with controls. Recently, increased IL‐7 levels have been detected in the synovial fluid of JIA patients, and these were related to clinical activity in that IL‐7 levels were higher in polyarthritis compared with the milder oligoarthritic forms.63 IL‐7 was found to abrogate the suppressive activity of regulatory T cells, altering the balance between proinflammatory effector and suppressor T cells, a balance that was related to clinical activity in these patients.63
A proinflammatory role of IL‐7 has also been suggested in another rheumatic autoimmune disorder—primary Sjögren's syndrome (pSS), which is characterised by lymphocyte infiltration in salivary and lachrymal glands. Levels of IL‐7 in saliva and salivary glands of patients with pSS were significantly higher compared with those of controls (van Woerkom et al, manuscript submitted for publication). Expression of IL‐7 correlated with the presence of local and peripheral disease activity parameters (lymphocyte focus score/IgA producing plasma cells and erythrocyte sedimentation rate/serum IgG levels, respectively). IL‐7 induced production of cytokines that can contribute to activation of proinflammatory Th 1 cells (IL‐12 and IL‐15), and induced cytokines produced by Th 1 cells (IFNγ) as well as chemokines that facilitate migration of such cells (MIG and IP‐10). This was in contrast to IL‐4, the major defining Th 2 cytokine, which was not significantly induced. These findings corroborate previous findings demonstrating a predominance of Th 1 cell activity in patients with pSS.64
IL‐7 is also increased in relapsing polychondritis, a systemic disorder in which there is recurrent, widespread chondritis of the auricular, nasal, and tracheal cartilages. The pathology is suspected to be autoimmune, on the basis of association with human leucocyte antigen (HLA)‐DR4 and evidence of humoral and cellular responses against cartilage components.43 IL‐7 levels are also significantly higher in the lesional regions of the skin of patients with psoriasis, compared with controls as well as non‐lesional skin of patients65; this has been found in skin biopsies as well as samples from the stratum cornea. Serum levels in patients were significant higher than those of controls. As circulating cells did not show an increased production compared with healthy controls, it was suggested that the increased skin and serum concentrations of IL‐7 were skin derived, most likely produced by keratinocytes.
Conclusion
IL‐7 is a potent immunoregulatory cytokine that is produced by cells of the immune system and tissue cells at the inflammatory site of several rheumatic disorders, correlating with parameters of disease. IL‐7 activates T cells and seems to cause primarily T cell dependent B cell and macrophage activation. In addition, IL‐7 can induce bone loss by stimulating RANKL dependent osteoclastogenesis. It is suggested that IL‐7 induces both TNFα dependent and independent inflammatory responses and immunopathology. Considering the lack of response or partial response to anti‐TNFα therapy in a considerable number of patients the elucidation of the role IL‐7 in immunopathology will be of major value. In this respect, study of the capacity of IL‐7 blockade to reduce arthritis and joint pathology in experimental animal models for arthritis is a necessity.
Abbreviations
IFN - interferon
IL - interleukin
IL‐7Rα - IL‐7 receptor‐α chain
JIA - juvenile idiopathic arthritis
MIG - macrophage induced gene
MIP - macrophage inflammatory protein
NK - natural killer
RA - rheumatoid arthritis
RANKL - receptor activator of nuclear factor κB ligand
Th - T helper
TNF - tumour necrosis factor
Footnotes
Competing interests: none declared
References
- 1.Feldmann M, Brennan F M, Maini R N. Rheumatoid arthritis. Cell 199685307–310. [DOI] [PubMed] [Google Scholar]
- 2.Roudier J. Association of MHC and rheumatoid arthritis. Association of RA with HLA‐DR4: the role of repertoire selection, Arthritis Res 20002217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabaud M, Durand J M, Buchs N, Fossiez F, Page G, Frappart L.et al Human interleukin‐17: A T cell‐derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 199942963–970. [DOI] [PubMed] [Google Scholar]
- 4.Morita Y, Yamamura M, Kawashima M, Harada S, Tsuji K, Shibuya K.et al Flow cytometric single‐cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum 1998411669–1676. [DOI] [PubMed] [Google Scholar]
- 5.Namekawa T, Wagner U G, Goronzy J J, Weyand C M. Functional subsets of CD4 T cells in rheumatoid synovitis. Arthritis Rheum 1998412108–2116. [DOI] [PubMed] [Google Scholar]
- 6.Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum 200043617–627. [DOI] [PubMed] [Google Scholar]
- 7.Burmester G R, Stuhlmuller B, Keyszer G, Kinne R W. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? Arthritis Rheum 1997405–18. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J C, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close D R.et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 20043502572–2581. [DOI] [PubMed] [Google Scholar]
- 9.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 199639115–124. [DOI] [PubMed] [Google Scholar]
- 10.Firestein G S, Alvaro‐Gracia J M, Maki R, Alvaro‐Garcia J M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol 19901443347–3353. [PubMed] [Google Scholar]
- 11.Kremer J M, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S.et al Treatment of rheumatoid arthritis by selective inhibition of T‐cell activation with fusion protein CTLA4Ig. N Engl J Med 20033491907–1915. [DOI] [PubMed] [Google Scholar]
- 12.Olsen N J, Stein C M. New drugs for rheumatoid arthritis. N Engl J Med 20043502167–2179. [DOI] [PubMed] [Google Scholar]
- 13.Sims J E, Williams D E, Morrissey P J, Garka K, Foxworthe D, Price V.et al Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med 2000192671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park L S, Martin U, Garka K, Gliniak B, Di Santo J P, Muller W.et al Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med 2000192659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey A, Ozaki K, Baumann H, Levin S D, Puel A, Farr A G.et al Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol 2000159–64. [DOI] [PubMed] [Google Scholar]
- 16.Fry T J, Mackall C L. Interleukin‐7: from bench to clinic. Blood 2002993892–3904. [DOI] [PubMed] [Google Scholar]
- 17.Sieling P A, Sakimura L, Uyemura K, Yamamura M, Oliveros J, Nickoloff B J.et al IL‐7 in the cell‐mediated immune response to a human pathogen. J Immunol 19951542775–2783. [PubMed] [Google Scholar]
- 18.Vasir B, Avigan D, Wu Z, Crawford K, Turnquist S, Ren J.et al Dendritic cells induce MUC1 expression and polarization on human T cells by an IL‐7‐dependent mechanism. J Immunol 20051742376–2386. [DOI] [PubMed] [Google Scholar]
- 19.Chu Y W, Memon S A, Sharrow S O, Hakim F T, Eckhaus M, Lucas P J.et al Exogenous IL‐7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood 20041041110–1119. [DOI] [PubMed] [Google Scholar]
- 20.Sempowski G, Thomasch J, Gooding M, Hale L, Edwards L, Ciafaloni E.et al Effect of thymectomy on human peripheral blood T cell pools in myasthenia gravis. J Immunol 20011662808–2817. [DOI] [PubMed] [Google Scholar]
- 21.Hakim F T, Memon S A, Cepeda R, Jones E C, Chow C K, Kasten‐Sportes C.et al Age‐dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest 2005115930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondrack R M, Harbertson J, Tan J T, McBreen M E, Surh C D, Bradley L M. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med 20031981797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol 20034680–686. [DOI] [PubMed] [Google Scholar]
- 24.Chou Y K, Bourdette D N, Barnes D, Finn T P, Murray S, Unsicker L.et al IL‐7 enhances Ag‐specific human T cell response by increasing expression of IL‐2R alpha and gamma chains. J Neuroimmunol 199996101–111. [DOI] [PubMed] [Google Scholar]
- 25.Alderson M R, Tough T W, Ziegler S F, Grabstein K H. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med 1991173923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehrotra P T, Grant A J, Siegel J P. Synergistic effects of IL‐7 and IL‐12 on human T cell activation. J Immunol 19951545093–5102. [PubMed] [Google Scholar]
- 27.Lum J J, Schnepple D J, Nie Z, Sanchez‐Dardon J, Mbisa G L, Mihowich J.et al Differential effects of interleukin‐7 and interleukin‐15 on NK cell anti‐human immunodeficiency virus activity. J Virol 2004786033–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giliani S, Mori L, de Saint B G, Le Deist F, Rodriguez‐Perez C, Forino C.et al Interleukin‐7 receptor alpha (IL‐7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev 2005203110–126. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin R G, Lupton S, Schmierer A, Hjerrild K J, Jerzy R, Clevenger W.et al Human interleukin 7: molecular cloning and growth factor activity on human and murine B‐lineage cells. Proc Natl Acad Sci U S A 198986302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puel A, Ziegler S F, Buckley R H, Leonard W J. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet 199820394–397. [DOI] [PubMed] [Google Scholar]
- 31.Standiford T J, Strieter R M, Allen R M, Burdick M D, Kunkel S L. IL‐7 up‐regulates the expression of IL‐8 from resting and stimulated human blood monocytes. J Immunol 19921492035–2039. [PubMed] [Google Scholar]
- 32.Ziegler S F, Tough T W, Franklin T L, Armitage R J, Alderson M R. Induction of macrophage inflammatory protein‐1 beta gene expression in human monocytes by lipopolysaccharide and IL‐7. J Immunol 19911472234–2239. [PubMed] [Google Scholar]
- 33.van Roon J A, Glaudemans K A, Bijlsma J W, Lafeber F P. Interleukin 7 stimulates tumour necrosis factor alpha and Th1 cytokine production in joints of patients with rheumatoid arthritis. Ann Rheum Dis 200362113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Roon J A, Verweij M C, Wijk M W, Jacobs K M, Bijlsma J W, Lafeber F P. Increased intraarticular interleukin‐7 in rheumatoid arthritis patients stimulates cell contact‐dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum 2005521700–1710. [DOI] [PubMed] [Google Scholar]
- 35.van Roon J A G, Wenting‐vanWijk M, Jahangier N, Bijlsma J W J, Lafeber F P J G. IL‐7 stimulates T cell‐dependent TNFα production by monocytes and persists upon anti‐TNFα therapy of RA patients [abstract]. Arthritis Rheum 200552S274 [Google Scholar]
- 36.Girard D, Beaulieu A D. Absence of the IL‐7 receptor component CDw127 indicates that gamma(c) expression alone is insufficient for IL‐7 to modulate human neutrophil responses. Clin Immunol Immunopathol 199783264–271. [DOI] [PubMed] [Google Scholar]
- 37.Komschlies K L, Gregorio T A, Gruys M E, Back T C, Faltynek C R, Wiltrout R H. Administration of recombinant human IL‐7 to mice alters the composition of B‐lineage cells and T cell subsets, enhances T cell function, and induces regression of established metastases. J Immunol 19941525776–5784. [PubMed] [Google Scholar]
- 38.Bolotin E, Smogorzewska M, Smith S, Widmer M, Weinberg K. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin‐7. Blood 1996881887–1894. [PubMed] [Google Scholar]
- 39.Li A L, Li C, Feng Y G, Yuan G H, Wang G M, Hao J.et al Antileukemic effect of interleukin‐7‐transduced bone marrow stromal cells in mice following allogeneic T‐cell‐depleted bone marrow transplantation. Transplant Proc 2005372297–2299. [DOI] [PubMed] [Google Scholar]
- 40.Lu H, Zhao Z, Kalina T, Gillespy T, III, Liggitt D, Andrews R G.et al Interleukin‐7 improves reconstitution of antiviral CD4 T cells. Clin Immunol 200511430–41. [DOI] [PubMed] [Google Scholar]
- 41.Moniuszko M, Fry T, Tsai W P, Morre M, Assouline B, Cortez P.et al Recombinant interleukin‐7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol 2004789740–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storek J, Gillespy T, III, Lu H, Joseph A, Dawson M A, Gough M.et al Interleukin‐7 improves CD4 T‐cell reconstitution after autologous CD34 cell transplantation in monkeys. Blood 20031014209–4218. [DOI] [PubMed] [Google Scholar]
- 43.Stabler T, Piette J C, Chevalier X, Marini‐Portugal A, Kraus V B. Serum cytokine profiles in relapsing polychondritis suggest monocyte/macrophage activation. Arthritis Rheum 2004503663–3667. [DOI] [PubMed] [Google Scholar]
- 44.De Benedetti F, Massa M, Pignatti P, Kelley M, Faltynek C R, Martini A. Elevated circulating interleukin‐7 levels in patients with systemic juvenile rheumatoid arthritis. J Rheumatol 1995221581–1585. [PubMed] [Google Scholar]
- 45.Ponchel F, Verburg R J, Bingham S J, Brown A K, Moore J, Protheroe A.et al Interleukin‐7 deficiency in rheumatoid arthritis: consequences for therapy‐induced lymphopenia. Arthritis Res Ther 20057R80–R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saint‐Vis B, Fugier‐Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait‐Yahia S.et al The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol 19981601666–1676. [PubMed] [Google Scholar]
- 47.van Eden W, van der Z R, Taams L S, Prakken A B, van Roon J, Wauben M H. Heat‐shock protein T‐cell epitopes trigger a spreading regulatory control in a diversified arthritogenic T‐cell response. Immunol Rev 1998164169–174. [DOI] [PubMed] [Google Scholar]
- 48.Sakkas L I, Scanzello C, Johanson N, Burkholder J, Mitra A, Salgame P.et al T cells and T‐cell cytokine transcripts in the synovial membrane in patients with osteoarthritis [see comments]. Clin Diagn Lab Immunol 19985430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maurice M M, van der Voort E A, Leow A, Levarht N, Breedveld F C, Verweij C L. CD28 co‐stimulation is intact and contributes to prolonged ex vivo survival of hyporesponsive synovial fluid T cells in rheumatoid arthritis. Eur J Immunol 1998281554–1562. [DOI] [PubMed] [Google Scholar]
- 50.McInnes I B, al Mughales J, Field M, Leung B P, Huang F P, Dixon R.et al The role of interleukin‐15 in T‐cell migration and activation in rheumatoid arthritis. Nat Med 19962175–182. [DOI] [PubMed] [Google Scholar]
- 51.McInnes I B, Leung B P, Sturrock R D, Field M, Liew F Y. Interleukin‐15 mediates T cell‐dependent regulation of tumor necrosis factor‐alpha production in rheumatoid arthritis. Nat Med 19973189–195. [DOI] [PubMed] [Google Scholar]
- 52.van Roon J A, Glaudemans C A, Bijlsma J W, Lafeber F P. Differentiation of naive CD4+ T cells towards T helper 2 cells is not impaired in rheumatoid arthritis patients. Arthritis Res Ther 20035R269–R276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Roon J A, van Roy J L, Duits A, Lafeber F P, Bijlsma J W. Proinflammatory cytokine production and cartilage damage due to rheumatoid synovial T helper‐1 activation is inhibited by interleukin‐4. Ann Rheum Dis 199554836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harada S, Yamamura M, Okamoto H, Morita Y, Kawashima M, Aita T.et al Production of interleukin‐7 and interleukin‐15 by fibroblast‐like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum 1999421508–1516. [DOI] [PubMed] [Google Scholar]
- 55.Weitzmann M N, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin‐7 stimulates osteoclast formation by up‐regulating the T‐cell production of soluble osteoclastogenic cytokines. Blood 2000961873–1878. [PubMed] [Google Scholar]
- 56.Chizzolini C, Parel Y, Scheja A, Dayer J M. Polarized subsets of human T‐helper cells induce distinct patterns of chemokine production by normal and systemic sclerosis dermal fibroblasts. Arthritis Res Ther 20058R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cope A P, Londei M, Chu N R, Cohen S B, Elliott M J, Brennan F M.et al Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti‐TNF antibodies in patients with rheumatoid arthritis. J Clin Invest 199494749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyaura C, Onoe Y, Inada M, Maki K, Ikuta K, Ito M.et al Increased B‐lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc Natl Acad Sci U S A 1997949360–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toraldo G, Roggia C, Qian W P, Pacifici R, Weitzmann M N. IL‐7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci U S A 2003100125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leistad L, Ostensen M, Faxvaag A. Detection of cytokine mRNA in human, articular cartilage from patients with rheumatoid arthritis and osteoarthritis by reverse transcriptase‐polymerase chain reaction. Scand J Rheumatol 19982761–67. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Huston G, Swain S L. IL‐7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med 20031981807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bebo B F, Jr, Schuster J C, Adlard K, Vandenbark A A, Offner H. Interleukin 7 is a potent co‐stimulator of myelin specific T cells that enhances the adoptive transfer of experimental autoimmune encephalomyelitis. Cytokine 200012324–331. [DOI] [PubMed] [Google Scholar]
- 63.Ruprecht C R, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A.et al Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 20052011793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Woerkom J M, Kruize A A, Wenting‐van Wijk M J, Knol E, Bihari I C, Jacobs J W.et al Salivary gland and peripheral blood T helper 1 and 2 cell activity in Sjögren's syndrome compared with non‐Sjögren's sicca syndrome. Ann Rheum Dis 2005641474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonifati C, Trento E, Cordiali‐Fei P, Carducci M, Mussi A, D'Auria L.et al Increased interleukin‐7 concentrations in lesional skin and in the sera of patients with plaque‐type psoriasis. Clin Immunol Immunopathol 19978341–44. [DOI] [PubMed] [Google Scholar]