Abstract
Background
Salivary gland dysfunction is one of the key manifestations of Sjögren's syndrome.
Objectives
(1) To assess prospectively loss of function of individual salivary glands in patients with primary and secondary Sjögren's syndrome in relation to disease duration and use of immunomodulatory drugs. (2) To study changes in sialochemical and laboratory values and subjective complaints over time.
Methods
60 patients with Sjögren's syndrome were included in this study. Whole and gland‐specific saliva (parotid and submandibular/sublingual (SM/SL)), samples were collected at baseline and after a mean of 3.6 (SD 2.3) years of follow‐up. Disease duration was recorded for all patients.
Results
Patients with Sjögren's syndrome with short disease duration had significantly higher stimulated flow rates at baseline than those with longer disease duration (p<0.05). When compared with healthy controls, the decrease in SM/SL flow rates at baseline was more prominent than that in parotid flow rates (p<0.05). Over time, there was a significant further decrease of stimulated flow rates, especially of the parotid gland, accompanied by increasing problems with swallowing dry food (p<0.05). The decrease was independent of the use of corticosteroids or disease‐modifying antirheumatic drugs (DMARDs). Sialochemical variables remained stable.
Conclusions
Early Sjögren's syndrome is characterised by a decreased salivary gland function (parotis>SM/SL), which shows a further decrease over time, regardless of the use of DMARDs or steroids. Patients with Sjögren's syndrome with longer disease duration are characterised by severely reduced secretions of both the parotid and SM/SL glands. These observations are relevant for identifying patients who would most likely benefit from intervention treatment.
Sjögren's syndrome is a progressive autoimmune disease characterised by lymphocytic infiltration of the exocrine glands, predominantly of the salivary and lacrimal glands. Within the wide spectrum of its clinical manifestations, salivary gland dysfunction is considered to be one of the key manifestations.1 As such, assessment of salivary flow rates (sialometry) is of diagnostic and, possibly, prognostic value. In addition, the amount and composition of saliva reflect the effects of the autoimmune process in the salivary glands, because glandular destruction leads to a disruption of the resorptive and secretory processes.2 Therefore, analysis of saliva (sialochemistry) may also be valuable in diagnosis, assessment of prognosis and evaluation of treatment.3
Sialometry and sialochemistry can be used as a diagnostic tool by collecting either whole saliva (the combined secretions of all salivary glands) or glandular saliva (gland‐specific saliva).4 Assessment of unstimulated whole saliva is currently proposed as one of the major criteria for evaluation of salivary gland dysfunction in patients with Sjögren's syndrome.5 However, when the disease develops, not all major salivary glands may yet manifest dysfunction, rendering whole saliva less valuable as a diagnostic tool or as a parameter for evaluating progression of disease or therapeutic intervention. By contrast, analysis of gland‐specific saliva can yield sequential involvement of particular glands, reflecting the ongoing autoimmune process in individual major salivary glands. By using glandular saliva, patients with Sjögren's syndrome may frequently be diagnosed at an earlier stage, and progression or effects of therapeutic intervention can be measured in a non‐invasive way.
Different sialometrical and sialochemical profiles have been proposed as being characteristic for an early or late salivary manifestation of Sjögren's syndrome.6 This cross‐sectional study showed that patients with a short duration of oral symptoms related to Sjögren's syndrome (<1 year) show either normal flow rates with changed salivary composition or reduced stimulated flow rates from the submandibular/sublingual (SM/SL) glands, accompanied by normal or subnormal flow rates from the parotid glands. These data suggest that the parotid gland is the last salivary gland to manifest hyposalivation during the disease course, which has been confirmed in other cross‐sectional studies.7,8,9,10 In this study, we assessed prospectively the loss of secretory potency of the parotid and SM/SL salivary glands in patients with Sjögren's syndrome in order to analyse the course of disease from onset to severe dysfunction of the salivary glands. We also studied changes in sialochemical and laboratory values as well as subjective complaints over time to further characterise the early and late stages of salivary gland involvement in patients with Sjögren's syndrome.
Patients and methods
Patients
Between 1994 and 2005, a cohort of patients with primary and secondary Sjögren's syndrome (pSS and sSS, respectively, diagnosed according to the American–European criteria5), who are being followed up at the Department of Rheumatology and Clinical Immunology and Department of Oral and Maxillofacial Surgery, University Medical Center Groningen, Groningen, The Netherlands, was established. All patients were seen at regular intervals (6 or 12 months depending on disease activity). A complete investigation for Sjögren's syndrome was available for all patients, including data on whole and gland‐specific (parotid and SM/SL) salivary secretion and composition, level of subjective complaints and disease duration. Disease duration was defined as the time from the first complaints related to oral dryness until the moment of measurement of glandular saliva. Early‐onset Sjögren's syndrome was defined as a disease duration of <1 year, established Sjögren's syndrome as a disease duration of 1–4 years and late Sjögren's syndrome as a disease duration of >4 years. To assess the loss of secretory potency as a function of time, it was decided to repeat collection of gland‐specific saliva in all patients seen between January and July 2005 for routine follow‐up. In addition, sialochemical analyses and laboratory studies were carried out, and subjective complaints were recorded. All patients provided written informed consent. Normal values were derived from historic controls (n = 36).10,11 Healthy controls were matched for age and sex and did not use any drugs.
Collection of whole and glandular saliva
Whole and glandular saliva was collected in a standardised manner. Whole saliva was collected according to the guidelines given in the revised American–European criteria.5 Patients were instructed not to eat, drink or smoke for 90 min before the sialometric assessment. All assessments were performed at a fixed time of the day, in our study between 13:00 and 15:00, to minimise fluctuations related to the circadian rhythm of salivary secretion and composition. Unstimulated salivary secretions were collected during 15 min. Glandular saliva specimens from both individual parotid glands and simultaneously from the SM/SL glands were collected by Lashley cups (placed over the orifices of Stenson's duct) and syringe aspiration (from the orifices of Warton's duct located anteriorly in the floor of the mouth), respectively. Stimulated salivary secretions were collected during 10 min. The salivary glands were stimulated with citric acid solution (2% wt/vol) applied with a cotton swab to the lateral borders of the tongue at 30‐s intervals.6,11 Sialochemical analysis was performed as described previously.3 The following salivary components were quantified: sodium, potassium, chloride, total protein and amylase.
Laboratory parameters
Systemic inflammatory activity was evaluated by assessing erythrocyte sedimentation rate, and levels of C reactive protein, rheumatoid factor (IgM‐Rf) and immunoglobulins (IgG, IgA and IgM). Levels of immunoglobulins and IgM‐Rf were measured by nephelometry.
Subjective complaints
Patients were instructed to express the severity of each of the following three complaints on a 100‐mm visual analogue scale (0, no complaints; 100, most severe complaints): (1) dry mouth during the day; (2) dry mouth during the night; and (3) problems with swallowing dry food without additional liquids.12
Statistical analysis
Data are presented as mean (standard deviation (SD), range) in table 1 and as mean (standard error of mean (SEM)) in figs 1 and 2. The significance (p<0.05) of change from baseline was measured by Wilcoxon's signed rank test. The Mann–Whitney U test was used to analyse differences between the various patient groups. A multivariate analysis was used to determine any influence of several possibly relevant cofactors on the outcome parameters in patients with Sjögren's syndrome (SPSS V.12.0).
Table 1 Characteristics of the patients at baseline and follow‐up.
| Patients with pSS (n = 32) | Patients with sSS (n = 28) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | p Value | Baseline | Follow‐up | p‐value | Normal values for controls (n = 36)3,6 | |
| Sex (M/F) | 2/30 | 5/23 | |||||
| Age (years) | 51 (17, 21–76) | 55 (17, 23–86) | 55 (13, 25–78) | 58 (13, 28–83) | |||
| Disease duration (months) | 31 (39, 0–156) | 77 (47, 10–194) | 43 (62, 0–312) | 82 (64, 6–352) | |||
| Anti‐SSA or anti‐SSB positive n(%)* | 24 (75%) | NA | 14 (50%) | NA | |||
| Sialochemistry: parotid glands | |||||||
| Sodium (mmol/l)† | 21 (19, 1–63) | 19 (25, 1–78) | NS | 24 (24, 1–94) | 11 (14, 2–49) | NS | 14 (2) |
| Potassium (mmol/l) | 26 (7, 11–38) | 25 (8, 14–46) | NS | 23 (7, 13–46) | 23 (5, 15–32) | NS | 24 (6) |
| Chloride (mmol/l) | 28 (14, 8–53) | 31 (20, 17–70) | NS | 38 (29, 10–114) | 22 (14, 10–55) | NS | 16 (12) |
| Total protein (g/l) | 1.16 (0.55, 0.50–2.19) | 1.0 (0.5, 0.6–2.3) | NS | 1.48 (1.13, 0.52–5.63) | 0.96 (0.30, 0.51–1.42) | NS | 0.6 (0.6) |
| Amylase (103 U/l) | 594 (447, 58–1713) | 540 (285, 248–1036) | NS | 670 (359, 38–1486) | 595 (256, 154–1319) | NS | 590 (520) |
| Sialochemistry: SM/SL glands | |||||||
| Sodium (mmol/l) | 18 (13, 1–55) | 18 (13, 2–49) | NS | 14 (12, 1–48) | 14 (16, 1–59) | NS | 11 (6) |
| Potassium (mmol/l) | 17 (7, 6–43) | 16 (4, 7–22) | NS | 17 (6, 4–31) | 19 (3, 14–28) | NS | 17 (6) |
| Chloride (mmol/l) | 26 (12, 10–52) | 23 (10, 7–43) | NS | 30 (27, 16–121) | 20 (11, 8–55) | NS | 16 (6) |
| Total protein (g/l) | 0.79 (0.98, 0.25–7.76) | 0.6 (0.4, 0.1–1.79) | NS | 0.77 (0.46, 0.16–1.84) | 0.74 (0.54, 0.17–2.56) | NS | 0.8 (0.6) |
| Amylase (103 U/l) | 97 (86, 26–390) | 95 (71, 14–220) | NS | 153 (264, 2–1152) | 148 (225, 36–977) | NS | ND |
| Laboratory tests | |||||||
| ESR (mm/h) | 32 (21, 5–80) | 29 (23, 3–80) | NS | 33 (33, 3–103) | 28 (27, 4–133) | NS | 0–10 |
| CRP (mg/l) | 9 (13, 0–52) | 7 (8, 3–40) | NS | 19 (32, 0–138) | 9 (9, 0–27) | NS | <5 |
| IgM‐Rf (kIU/l) | 209 (252, 2–1160) | 216 (230, 11–650) | NS | 189 (238, 11–768) | 165 (174, 3–565) | NS | <11 |
| IgG (g/l) | 20.9 (8.0, 3.6–39) | 19.7 (7.8, 9.9–39.1) | <0.05 | 19.1 (9.1, 10.1–46.6) | 15.6 (5.7, 7.4–28.8) | <0.05 | 8.5–15 |
| IgA (g/l) | 2.9 (1.6, 0.4–6.9) | 2.8 (1.2, 0.8–5.1) | <0.05 | 3.5 (2.0, 1.2–8.8) | 2.9 (1.4, 0.8–5.0) | <0.10 | 0.7–4.0 |
| IgM (g/l) | 2.8 (4.0, 0.7–22.6) | 2.1 (1.8, 0.4–7.2) | <0.05 | 1.8 (0.8, 0.8–3.9) | 1.6 (1.1, 0.5–3.7) | <0.05 | 0.4–2.3 |
| Subjective VAS scores (100‐mm scale | |||||||
| Oral dryness during the day | 55 (28, 0–100) | 48 (24, 0–90) | NS | 56 (33, 0–100) | 51 (37, 0–95) | NS | |
| Oral dryness during the night | 67 (31, 0–100) | 60 (26, 0–98) | NS | 70 (34, 0–100) | 68 (34, 0–98) | NS | |
| Difficulty swallowing food without additional liquids | 61 (33, 0–100) | 55 (31, 0–100) | NS | 59 (36, 0–99) | 55 (39, 0–97) | NS | |
CRP, C reactive protein; ESR, erythrocyte sedimentation rate; F, female; Ig, immunoglobulin; IgM‐Rf, rheumatoid factor; M, male; NA, not available; ND, not defined; NS, not significant; pSS, primary SS; sSS, secondary SS; SM/SL, submandibular/sublingual glands; VAS, visual analogue scale.
Values are numbers of patients and mean (SD, range). For sialometry data, see figs 1 and 2.
*Significant difference between pSS and sSS at baseline and follow‐up (p<0.05).
†Sodium concentrations are dependent on flow rate. The mean (SD) flow rate normal values are 6 (7) and 5 (5) mmol/l at baseline and follow‐up for pSS, and 8 (7) and 7 (5) mmol/l for sSS, respectively.
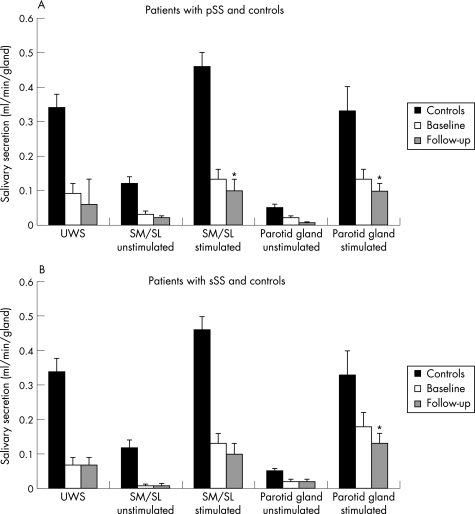
Figure 1 Mean (SEM) salivary flow rates of (A) 32 patients with primary Sjögren's syndrome (pSS) and (B) 28 patients with secondary Sjögren's syndrome (sSS) at baseline and follow‐up. Normal values are derived from historic controls (n = 36).10,11 SM/SL, submandibular/sublingual glands; UWS, unstimulated whole saliva. *p<0.05 versus baseline, by Wilcoxon's signed rank test.
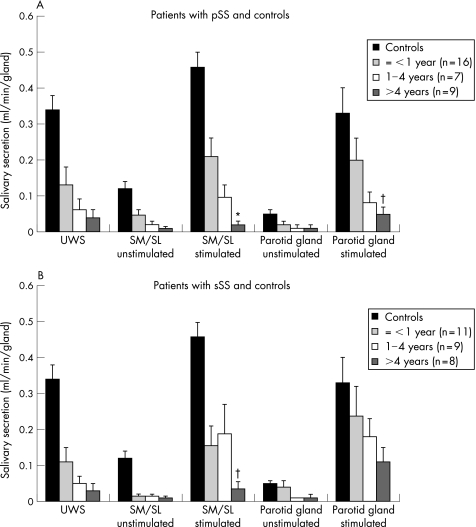
Figure 2 Relationship between disease duration (the time from first complaints induced by or related to oral dryness until referral) and mean (SEM) salivary flow rates in patients with (A) primary Sjögren's syndrome (pSS) and in those with (B) secondary Sjögren's syndrome (sSS). Normal values are derived from historic controls (n = 36).10,11 SM/SL, submandibular/sublingual glands; UWS, unstimulated whole saliva. *Significant difference versus patients with early‐onset Sjögren's syndrome (⩽1‐year oral complaints; p<0.005) by the Mann–Whitney U test. †Significant difference versus patients with early‐onset Sjögren's syndrome (p<0.05) by the Mann–Whitney U test.
Results
Patients
Table 1 summarises the characteristics of the patients. Of the 60 consecutive patients with pSS and sSS included in this study, 32 (53%) were diagnosed as having pSS and 28 (47%) as having sSS. The mean (SD) follow‐up time after the first visit was 3.6 (2.3) years.
We observed no differences between patients with pSS and those with sSS at baseline, except for the frequency of autoantibodies. At baseline, 27 (45%; pSS, n = 16; sSS, n = 11) patients had early‐onset Sjögren's syndrome, 16 (27%; pSS, n = 7; sSS, n = 9) had established Sjögren's syndrome and 17 (28%; pSS, n = 9; sSS, n = 8) had late Sjögren's syndrome. The duration of follow‐up was comparable between these three groups.
At baseline, only 2 (6%) patients with pSS were using hydroxychloroquine. At the last visit, 8 (25%) patients with pSS were using hydroxychloroquine, including the patients who already used this at baseline. Of the 28 patients with sSS, 19 (68%) were using disease‐modifying antirheumatic drugs (DMARDs) at follow‐up: methotrexate, n = 5; sulfasalazine, n = 2; combination of methotrexate and sulfasalazine, n = 3; hydroxychloroquine, n = 4; leflunomide, n = 3; azathioprine, n = 1; and cyclophosphamide, n = 1. Except for one patient, all these 19 patients were already using a DMARD at baseline. Prednisolone was being used during the whole period by 12 patients (pSS, n = 3; sSS, n = 9) in dosages of 5–7.5 mg daily.
Xerogenic drugs were used in 31% of the patients with pSS and in 48% of the patients with sSS (p>0.05). The usage was equally distributed between early, established and late Sjögren's syndrome, and in patients with or without anti‐SSA/anti‐SSB antibodies.
Salivary flow and sialochemical analysis
Although unstimulated flow rates remained at their already rather low level, stimulated flow rates of glandular saliva had significantly dropped at follow‐up in patients with pSS and in those with sSS (p<0.05, fig 1). Patients with early‐onset Sjögren's syndrome had significantly higher stimulated flow rates than those with late Sjögren's syndrome at baseline (p<0.05, fig 2). At the time of referral, the relative decrease in unstimulated and stimulated glandular saliva was more prominent in SM/SL flow rates than in parotid flow rates in patients with early‐onset Sjögren's syndrome, for both pSS and sSS (fig 2; p<0.05). After correction for follow‐up duration, patients with early‐onset Sjögren's syndrome showed a decrease in the stimulated parotid flow rate of 0.02 ml/min/6 months, whereas those with established and late Sjögren's syndrome showed a decrease of <0.01 ml/min/6 months. This did not apply for stimulated SM/SL flow rates; patients with early‐onset, established and late Sjögren's syndrome showed a rather continuous decrease in the flow rate of about 0.01–0.02 ml/min/6 months. We found no significant differences in glandular flow rates between patients treated with DMARDs or steroids and those who did not receive such treatment.
With regard to sialochemical values, we observed a significant increase in the concentrations of sodium and chloride in parotid and SM/SL salivary secretions in patients with pSS and in those with sSS at baseline (p<0.05). No significant differences in sialochemical values were observed between baseline and follow‐up after correction for the flow rate (sodium reabsorption is dependent on salivary flow rate) in patients with pSS and in those with sSS, between patients with early‐onset, established or late Sjögren's syndrome. Although not significant, patients with early‐onset Sjögren's syndrome tended to have the highest concentrations of sodium in parotid saliva, even when corrected for flow rate (maximum 94 mmol/l).
Laboratory values
Whereas the erythrocyte sedimentation rate and IgM‐Rf levels remained stable, raised levels of immunoglobulins showed a significant decline at follow‐up, in patients with pSS and in those with sSS (table 1). This decline was independent of disease duration at diagnosis. Changes in levels of immunoglobulins could not be attributed to treatment alone, because patients who never received DMARDs or steroids (n = 36), mostly those with pSS, also showed a significant decline in mean (SD) serum levels of IgG (20.1 (7.5) v 18.8 (7.0) g/l; p<0.05) and IgM (3.1 (4.2) v 2.7 (2.7) g/l; p<0.05). In the group without treatment, patients with early‐onset Sjögren's syndrome had significantly higher mean (SD) IgG levels than those with late Sjögren's syndrome at baseline (20.1 (7.5) v 17.3 (6.2) g/l; p<0.05). In multivariate analysis, however, the use of DMARDs and steroids was shown to be the only significant variable reducing levels of immunoglobulins (p<0.05).
Patients with early‐onset Sjögren's syndrome and anti‐SSA/anti‐SSB antibodies had significantly higher mean (SD) unstimulated and stimulated SM/SL salivary flow rates than those with early‐onset Sjögren's syndrome without autoantibodies (0.11 (0.12) v 0.02 (0.03) ml/min and 0.53 (0.47) v 0.22 (0.17) ml/min, respectively). This might be explained by differences in disease duration, as patients with autoantibodies tended to have shorter disease duration than those without autoantibodies. We observed no association between the presence of autoantibodies and salivary flow in patients with established or late Sjögren's syndrome.
Subjective complaints
No differences were observed in visual analogue scale scores for oral subjective complaints at baseline and follow‐up (table 1). When corrected for the duration of oral symptoms, patients with a disease duration of <1 year had significantly less problems swallowing dry food without liquids when compared with patients with late Sjögren's syndrome at baseline (p<0.05). This difference disappeared during follow‐up; patients with short disease duration at baseline now had the same amount of problems swallowing dry foods as those with late Sjögren's syndrome.
Discussion
This longitudinal study shows that patients with pSS and those with sSS lose stimulated salivary gland function over time, irrespectively of the type of treatment. Although we found some variation in flow rates on repeated measurements, this variation was of a rather low magnitude, especially in patients with low flow rates.11 When considering this technique‐related variation, the observed decrease of stimulated flow rates in this study was still found to be statistically and clinically significant (fig 1).
Loss of salivary gland function is already prominent in early‐onset Sjögren's syndrome, and progression of this functional loss seems to be of a lower magnitude after a longer period of disease duration.
The parotid gland seems to be the last gland to be affected in patients with Sjögren's syndrome. It is unclear why the parotid gland has a longer‐lasting secretory capacity, although the same effect has been observed in healthy elderly people in whom the SM/SL glands show a physiological decline in secretory capacity and the parotid glands show no loss of function.13 In the more progressive stages of the disease, both unstimulated and stimulated SM/SL and parotid gland functions fall to a low level. Patients then start having considerably more problems swallowing dry food, which is also considered to be one of the main oral symptoms in the diagnostic criteria for Sjögren's syndrome.5
Although we did not observe an influence of drugs on salivary flow rates, evidence suggests that prednisone may improve salivary flow and the main clinical and histological features in selected patients.14,15,16 We were not able to show such an effect, which may be due to the insufficient power of this study to determine an effect of drugs in this rather small and heterogeneous group of patients. The use of xerogenic drugs (antihypertensives, antihistamines and psychotropics) may cause a suppressive effect on salivary secretion. This suppression may lead to a decrease in unstimulated flow rates, predominantly of the SM/SL glands, which are the most active glands under resting conditions. Although a possible effect of the drugs is usually only shown regarding the subjective feeling of a dry mouth and in many cases is not proved by the objective measurement of the unstimulated salivary secretion, these drug‐related effects on salivary secretion might partly explain the observed difference between healthy controls and patients with Sjögren's syndrome.3 The loss of stimulated salivary gland function cannot (or at the most to a minor extent) be attributed to the use of xerogenic drugs, and is therefore most probably associated with disease duration.
Few studies have investigated the long‐term course of salivary gland function in patients with Sjögren's syndrome; hence, little is known yet about the natural course of the disease and the onset of salivary gland dysfunction.1,17,18,19,20,21,22 Gannot et al17 suggested that exocrine function, once already compromised, remains stable over time. This reflects the natural disease course only partially, as changes in the early stage of the disease—when a major loss of exocrine function actually takes place—seem unduly overlooked. Theander et al23 described a 5‐year follow‐up of 58 patients with pSS and showed a rather stable course of manifestations of the main disease. Disease duration did not influence changes during follow‐up, which is in contrast with our findings. Theander et al23 did not study patients with early‐onset disease, which, as mentioned by the authors in their discussion, might explain these differences.
Although most studies used the time since definitive diagnosis as a measure for disease duration, we defined disease duration as the time since the first complaints related to oral dryness. Although this is somewhat arbitrary, it is in our opinion the only method to investigate patients with early disease onset.
High levels of sodium and chloride in saliva of patients with Sjögren's syndrome are associated with higher levels of disease activity or a more progressive disease.24,25 The patients had increased levels of sodium and chloride, which remained unaffected during follow‐up, indicating ongoing sialoadenitis. Further, patients with early‐onset Sjögren's syndrome tended to have the highest concentrations of sodium. Patients with early Sjögren's syndrome may have more active disease, which is also reflected in higher serum levels of immunoglobulins.
In the course of the disease, immunoglobulin levels were found to decrease, although they were still above normal. Patients treated with DMARDs had the largest decrease in immunoglobulin levels. However, patients without drugs also showed a decline in serum levels of immunoglobulins, possibly reflecting reduction in inflammatory activity over time. This has been confirmed by other studies, where the erythrocyte sedimentation rate and immunoglobulin levels of patients remained stable or decreased, independent of DMARD or steroid use.17,20,22 Despite this decrease in immunoglobulin levels, patients with Sjögren's syndrome still have increased B cell activity, reflected by raised levels of IgG and IgM‐Rf (table 1).
Determination of glandular flow rates is not only important in the diagnostic investigation of Sjögren's syndrome but is, possibly, also a parameter for assessing the potential for new intervention treatment. Sialochemistry might be beneficial in identifying patients with active glandular disease, and might be a new parameter for assessing the effect of treatment on the level of the salivary gland function. A recent intervention study with B cell depletion in patients with Sjögren's syndrome showed that only patients with sufficient residual gland function (ie, those with early Sjögren's syndrome) responded well to treatment.26 Therefore, gland‐specific sialometry is of paramount importance for diagnosing patients with early‐onset Sjögren's syndrome and is also crucial in identifying patients who may benefit from intervention treatment. Future studies should also focus on other salivary components such as salivary immunoglobulins and saliva levels of anti‐SSA and anti‐SSB antibodies to further characterise disease progression.27
Abbreviations
DMARD - disease‐modifying antirheumatic drug
IgM‐Rf - rheumatoid factor
pSS - primary Sjögren's syndrome
SM/SL - submandibular/sublingual
sSS - secondary Sjögren's syndrome
Footnotes
Competing interests: None.
References
- 1.Mignogna M D, Fedele S, Russo L L, Lo Muzio L, Wolff A. Sjögren's syndrome: the diagnostic potential of early oral manifestations preceding hyposalivation/xerostomia. J Oral Pathol Med 2005341–6. [DOI] [PubMed] [Google Scholar]
- 2.Thorn J J, Prause J U, Oxholm P. Sialochemistry in Sjögren's syndrome: a review. J Oral Pathol Med 198918457–468. [DOI] [PubMed] [Google Scholar]
- 3.Kalk W W I, Vissink A, Spijkervet F K L, Bootsma H, Kallenberg C G M, Nieuw Amerongen A V. Sialometry and sialochemistry: diagnostic tools for Sjögren's syndrome. Ann Rheum Dis 2001601110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veerman E C I, Van den Keybus P A, Vissink A, Nieuw Amerongen A V. Human glandular salivas: their separate collection and analysis. Eur J Oral Sci 1996104346–352. [DOI] [PubMed] [Google Scholar]
- 5.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos H M, Alexander E L, Carsons S E.et al Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 200261554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalk W W I, Vissink A, Stegenga B, Bootsma H, Nieuw Amerongen A V, Kallenberg C G M. Sialometry and sialochemistry: a non‐invasive approach for diagnosing Sjögren's syndrome. Ann Rheum Dis 200261137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson J C, Travis W D, Pillemer S R, Bermudez D, Wolff A, Fox P C. Major salivary gland function in primary Sjögren's syndrome and its relationship to clinical features. J Rheumatol 199017318–322. [PubMed] [Google Scholar]
- 8.Carpenter G H, Proctor G B, Pankhurst C L, O'Donohue J, Scott D, Hunnable M P. Sialochemical markers of salivary gland involvement with Sjögren's syndrome secondary to rheumatoid arthritis and primary biliary cirrhosis. J Oral Pathol Med 200029452–459. [DOI] [PubMed] [Google Scholar]
- 9.Lindvall A M, Jonsson R. The salivary gland component of Sjögren's syndrome: an evaluation of diagnostic methods. Oral Surg Oral Med Oral Pathol 19866232–42. [DOI] [PubMed] [Google Scholar]
- 10.Vissink A, Panders A K, Nauta J M, Ligeon E E, Nikkels P G, Kallenberg C G M. Applicability of saliva as a diagnostic fluid in Sjögren's syndrome. Ann N Y Acad Sci 1993694325–329. [DOI] [PubMed] [Google Scholar]
- 11.Burlage F R, Pijpe J, Coppes R P, Hemels M E W, Meertens H, Canrinus A.et al Variability of flow rate when collecting stimulated human parotid saliva. Eur J Oral Sci 2005113386–390. [DOI] [PubMed] [Google Scholar]
- 12.Fox P C, Busch K A, Baum B J. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc 1987115581–584. [DOI] [PubMed] [Google Scholar]
- 13.Vissink A, Spijkervet F K L, Van Nieuw A A V. Aging and saliva: a review of the literature. Spec Care Dentist 19961695–103. [DOI] [PubMed] [Google Scholar]
- 14.Fox P C, Datiles M, Atkinson J C, Macynski A A, Scott J, Fletcher D.et al Prednisone and piroxicam for treatment of primary Sjögren's syndrome. Clin Exp Rheumatol 199311149–156. [PubMed] [Google Scholar]
- 15.Miyawaki S, Nishiyama S, Matoba K. Efficacy of low‐dose prednisolone maintenance for saliva production and serological abnormalities in patients with primary Sjögren's syndrome. Intern Med 199938938–943. [DOI] [PubMed] [Google Scholar]
- 16.Zandbelt M M, Van den Hoogen F H, De Wilde P C, Van den Berg P J, Schneider H G, Van de Putte L B. Reversibility of histological and immunohistological abnormalities in sublabial salivary gland biopsy specimens following treatment with corticosteroids in Sjögren's syndrome. Ann Rheum Dis 200160511–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gannot G, Lancaster H E, Fox P C. Clinical course of primary Sjögren's syndrome: salivary, oral, and serologic aspects. J Rheumatol 2000271905–1909. [PubMed] [Google Scholar]
- 18.Haga H J. Clinical and immunological factors associated with low lacrimal and salivary flow rate in patients with primary Sjögren's syndrome. J Rheumatol 200229305–308. [PubMed] [Google Scholar]
- 19.Jonsson R, Kroneld U, Backman K, Magnusson B, Tarkowski A. Progression of sialadenitis in Sjögren's syndrome. Br J Rheumatol 199332578–581. [DOI] [PubMed] [Google Scholar]
- 20.Kruize A A, Hene R J, Van der Heide A, Bodeutsch C, De Wilde P C, Van Bijsterveld O P.et al Long‐term followup of patients with Sjögren's syndrome. Arthritis Rheum 199639297–303. [DOI] [PubMed] [Google Scholar]
- 21.Leroy J P, Pennec Y L, Soulier C, Berthelot J M, Letoux G, Youinou P. Follow up study of labial salivary gland lesions in primary Sjögren's syndrome. Ann Rheum Dis 199251777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pertovaara M, Pukkala E, Laippala P, Miettinen A, Pasternack A. A longitudinal cohort study of Finnish patients with primary Sjögren's syndrome: clinical, immunological, and epidemiological aspects. Ann Rheum Dis 200160467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theander E, Andersson S I, Manthorpe R, Jacobsson L T. Proposed core set of outcome measures in patients with primary Sjögren's syndrome: 5 year follow up. J Rheumatol 2005321495–1502. [PubMed] [Google Scholar]
- 24.Pedersen A M, Reibel J, Nordgarden H, Bergem H O, Jensen J L, Nauntofte B. Primary Sjögren's syndrome: salivary gland function and clinical oral findings. Oral Dis 19995128–138. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen A M, Bardow A, Nauntofte B. Salivary changes and dental caries as potential oral markers of autoimmune salivary gland dysfunction in primary Sjögren's syndrome. BMC Clin Pathol 200554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pijpe J, Van Imhoff G W, Spijkervet F K L, Roodenburg J L N, Wolbink G J, Mansour K.et al Rituximab treatment in patients with primary Sjögren's syndrome: an open‐label phase II study. Arthritis Rheum 2005522740–2750. [DOI] [PubMed] [Google Scholar]
- 27.Halse A K, Marthinussen M C, Wahren‐Herlenius M, Jonsson R. Isotype distribution of anti‐Ro/SS‐A and anti‐La/SS‐B antibodies in plasma and saliva of patients with Sjögren's syndrome. Scand J Rheumatol 20002913–19. [DOI] [PubMed] [Google Scholar]