Abstract
Background
B cell activation may result in an increased secretion of immunoglobulin free light chains (FLCs) in autoimmune diseases.
Objective
To analyse serum FLC levels in patients with rheumatoid arthritis and in those with primary Sjögren's syndrome (pSS).
Patients and methods
Blood samples were collected from 80 healthy blood donors, 50 patients with rheumatoid arthritis and 139 patients with pSS. Serum FLC level was measured using a new quantitative immunoassay.
Results
Mean (standard error (SE)) serum κ and λ FLC levels were significantly higher in patients with rheumatoid arthritis and in those with pSS than in controls (κ : 18.9 (1.1) and 16.3 (1.4) v 10.5 (0.4) mg/l, p<0.001 and p = 0.001, respectively; λ: 16.7 (1.2) and 19.3 (1.5) v 11.6 (0.6) mg/l, p<0.001 for both). 18 (36%) patients with rheumatoid arthritis and 31 (22.3%) patients with pSS had abnormal serum FLC levels (increased κ or λ levels and abnormal ratio of κ:λ). Serum κ and λ levels were correlated with other B cell activation markers in both diseases. FLC levels increased with disease activity, because, unlike total gammaglobulin and immunoglobulin G levels, they were significantly correlated with Disease Activity Score 28 in patients with rheumatoid arthritis (p = 0.004 for κ, p = 0.05 for λ) and with extraglandular involvement in pSS (p = 0.01 for κ, p = 0.04 for λ).
Conclusion
FLC levels are increased and correlate with disease activity in patients with rheumatoid arthritis and in those with pSS, two diseases in which increased risk of lymphoma could result from persistent B cell activation and disease activity. Further studies are required to determine whether FLC assessment could represent a relevant biomarker for response to treatment (especially B cell depletion) and for the risk of lymphoma in autoimmune diseases.
Immunoglobulin light chains and heavy chains are combined together during the synthesis of immunoglobulins; however, more light chains than heavy chains are produced. Thus, light chains that are not bound to intact immunoglobulins can be detected as circulating free light chains (FLCs) under physiological conditions. Increased FLC levels have been reported in several immunopathological conditions but until very recently, serum immunoassays required the separation of FLCs from intact immunoglobulins and were impractical for routine use. A new automated immunoassay now allows for sensitive and specific FLC assessment using antibodies directed against the “hidden” epitopes of FLC molecules, located at the interface between the light and heavy chains of intact immunoglobulins.1,2 To date, this assay has essentially been used to assess the excess of one light chain over another, using κ:λ ratio as a surrogate for clonal expansion. Thus, assessment of quantitative FLC levels already represents a major breakthrough in the routine monitoring of non‐secretory myeloma,3 light‐chain myeloma,4 primary amyloidosis5 and monoclonal gammapathy of undetermined significance (MGUS).6 However, assessment of serum FLC levels might also prove useful in autoimmune diseases. The interest in B cell activation markers has undergone a renaissance over the past few years, given the pivotal role of B cells in the pathogenesis of autoimmune diseases7 and the proved efficacy of B cell‐targeted treatment in patients with rheumatoid arthritis.8 We therefore investigated FLC levels in patients with rheumatoid arthritis and in those with primary Sjögren's syndrome (pSS), two diseases in which the pathogenic role of B cell activation has been shown well.9,10,11
Patients and methods
Patients
Blood samples were collected from 80 healthy blood donors (mean age 45 years), from 50 patients with rheumatoid arthritis according to the American College of Rheumatology criteria and from 139 Caucasian patients with pSS as defined by the American–European consensus group criteria (including a focus score ⩾1 on labial salivary gland biopsy or the presence of anti‐SSA/Ro or anti‐SSB/La antibodies).12 The patients successively attended the Department of Rheumatology, Hôpital de Bicêtre, Le Kremlin Bicêtre, France, and the Department of Rheumatology, Hôpital de Hautepierre, Strasbourg, France. Informed consent was obtained from all patients, and ethics committees of the two hospitals approved the study.
Patients with rheumatoid arthritis had a mean (standard error (SE)) age of 53 (14) years and a disease duration of 15 (9) years. In two patients, rheumatoid arthritis was associated with Sjögren's syndrome. Patients with rheumatoid arthritis were treated with methotrexate (n = 11), anti‐tumour necrosis factors (adalimumab, n = 7; infliximab, n = 17; etanercept, n = 8), or other disease‐modifying antirheumatic drugs (n = 7).
Patients with pSS had been previously included in a study evaluating B cell activation markers.13
Table 1 summarises the clinical and immunological features of the patients with pSS (mean (SE) age 56(12.5) years, disease duration 14 (8.6) years). Extraglandular involvement was defined as the presence or confirmed records of purpura, lung or neurological involvement, synovitis, myositis, vasculitis, lymphadenopathy, enlarged spleen or previous lymphoma during the evolution of the disease. Extraglandular involvement was present in 62 (44.6%) patients.
Table 1 Clinical and immunological features of 139 patients with primary Sjögren's syndrome.
| Enlarged parotid glands | 45 (32.4) |
| Raynaud's phenomenon | 49 (36.2) |
| Extraglandular involvement | 62 (44.6) |
| Purpura | 9 (6.4) |
| Synovitis | 21 (15.1) |
| Myositis | 3 (2.1) |
| Lung involvement | 15 (10.8) |
| CNS involvement/peripheral neuropathy | 1 (0.7)/9 (6.4) |
| Previous lymphoma* | 4 (2.8) |
| Medium‐size vessel vasculitis/lymphadenopathy | 1 (0.7)/3 (2.1) |
| κ FLC serum level, mg/l† | 16.3 (1.4) |
| λ FLC serum level, mg/l† | 19.3 (1.5) |
| κ:λ ratio† | 1 (0.3) |
| Positive RF results (>20 IU/ml) | 82 (58.9) |
| RF, IU/ml† | 165 (42) |
| β2‐microglobulin value, mg/l, n = 129† | 1.8 (0.2) |
| BAFF, ng/ml, n = 107† | 5.7 (0.9) |
| Gammaglobulin serum level, g/l† | 13.6 (1) |
| IgG serum level, g/l† | 16 (1.3) |
| IgA serum level, g/l† | 2.7 (0.3) |
| IgM serum level, g/l† | 2.4 (0.6) |
| No anti‐SSA or anti‐SSB antibodies | 43 (30.9) |
| Anti‐SSA antibody only | 47 (33.8) |
| Anti‐SSA and anti‐SSB antibodies | 49 (35.2) |
| Focus score ⩾1 on labial salivary gland biopsy (n = 125) | 112 (89.6) |
| Serum creatinine level, μmol/l† | 85.6 (3.2) |
BAFF, B cell‐activating factor; CNS, central nervous system; FLC, free light chain; Ig, immunoglobulin; RF, rheumatoid factor.
Values are n (%) unless otherwise mentioned.
*All patients with lymphoma were in remission and had normal FLC levels.
†Values are mean (SE).
Laboratory analysis
Serum FLC levels were measured using a latex‐enhanced immunoassay (Freelite, The Binding Site, Birmingham, UK) using a Behring nephelometric analyzer (Dade Behring, Deerfield, Illinois, USA). The immunoassay consisted of two separate measurements, one to detect free κ (normal range: 3.3–19.4 mg/l) and the other to detect free λ (normal range: 5.7–26.3 mg/l). The diagnostic ranges had been previously established by the manufacturer to include 100% of a reference population of 282 serum samples.2 A ratio of κ:λ<0.26 or >1.65 is abnormal, according to the manufacturer's recommendations.2 An abnormal FLC level was defined as an increased κ or an λ level or abnormal κ:λ ratio.
In patients with rheumatoid arthritis, levels of rheumatoid factor and of anticyclic citrullinated peptide (anti‐CCP) antibodies were assessed using nephelometry and ELISA (anti‐CCP2 kit; Immunoscan RA, Arnhem, The Netherlands, Eurodiagnostica, Arnhem, The Netherlands), respectively. Serum samples were diluted 1:50 and were considered to be positive for anti‐CCP if the antibody titre was >50 AU, as determined by dilution of a positive standard serum.
In patients with pSS, the results of the following biological markers of B cell activation have been previously reported: anti‐SSA and anti‐SSB antibodies, rheumatoid factor, serum levels of immunoglobulin (Ig) A, IgG and IgM, serum B cell‐activating factor level in 107 patients and serum β2‐microglobulin value in 129 patients.13
The presence of monoclonal gammapathy, analysed in all patients by serum and urine electrophoresis together with immunofixation, and in controls by serum electrophoresis and immunofixation, was observed in three patients with rheumatoid arthritis and in 15 patients with pSS. The clonality status of blood B cell populations was analysed in 13 patients with pSS by amplification of VH–(DH)–JH junctional regions by polymerase chain reaction using the VH framework region 3 (FR3) and JH FR4 consensus primers. Polymerase chain reaction products were analysed on an ethidium bromide‐stained polyacrylamide gel after high‐resolution electrophoresis.
Statistical analysis
Continuous values are shown as mean (SE) and qualitative values as n (%). The χ2 test (with Yates' correction, when appropriate) was used to assess the differences in frequencies for qualitative values. Analysis of variance was used to compare FLC levels between controls, patients with rheumatoid arthritis and those with pSS. Correlation was evaluated using Spearman's test. Data were analysed using SPSS V.11.5 program.
Results
Serum FLC assessment in patients with rheumatoid arthritis or pSS
No healthy participant had increased κ or λ levels, or abnormal κ:λ ratio.
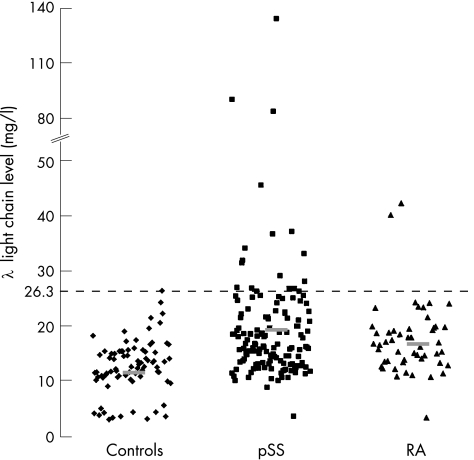
In the 50 patients with rheumatoid arthritis, mean (SE) serum κ and λ FLC levels were significantly higher than in controls (κ: 18.9 (1.1) v 10.5 (0.4) mg/l, p<0.001; λ: 16.7 (1.2) v 11.6 (0.6) mg/l, p<0.001; figs 1, 2). The κ:λ ratio was not significantly different between patients and controls (mean (SE) 1.4 (0.2) v 1.1 (0.1), p = 0.4). In all, 18 (36%) patients had abnormal FLC serum levels: 15 (30%) had increased κ levels (including 3 (6%) patients with increased κ:λ ratio) and 3 (6%) had increased λ levels.
Figure 1 Serum κ levels in patients with rheumatoid arthritis (RA) or primary Sjögren's syndrome (pSS) and in healthy controls. Bars represent mean values. Increased κ levels are defined by values >19.4 mg/l.
Figure 2 Serum λ levels in patients with rheumatoid arthritis (RA) or primary Sjögren's syndrome (pSS) and in healthy controls. Bars represent mean values. Increased λ levels are defined by values >26.3 mg/l.
In the 139 patients with pSS, mean (SE) serum κ and λ FLC levels were significantly higher than in controls (κ: 16.3 (1.4) v 10.5 (0.4) mg/l, p = 0.001; λ: 19.3 (1.5) v 11.6 (0.6) mg/l, p<0.001; table 1; figs 1, 2). The κ:λ ratio was not significantly different between patients and controls (1 (0.3) v 1.14 (0.1), p = 0.15). In all, 31 (22.3%) patients had abnormal FLC serum levels, including 17 (12.2%) patients with increased κ levels (5/17 had abnormal κ:λ ratio), 6 (35.3%) patients with increased λ levels (5/6 with abnormal κ:λ ratio), 7 (5%) patients with increased κ and λ levels and 1 (0.7%) patient with only an abnormal κ:λ ratio.
Abnormal FLC levels were more often detected in the 15 patients with pSS and MGUS compared with patients with pSS without monoclonal gammapathy (33% v 20%, p = 0.2).
Analysis of IgH gene rearrangements by high‐resolution gene electrophoresis did not show a difference between the five patients with pSS without monoclonal gammapathy who had an abnormal κ:λ ratio and eight patients, randomly selected from those with normal FLCs, who all had a polyclonal pattern. Thus, no blood monoclonal B cell populations could be detected in patients with pSS with abnormal κ:λ ratio.
We found no significant difference between patients with rheumatoid arthritis and patients with pSS regarding the frequency of abnormal FLCs and levels of κ and λ light chains.
Correlation between FLC levels and immunological features
In patients with rheumatoid arthritis, κ and λ FLC levels were correlated with serum levels of gammaglobulin (κ: r = 0.38, p = 0.04; λ: r = 0.4, p = 0.03) and IgG (κ: r = 0.37, p = 0.04; λ: r = 0.38, p = 0.04). Moreover, a significant correlation was observed between κ and rheumatoid factor levels (r = 0.3, p = 0.03) but not between λ and rheumatoid factor levels (r = 0.21, p = 0.17). Anti‐CCP levels were not correlated with FLC levels (κ: r = 0.23, p = 0.2; λ: r = 0.12, p = 0.7).
In patients with pSS, κ and λ FLC levels were correlated with serum levels of gammaglobulin (κ: r = 0.37, p<0.002; λ: r = 0.28, p = 0.001); IgG (κ: r = 0.40, p<0.001; λ: r = 0.28, p = 0.001); rheumatoid factor (κ: r = 0.24, p = 0.008; λ: r = 0.28, p = 0.002); β2‐microglobulin (κ: r = 0.4, p<0.001; λ: r = 0.4, p<0.001) and B cell‐activating factor levels (κ: r = 0.17, p = 0.08; λ: r = 0.27, p = 0.005). Mean serum FLC concentrations were higher in patients with autoantibodies. Thus, patients with anti‐SSB had higher mean (SE) serum FLC levels (κ: 22.3 (3.1) mg/l; λ: 24.7 (2.8) mg/l) than patients with anti‐SSA antibody alone (κ: 14 (1) mg/l; λ: 17.1 (0.8) mg/l, p = 0.002 and p = 0.04, respectively) and than patients without autoantibodies (κ: 12 (2) mg/l; λ: 17.3 (1.9) mg/l, p = 0.003 and p = 0.09, respectively). Likewise, the frequency of abnormal FLC levels was higher in patients with anti‐SSB (36%) than in patients with anti‐SSA antibody alone (11.2%) or in patients without autoantibodies (10.1%; p = 0.01). We found no correlation between κ or λ levels and age, serum creatinine level or disease duration (data not shown).
Association between FLC level and disease activity
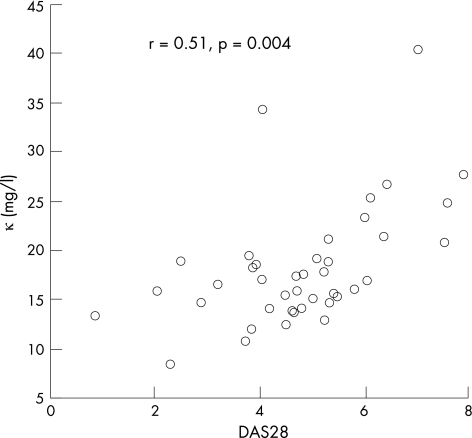
In patients with rheumatoid arthritis, a significant correlation was observed between disease activity assessed by the Disease Activity Score 28 (DAS28) and κ (r = 0.51, p = 0.004; fig 3) or λ levels (r = 0.31, p = 0.05). Patients with abnormal κ levels had a higher DAS28 than those with normal λ levels (mean (SE) 6.2 (0.4) v 4.2 (0.2), p = 0.006). No correlation was observed between DAS28 and serum gammaglobulin or IgG levels (r = 0.2, p = 0.2 and r = 0.18, p = 0.36, respectively). The mean C reactive protein value was 18.2 (SE 3) mg/l. κ and λ levels were significantly correlated with C reactive protein levels (κ: r = 0.34, p = 0.03; λ: r = 0.3, p = 0.04). FLC levels were not significantly different between patients treated with methotrextate and those treated with anti‐tumour necrosis factor (data not shown).
Figure 3 Correlation between κ levels and Disease Activity Score (DAS)28 in patients with rheumatoid arthritis.
In pSS, mean (SE) serum FLC levels were higher in patients with systemic features than in patients with only glandular involvement (κ: 19 (1.8) v 13 (1.1) mg/l, p = 0.01; λ: 21.6 (2.5) v 16.7 (0.6) mg/l, p = 0.04). Likewise, the frequency of abnormal FLC levels was significantly higher in patients with extraglandular involvement than in those without (20/62 (32.2%) patients with extraglandular involvement v 11/77 (14.3%) patients with no extraglandular involvement, p = 0.01). Among the 124 patients without MGUS, extraglandular involvement remained associated with abnormal FLC levels (31.3% v 12.3% in patients without extraglandular involvement, p = 0.02). Extraglandular involvement was associated with abnormal FLC levels and also with anti‐SSB (58.3% v 36% in patients without anti‐SSB, p = 0.02). This association between anti‐SSB and extraglandular involvement was not observed in patients with increased FLC levels (59% in patients with anti‐SSB v 56% in patients without anti‐SSB, p = 0.7). In anti‐SSB‐negative patients, increased FLC levels remained significantly associated with extraglandular involvement (29.4% v 8.5% in patients without extraglandular involvement, p = 0.02). As previously reported,13 the mean (SE) serum IgG (16.8 (7) v 14.2 (6) g/l, in patients with and without extraglandular involvement, respectively, p = 0.3) and gammaglobulin levels (13.9 (7.1) v 12.9 (5.5) g/l in patients with and without extraglandular involvement, respectively, p = 0.8) were not significantly associated with extraglandular involvement.
Discussion
Using a new quantitative immunoassay, we showed that one third of patients with rheumatoid arthritis and one fifth of those with pSS had abnormal FLC levels. Interestingly, patients with increased FLC levels had considerably higher disease activity in rheumatoid arthritis and higher frequency of extraglandular involvement in pSS.
Our study is, to our knowledge the first to evaluate the level of FLCs in patients with rheumatoid arthritis and in those with pSS, and does not deal with their clonality directly. Previous studies on autoimmune diseases used older qualititative immunoassay methods (high‐resolution electrophoresis or isoelectric focusing, followed by immunofixation) to detect monoclonal immunoglobulin FLCs. In patients with rheumatoid arthritis, monoclonal κ and λ chains were detected in the serum of patients and were associated with increased disease activity.14 In studies on a small number of patients with pSS, 25–40% had serum monoclonal light chains and had a more frequent extraglandular involvement than patients without serum monoclonal light chains.15,16,17
A strong correlation was observed between FLCs and other markers of B cell activation. FLCs were correlated with gammaglobulin, IgG and rheumatoid factor levels, but not with anti‐CCP in patients with rheumatoid arthritis. This might be related to the fact that gammaglobulin, IgG and rheumatoid factor levels reflect polyclonal B cell activation rather than a specific B cell response such as anti‐CCP antibodies. Likewise in pSS, FLC levels were correlated with B cell‐activating factor and non‐specific markers of B cell activation, such as β2‐microglobulin, gammaglobulin and IgG.
Interestingly, FLC levels markedly increased with DAS28, which supports the potential relationship between B cell and disease activity in patients with rheumatoid arthritis. Interestingly, no major correlation was observed between DAS28 and serum immunoglobulin or IgG, markers of B cell activation, which have a much longer half life (20–25 days) than FLCs (2–4 h).4 The faster turnover of FLCs might account for their predominant potential as disease activity markers, which suggests that they might be a good and early surrogate marker for response to treatments. Thus, evaluation of the role of FLCs as prognostic markers for response to treatments (particularly to B cell depletion, such as rituximab) and of structural evolution deserves further investigation.
Increased FLC levels were associated with extraglandular involvement in patients with pSS. Regarding disease activity, serum FLC levels might add a definite association to routine measurements, such as erythrocyte sedimentation rate, quantitative immunoglobulin values13 or detection of monoclonal immunoglobulin, which were not markedly associated with extraglandular involvement either in our study or in a recent survey of 237 patients with pSS (except for monoclonal immunoglobulin and pulmonary involvement in the survey).18 Our results confirm the increase in FLC levels in one third of patients with MGUS.6 Serum FLC levels are associated with extraglandular involvement, especially in patients without anti‐SSB, and could be a potentially useful marker of systemic complications in this subgroup of patients. Our results reinforce the hypothesis that extraglandular involvement in patients with pSS might be the result of more intense stimulation of B cells, suggested by the reported association of anti‐SSB and increased β2‐microglobulin with extraglandular involvement in patients with pSS.13,19
In systemic lupus eythematosus, increased FLC levels have also been reported to be associated with disease activity, as a strong correlation was observed between FLC levels and lupus disease activity assessed by the European Consensus Lupus Activity Measure score.20
The increase in FLC levels with disease activity may passively result from polyclonal B cell activation, but FLCs could also have an intrinsic pathogenic role. Indeed, there is compelling evidence that FLCs do have the ability to bind antigen, albeit generally with lower affinities. IgM expressed by CD5 cells binds FLCs, but no information is available on intracellular signalling after binding.21 Moreover, FLCs induced mast‐cell activation and elicited allergic asthma in a murine experimental model.22 Interestingly, mast cells could have a key role in the early phases of autoimmune disorders, notably in patients with rheumatoid arthritis.23
Patients with rheumatoid arthritis,24 pSS,25 or systemic lupus erythematosus26 share an increased risk of developing lymphoma as a possible result of a persistent B cell activation and disease activity. To date, no biological marker is available to evaluate the individual risk for lymphoma in these patients. Of note, FLC levels are increased in these three diseases and correlate with disease activity. An abnormal κ:λ ratio indicates a loss of control over the proportion of heavy and light chains synthesised, and it was recently shown to be a relevant clinical marker of malignant evolution in MGUS.6 Among the five patients with pSS without MGUS who had abnormal κ:λ ratio, one had purpura, two had decreased C4 levels, which are two known risk factors for lymphoma. No clonal B cell populations could be detected in the blood of these patients, which suggests that an abnormal κ:λ ratio could be a more sensitive marker of clonality that could be restricted initially to the site of autoimmunity.27 The predictive value of abnormal κ:λ ratio regarding the occurrence of lymphoma deserves to be investigated in a longitudinal study on patients with rheumatoid arthritis, pSS or systemic lupus erythematosus.
Conclusion
Increased FLC levels is a common feature of autoimmune diseases and correlates with disease activity, which supports the relationship between B cell activation and autoimmune disease activity. Prospective longitudinal studies are necessary to determine the potential utility of FLC assessment as a biomarker of response to treatment (especially B cell depletion). Lastly, because of the possible relationship of persistent B cell activation and disease activity with lymphoma, further studies are required to determine whether FLC assessment could represent a relevant biomarker for the risk of lymphoma in autoimmune diseases.
Abbreviations
CCP - cyclic citrullinated peptide
DAS - Disease Activity Score
FLC - free light chain
MGUS - monoclonal gammapathy of undetermined significance
pSS - primary Sjögren's syndrome
Footnotes
Competing interests: None.
References
- 1.Bradwell A R, Carr‐Smith H D, Mead G P, Tang L X, Showell P J, Drayson M T.et al Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 200147673–680. [PubMed] [Google Scholar]
- 2.Katzmann J A, Clark R J, Abraham R S, Bryant S, Lymp J F, Bradwell A R.et al Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem 2002481437–1444. [PubMed] [Google Scholar]
- 3.Drayson M, Tang L X, Drew R, Mead G P, Carr‐Smith H, Bradwell A R. Serum free light‐chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001972900–2902. [DOI] [PubMed] [Google Scholar]
- 4.Bradwell A R, Carr‐Smith H D, Mead G P, Harvey T C, Drayson M T. Serum test for assessment of patients with Bence Jones myeloma. Lancet 2003361489–491. [DOI] [PubMed] [Google Scholar]
- 5.Abraham R S, Katzmann J A, Clark R J, Bradwell A R, Kyle R A, Gertz M A. Quantitative analysis of serum free light chains. A new marker for the diagnostic evaluation of primary systemic amyloidosis. Am J Clin Pathol 2003119274–278. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar S V, Kyle R A, Therneau T M, Melton LJ I I I, Bradwell A R, Clark R J.et al Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005106812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looney R J, Anolik J, Sanz I. B cells as therapeutic targets for rheumatic diseases. Curr Opin Rheumatol 200416180–185. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J C, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close D R.et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 20043502572–2581. [DOI] [PubMed] [Google Scholar]
- 9.Silverman G J, Carson D A. Roles of B cells in rheumatoid arthritis. Arthritis Res Ther 20035(Suppl 4)S1–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay F, Sierro F, Grey S T, Gordon T P. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr Dir Autoimmun 20058243–265. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson R, Gordon T P, Konttinen Y T. Recent advances in understanding molecular mechanisms in the pathogenesis and antibody profile of Sjogren's syndrome. Curr Rheumatol Rep 20035311–316. [DOI] [PubMed] [Google Scholar]
- 12.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos H M, Alexander E L, Carsons S E.et al Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 200261554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottenberg J E, Busson M, Cohen‐Solal J, Lavie F, Abbed K, Kimberly R P.et al Correlation of serum B lymphocyte stimulator and beta2 microglobulin with autoantibody secretion and systemic involvement in primary Sjogren's syndrome. Ann Rheum Dis 2005641050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solling K, Solling J, Romer F K. Free light chains of immunoglobulins in serum from patients with rheumatoid arthritis, sarcoidosis, chronic infections and pulmonary cancer. Acta Med Scand 1981209473–477. [DOI] [PubMed] [Google Scholar]
- 15.Moutsopoulos H M, Steinberg A D, Fauci A S, Lane H C, Papadopoulos N M. High incidence of free monoclonal lambda light chains in the sera of patients with Sjogren's syndrome. J Immunol 19831302663–2665. [PubMed] [Google Scholar]
- 16.Moutsopoulos H M, Costello R, Drosos A A, Mavridis A K, Papadopoulos N M. Demonstration and identification of monoclonal proteins in the urine of patients with Sjogren's syndrome. Ann Rheum Dis 198544109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youinou P, Papadopoulos N M, Katsikis P, Pennec Y L, Jouquan J, Lelong A.et al Monoclonal immunoglobulins in the serum of patients with primary Sjogren's syndrome. Clin Exp Rheumatol 19886247–252. [PubMed] [Google Scholar]
- 18.Brito‐Zeron P, Ramos‐Casals M, Nardi N, Cervera R, Yague J, Ingelmo M.et al Circulating monoclonal immunoglobulins in Sjogren syndrome: prevalence and clinical significance in 237 patients. Medicine (Baltimore) 20058490–97. [DOI] [PubMed] [Google Scholar]
- 19.Harley J B, Alexander E L, Bias W B, Fox O F, Provost T T, Reichlin M.et al Anti‐Ro (SS‐A) and anti‐La (SS‐B) in patients with Sjogren's syndrome. Arthritis Rheum 198629196–206. [DOI] [PubMed] [Google Scholar]
- 20.Urban S, Oppermann M, Reucher S W, Schmolke M, Hoffmann U, Hiefinger‐Schindlbeck R.et al Free light chains (FLC) of immunoglobulies as parameter resembling disease activity in autoimmune rheumatic diseases. Ann Rheum Dis 200463(Suppl 1)141 [Google Scholar]
- 21.Redegeld F A, Nijkamp F P. Immunoglobulin free light chains and mast cells: pivotal role in T‐cell‐mediated immune reactions? Trends Immunol 200324181–185. [DOI] [PubMed] [Google Scholar]
- 22.Kraneveld A D, Kool M, van Houwelingen A H, Roholl P, Solomon A, Postma D S.et al Elicitation of allergic asthma by immunoglobulin free light chains. Proc Natl Acad Sci USA 20051021578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolley D E. The mast cell in inflammatory arthritis. N Engl J Med 20033481709–1711. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti‐tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum 2004501740–1751. [DOI] [PubMed] [Google Scholar]
- 25.Kassan S S, Thomas T L, Moutsopoulos H M, Hoover R, Kimberly R P, Budman D R.et al Increased risk of lymphoma in Sicca syndrome. Ann Intern Med 197889888–892. [DOI] [PubMed] [Google Scholar]
- 26.Bernatsky S, Boivin J F, Joseph L, Rajan R, Zoma A, Manzi S.et al An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum 2005521481–1490. [DOI] [PubMed] [Google Scholar]
- 27.Gasparotto D, De Vita S, De Re V, Marzotto A, De Marchi G, Scott C A.et al Extrasalivary lymphoma development in Sjogren's syndrome: clonal evolution from parotid gland lymphoproliferation and role of local triggering. Arthritis Rheum 2003483181–3186. [DOI] [PubMed] [Google Scholar]