Abstract
Aim
To describe how patients presenting with inflammatory back pain (IBP) of short duration can be classified by different sets of classification criteria for spondyloarthritis (SpA) and ankylosing spondylitis, and which clinical and imaging features are of discernible importance.
Methods
68 patients with IBP of a maximum of 2 years' duration were included in the early spondyloarthritis cohort. Detailed history, clinical examination and imaging of sacroiliac joints by plain radiography and magnetic resonance imaging (MRI) were obtained. The Berlin criteria set for SpA that has a prominent place for MRI and human leucocyte antigen B27 was used to quantify the relative contribution of MRI in classifying SpA.
Results
14 of the 68 patients had ankylosing spondylitis according to the modified New York criteria, 57 patients fulfilled the European Spondylarthropathy Study Group (ESSG) criteria for SpA, 48 patients fulfilled the Amor criteria for SpA (43 patients fulfilled both criteria sets) and 44 patients fulfilled the Berlin criteria for SpA. Only four patients did not fulfil any criteria set; 36 patients fulfilled ESSG, Amor and Berlin criteria. The 14 patients with ankylosing spondylitis fulfilled all three SpA criteria sets.
Conclusion
Among our selected cohort of patients with early IBP, the prevalence of SpA according to three different criteria sets is high. The ESSG criteria were the most sensitive, followed by the Amor criteria and the Berlin criteria. The modified New York criteria for ankylosing spondylitis appeared to be the most specific. In this cohort, the contribution of MRI and human leucocyte antigen B27 to purely clinical criteria in making a diagnosis of axial SpA was limited.
Diseases belonging to the group of spondyloarthropathy or spondyloarthritis (SpA) share clinical and genetic characteristics, which distinguish them from rheumatoid arthritis.1 The most prominent clinical feature of SpA is chronic inflammatory back pain (IBP). Other common features are arthritis, enthesitis, uveitis and inflammatory bowel disease (IBD). The common genetic feature is human leucocyte antigen B27 (HLA‐B27). Included in the spectrum of SpA are ankylosing spondylitis, psoriatic arthritis, SpA related to IBD, reactive arthritis and undifferentiated spondylarthropathy. An early diagnosis of SpA may move forward the initiation of effective treatment, and thus diminish the burden of illness, as well as avoid costs.
Current criteria for classification of SpA are the European Spondylarthropathy Study Group criteria (ESSG)2 and the Amor criteria,3 and those for the classification of ankylosing spondylitis, the modified New York criteria.4 Especially in the case of early ankylosing spondylitis, criteria sets fall short, because the classification of ankylosing spondylitis depends on the presence of radiological sacroiliitis, which often appears late in the course of the disease. Thus, a long delay may exist between the start of symptoms and establishing a diagnosis.5 In a recent paper, Rudwaleit et al6 underscored the need for new classification criteria for axial SpA. Axial SpA is a new concept that recognises the involvement of the spine. Rudwaleit et al from Berlin have recently proposed a diagnostic algorithm for axial SpA, to be used in the individual patient. Another approach proposed by the Berlin group, that served as a template for their diagnostic algorithm, was based on calculation of the likelihood ratio (LR) product of currently available diagnostic tests for SpA.6 Magnetic resonance imaging (MRI) and HLA‐B27 testing have a prominent place in both the diagnostic algorithm and the LR product method.
In this paper, we describe how a group of patients presenting with IBP (the early spondyloarthritis clinic (ESPAC) cohort) could be classified according to different sets of classification criteria for SpA and ankylosing spondylitis, and which features were of discernible importance. Subsequently, we challenged the diagnostic value of the Berlin algorithm by changing the contribution of MRI and HLA‐B27.
Methods
Patients
Inclusion/exclusion criteria of the cohort
Patients with IBP of a maximum duration of 2 years were eligible. IBP was defined according to Calin et al.7 IBP is considered present if four of the following five characteristics are present: age at onset of back pain <40 years, insidious onset, duration of back pain >3 months, association with morning stiffness and improvement of back pain with exercise. Chronic back pain that awakens a patient at night is also suggestive of IBP, and is included in the Amor criteria set. Therefore, we also included patients fulfilling only three of the Calin criteria along with night pain.
All practising rheumatologists from the region of Limburg in both The Netherlands and Belgium and orthopaedic surgeons of the University Hospital Maastricht were asked to refer patients whom they considered eligible. All patients with a known history of psoriasis, who had visited the dermatology outpatient clinic between 2000 and 2002, received a questionnaire inquiring about the presence of IBP. Patients with a known diagnosis of IBD received a questionnaire when visiting the outpatient clinic, and the ophthalmologist handed out this questionnaire to patients with a diagnosis of acute anterior uveitis. Members of the regional branch of the ankylosing spondylitis society were informed by means of a leaflet included in their ankylosing spondylitis journal, and were encouraged to refer family members with IBP of short duration.
Assessments
Clinical evaluation
All patients completed the Bath Ankylosing Spondylitis Disease Activity Index,8 the Ankylosing Spondylitis Quality of Life questionnaire,9 the Bath Ankylosing Spondylitis Functional Index,10 a numeral rating scale for night pain (range 0–3), and visual analogue scales for spinal pain in the past week, global assessment for well‐being in the past week and fatigue in the past week (range from 0 to 10). Duration of morning stiffness in the past week (min) was recorded, and a 44‐joint swollen joint count was performed. Chest expansion (cm), modified Schober (cm), occiput‐to‐wall distance (cm) and lateral spinal flexion (cm) were assessed.
Erythrocyte sedimentation rate (Westergren's method; normal range ⩽7 for males; ⩽12 for females) and C reactive protein (turbidimetric method; normal range 2–9 mg/l) were measured. HLA‐B27 typing was performed.
Radiological evaluation
Conventional pelvic radiographs in the anterior–posterior view were performed, and sacroiliac joints were scored by two independent observers without knowledge of clinical characteristics according to the modified New York criteria (from 0, normal to 4, complete fusion). Sacroiliac joints with a score of 0 or 1 were considered normal; sacroiliac joints with a score of ⩾2 were considered abnormal (radiographic sacroiliitis). In case of disagreement between readers, the independent judgement of a third reader was regarded as conclusive. Patients with bilateral grade ⩾2, or unilateral grade 3 or 4 were classified as fulfilling the radiological criterion of the modified New York criteria.
An MRI scan of the sacroiliac joints was performed using a 1.5 Tesla Philips Gyro scan ACS‐NT (Philips, Best, The Netherlands). The following sequences were used: T1‐weighted spin echo, short τ inversion recovery, T2‐weighted fast spin echo, dynamic T1‐gradient sequence and T1‐weighted spin echo with fat suppression after the administration of contrast medium (gadolinium diethylenetriaminepentate, 0.1 mmol/kg body weight). MRI scans were independently scored by two readers (LH‐D and RW) who were blinded to the patient's identity, and to clinical, laboratory and radiological data. Both inflammation and structural changes (erosions, sclerosis and ankylosis) were scored. The MRI was considered normal or abnormal (for inflammation or structural changes) if both observers agreed. In case of disagreement between both readers, the independent judgement of a third reader was regarded as conclusive.11
Classification of patients
Patients were classified according to the modified New York criteria for ankylosing spondylitis, according to the ESSG and Amor classification criteria for SpA, and according to the Berlin criteria for axial SpA. A Berlin classification was made both by the LR product method and by the Berlin diagnostic algorithm (two different methods).
Patients were classified according to the ESSG criteria, Amor criteria and Berlin likelihood ratio product criteria for SpA, as well as according to the modified New York criteria for ankylosing spondylitis (appendix A; http://www.annrheumdis.com/supplimental).
Patients can be classified as having axial SpA according to the Berlin diagnostic algorithm immediately if they have IBP and if ⩾3 features of SpA are present. If they have IBP and if ⩾2 features of SpA are present, HLA‐B27 typing is performed. Patients with one or two features of SpA are classified as having axial SpA if HLA‐B27 is present. Patients with no SpA features in whom HLA‐B27 is present should undergo MRI of the sacroiliac joints, and are classified as having axial SpA only if MRI shows inflammation of the sacroiliac joints. Patients with no, one or two features of SpA in whom HLA‐B27 is absent are not classified as having axial SpA. The clinical SpA features include enthesitis, positive family history, uveitis, alternating buttock pain, peripheral arthritis, dactylitis, psoriasis, Crohn's disease and positive response to non‐steroidal anti‐inflammatory drugs (NSAIDs).
A classification according to the Berlin LR product method requires multiplication of the LRs of those SpA features that are present. In case of an LR product ⩾200, a classification of axial SpA is made, as an LR product ⩾200 results in a positive predictive value of approximately 90% in a population of patients with chronic low‐back pain and an assumed disease prevalence of axial SpA of 5% (pretest probability). For example, a patient with chronic IBP (LR+ 3.1), acute anterior uveitis (LR+ 7.3) and HLA‐B27 (LR+ 9.0) has an LR product of 3.1×7.3×9.0 = 203.7, and can therefore be classified as having axial SpA.
Results
Most patients were referred by rheumatologists. Other patients were referred by dermatologists, gastroenterologists, ophthalmologists and orthopaedic surgeons, or via the local branch of the ankylosing spondylitis society (detailed information in appendix B; http://www.annrheumdis.com/supplimental). The most important reason for not including referred patients was that they either denied back pain or reported back pain with a non‐inflammatory character. Patients from the rheumatology, orthopaedic and ophthalmology outpatient clinics did not receive a questionnaire but were referred immediately in case of IBP. Of the 94 patients who were considered eligible, 68 (72%) consented to participate. Of these 68 patients, only one patient did not return questionnaires but participated in the clinimetric and radiological parts of the study.
Table 1 shows the main demographic data and data about disease history. The median duration of complaints was 18 months (interquartile range 12–24 months). Of all patients, responses of 97% of the patients were confirmatory of at least four of the five questions about IBP (Calin criteria). The only two patients mentioning only three of the five questions reported night pain disturbing their sleep. Of all patients, 46% were HLA‐B27 positive, 15% had IBD confirmed by a gastroenterologist, 15% reported uveitis, 24% had psoriasis either confirmed by a dermatologist or present at physical examination, and 54% had a positive family history for ankylosing spondylitis (first‐degree or second‐degree relative). NSAIDs were used by 29 patients and disease‐modifying drugs by 10 patients (five patients for IBD and five other patients for arthritis). Of the disease‐modifying drug users, three patients used salazopyrine (one of them in combination with methotrexate), one patient used glucocorticoids and one patient used methotrexate in combination with IBD‐related drugs.
Table 1 Demographics, disease characteristics and clinimetry data of 68 patients included in the early spondyloarthritis clinic cohort.
| Characteristic | % of patients | Clinimetry | Clinimetry data |
|---|---|---|---|
| Male | 38 | Spinal pain the past week (on a 10‐cm VAS) | 4.7 (2.6)* |
| HLA‐B27+ | 46 | Night pain (on a Likert scale (0–3)) | 0:18%; 1:40%; 2:38%; 3:4% |
| IBD‡ | 15 | Global well‐being the past week (on a 10 cm VAS) | 3.8 (3.8) |
| Uveitis§ | 15 | Duration of morning stiffness the past week (in min; median (IQR)) | 23 (10; 60) |
| Psoriasis§ | 24 | Severity of fatigue the past week (on a 10 cm VAS) | 4.5 (2.6) |
| Family history of AS§ | 54 | 44‐swollen joint count | 0.19 (0.6) |
| Dactylitis§ | 10 | Finger to floor distance (in cm; median (IQR)) | 0.0 (0–12.5) |
| Heel pain§ | 6 | Chest expansion (in cm; % of patients <4 cm) | 4.9 (2.0; 43%) |
| Peripheral arthritis§ | 28 | Lateral spinal flexion (in cm; % of patients <20 cm) | 15.2 (4.8; 90%) |
| IBP improving with NSAIDs§ | 59 | Modified Schober (in cm; % of patients <5 cm) | 15.0 (1.1; 50%) |
| Buttock pain§ | 71 | Occiput‐to‐wall distance (in cm; % of patients >0 cm) | 0.4 (1.7; 7%) |
| Current NSAID use | 43 | Cervical rotation (in°; % of patients <70°) | 73 (14; 32%) |
| Current DMARD use | 9 | BASDAI | 3.6 (2.1) |
| Current glucocorticoid use | 1.5 | BASFI | 2.6 (2.1) |
| Current use of IBD‐related drugs | 6 | BASMI | 1.1 (1.2) |
| Criteria for inflammatory low‐back pain: | MASES | 2.8(2.4) | |
| 3 Criteria present | 3 | ASQoL | 6.4 (4.8) |
| 4 Criteria present | 41 | ESR; in mm; median (IQR)) | 6 (4; 16) |
| 5 Criteria present | 56 | CRP (in mg/l; median (IQR)) | 7 (3; 9) |
| Patients with raised acute‐phase reactants (in %) | 41 |
ASQoL, ankylosing spondylitis quality of life; BASDAI, Bath Ankylosing Spondylitis Activity Index; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; CRP, C reactive protein; DMARD, disease‐modifying anti‐rheumatic drug; ESR, erythrocyte sedimentation rate; HLA‐B27, human leucocyte antigen B27; IBD, inflammatory bowel disease; IQR, interquartile range; MASES, Maastricht Ankylosing Spondylitis Enthesopathy Index; NSAID, non‐steroidal anti‐inflammatory drug; VAS, visual analogue scale.
*Figures are means (standard deviations) unless otherwise indicated. ‡History or presence of this symptom (% of patients).
A high proportion of patients had a reduced Schober test, chest expansion or cervical rotation. The mean Bath Ankylosing Spondylitis Disease Activity Index was moderately high. In all, 40% of the patients had raised acute‐phase reactants (erythrocyte sedimentation rate or C reactive protein). The percentage of patients with IBD in the group of patients with normal acute‐phase reactants (18%) was comparable to the percentage of patients with IBD in the group with raised acute‐phase reactants (11%).
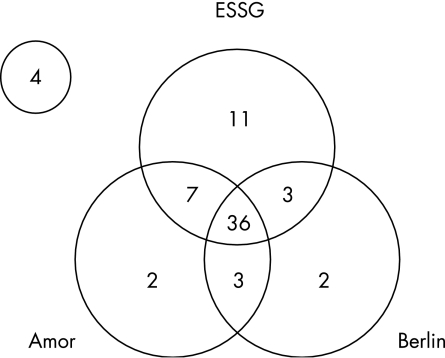
Figure 1 shows the number of patients fulfilling three different criteria sets for SpA. A total of 36 (53%) patients fulfilled all three criteria sets, 13 (19%) patients fulfilled two criteria sets and 15 (22%) patients fulfilled only one criteria set. Only 4 of the 68 (6%) patients did not fulfil any criteria set for ankylosing spondylitis or SpA. The highest classification rate was found with the ESSG criteria (84%), followed by the Amor criteria (71%) and by the Berlin criteria (65%). The Amor criteria and the Berlin criteria have a high level of conceptual similarity, but differ with respect to the contribution of MRI. We therefore investigated the contribution of MRI in explaining mismatches between an Amor and a Berlin classification. Of the five patients who were negative for Amor and positive for Berlin, only one patient had a positive MRI. This patient would have lost the Berlin classification if this MRI had been negative. All nine patients who were positive for Amor and negative for Berlin had a negative MRI. A positive MRI would have changed the classification in seven of the nine patients. In summary, MRI was of distinctive contribution in eight of the 14 patients with a mismatch, and irrelevant or redundant in six of them.
Figure 1 Venn diagram representing number of patients from the early spondyloarthritis clinic cohort meeting different criteria sets for spondyloarthritis. See text for further explanation. ESSG, European Spondylarthropathy Study Group.
In all, 14 of the 68 (20%) patients met the modified New York criteria for ankylosing spondylitis and fulfilled all the three criteria set for SpA.
Of the 36 patients fulfilling all three criteria sets for SpA, 21 (58%) showed inflammation on MRI and 23 (64%) were HLA‐B27 positive. Of the 13 patients fulfilling two criteria sets for SpA, no patient showed inflammation on MRI and 5 (39%) patients were HLA‐B27 positive. Of the 15 patients fulfilling one criteria set for SpA, 1 (7%) patient showed inflammation on MRI and 3 (20%) were HLA‐B27 positive. A detailed description of all MRI findings has been reported elsewhere .12 None of the four patients who did not fulfil any criteria set showed inflammation on MRI, and none of these patients were HLA‐B27 positive. But two of these patients had grade 2 radiographic sacroiliitis in one sacroiliac joint only. Of note, one additional patient would fulfil the Amor classification criteria if the radiographic sacroiliitis criterion could also be fulfilled by a positive MRI scan (chronic or inflammatory lesions were considered positive).
In summary, 22 (32%) patients showed inflammation on MRI and 31 (46%) patients were HLA‐B27 positive. Of the 14 patients meeting the modified New York criteria for ankylosing spondylitis, 12 (86%) patients were HLA‐B27 positive and 12 (86%) patients, including both HLA‐B27‐negative patients who met the criteria for ankylosing spondylitis, showed inflammation on MRI. One of these HLA‐B27‐negative patients had a history of psoriasis. Both patients with ankylosing spondylitis and patients without inflammation on MRI were HLA‐B27 positive.
We have analysed whether HLA‐B27‐positive patients differed from HLA‐B27‐negative patients, and whether patients with inflammation on MRI differed from those without inflammation on MRI (table 2). This comparison disclosed a significant male predominance in patients who are HLA‐B27 positive (p = 0.01), or have inflammation on MRI (p = 0.004). HLA‐B27‐positive patients (p = 0.002) or patients with inflammation on MRI (p<0.001) reported significantly more often improvement on NSAIDs compared with patients without these characteristics. In addition, HLA‐B27 was significantly over‐represented in patients with inflammation on MRI (p<0.001). All other demographic and clinical characteristics were equally distributed among HLA‐B27‐positive and HLA‐B27‐negative patients, and among those with and without inflammation on MRI. These differentiating results were somewhat more obvious if the analysis was applied to patients who were HLA‐B27 positive or had inflammation on MRI.
Table 2 Comparison between human leucocyte antigen B27 (HLA‐B27)‐positive and HLA‐B27‐negative patients and between patients with and without inflammation on magnetic resonance imaging of the sacroiliac joints in the early spondyloarthritis clinic cohort.
| HLA‐B27 | Inflammation on MRI | HLA‐B27 or inflammation | ||||
|---|---|---|---|---|---|---|
| Present (n = 31) | Absent (n = 37) | Present (n = 22) | Absent (n = 46) | Present (n = 37) | Absent (n = 31) | |
| Male | 52 | 24 | 64 | 24 | 54 | 16 |
| Inflammation on MRI | 52 | 16 | NA | NA | 59 | 0 |
| HLA‐B27 positive | X | X | 73 | 33 | 84 | 0 |
| History or presence of IBD | 7 | 23 | 5 | 20 | 5 | 26 |
| History or presence of acute anterior uveitis | 19 | 11 | 14 | 15 | 19 | 10 |
| History or presence of psoriasis | 13 | 32 | 18 | 26 | 16 | 32 |
| Family history of ankylosing spondylitis | 48 | 27 | 32 | 39 | 54 | 55 |
| Raised acute‐phase reactants | 45 | 38 | 46 | 44 | 51 | 31 |
| Improvement on NSAIDs (%) | 81 | 41 | 91 | 44 | 84 | 29 |
| Mean BASDAI (SD) | 3.6 (1.8) | 3.7 (4.4) | 3.8 (2.1) | 3.6 (2.2) | 3.7 (1.9) | 3.6 (3.3) |
| Mean BASFI (SD) | 2.5 (1.8) | 2.7 (2.3) | 2.7 (2.0) | 2.6 (2.2) | 2.6 (1.8) | 2.6 (2.2) |
| Checklist IBP | ||||||
| <5 criteria present | 30 | 57 | 36 | 48 | 35 | 61 |
| 5 criteria present | 70 | 43 | 64 | 52 | 65 | 39 |
| Presence of extra‐spinal SpA features | ||||||
| 0 SpA features | 39 | 24 | 36 | 28 | 30 | 19 |
| 1 or 2 SpA features | 55 | 67 | 55 | 65 | 62 | 74 |
| ⩾3 SpA features | 6 | 8 | 9 | 7 | 8 | 6 |
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; HLA‐B27, human leucocyte antigen B27; IBD, inflammatory bowel disease; IBP, inflammatory bowel pain; MRI, magnetic resonance imaging; NA, not applicable; NSAID, non‐steroidal anti‐inflammatory drug; SpA, spondyloarthritis.
Extra‐spinal SpA features: asymmetric arthritis, Achilles tendinitis, dactylitis, inflammatory bowel disease, acute anterior uveitis and psoriasis.
Data are percentages unless otherwise indicated.
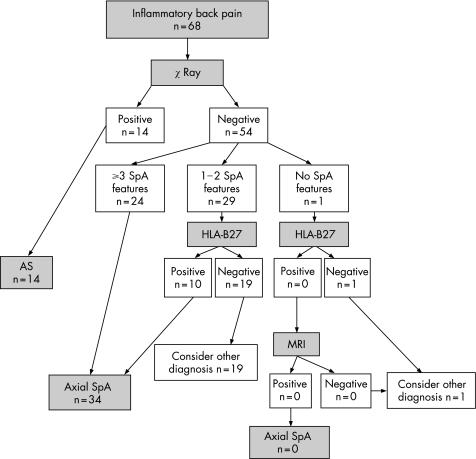
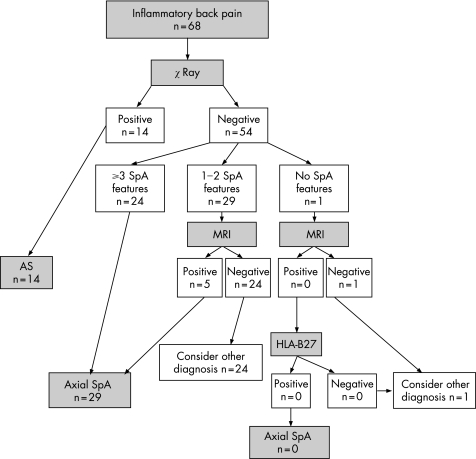
Figure 2 shows the performance of the Berlin algorithm. Fourteen patients were directly classified as having ankylosing spondylitis, an additional 24 patients fulfilled the Berlin criteria for axial SpA on clinical grounds (presence of ⩾3 SpA features), and another 10 patients with one or two SpA features were HLA‐B27 positive and thus fulfilled the Berlin classification criteria; so a total of 48 patients were classified as having axial SpA. In our cohort, only one patient had not even one single SpA feature. This patient was HLA‐B27 negative, and thus should—according to the Berlin algorithm—not be further investigated by MRI. If we modify the algorithm to apply MRI first instead of HLA‐B27 (fig 3), only five patients would be selected on the basis of 1 or 2 clinical SpA criteria in combination with a positive MRI.
Figure 2 Flow diagram of classification of patients included in the early spondyloarthritis (SpA) clinic cohort according to the Berlin algorithm. For details see text. AS, ankylosing spondylitis; HLA‐B27, human leucocyte antigen B27; MRI, magnetic resonance imaging.
Figure 3 Flow diagram of classification of patients included in the early spondyloarthritis (SpA) clinic cohort according to a modification of the Berlin algorithm. The order of human leucocyte antigen B27 (HLA‐B27) testing and magnetic resonance imaging (MRI) of the sacroiliac joints is changed. AS, ankylosing spondylitis.
Table 3 shows the diagnostic performance of the modified Berlin algorithm, both with and without the patients classified as having ankylosing spondylitis at inclusion. The diagnostic performance of the modification of the Berlin algorithm was better than the original algorithm, when taking fulfilment of both the ESSG criteria and the Amor criteria as an arbitrary gold standard. According to this gold standard, 11 patients were classified as false‐positive for axial SpA in the original algorithm (six with ⩾3 SpA features, and five with one or two SpA features who were HLA‐B27 positive), and six patients were classified as false‐negative (all with one or two SpA features who were HLA‐B27 negative). If MRI was applied first (modified algorithm), seven patients were classified as false‐positive (six with ⩾3 SpA features and one with one or two SpA features who had inflammation on MRI); and seven patients as false‐negative (all had one or two SpA features and had no inflammation on MRI). The modified algorithm outperforms the original algorithm in terms of correct classification, but still results in misclassifications.
Table 3 Test performance of modifications of the Berlin algorithm in the early spondyloarthritis clinic cohort with “fulfilment of Amor and European Spondylarthropathy Study Group criteria for spondyloarthritis” as gold standard.
| Order of application | ||
|---|---|---|
| HLA‐B27 first | MRI first | |
| All patients (n = 68) | ||
| Sensitivity | 77% | 84% |
| Specificity | 56% | 72% |
| NPV | 74% | 72% |
| PPV | 69% | 84% |
| LR‐positive test | 1.8 | 3.0 |
| LR‐negative test | 0.41 | 0.22 |
| Only patients who are not fulfilling the modified New York criteria for AS (n = 54) | ||
| Sensitivity | 68% | 76% |
| Specificity | 70% | 72% |
| NPV | 56% | 72% |
| PPV | 79% | 76% |
| LR‐positive test | 2.3 | 2.7 |
| LR‐negative test | 0.46 | 0.33 |
AS, ankylosing spondylitis; HLA‐B27, human leucocyte antigen B27; LR, likelihood ratio; MRI, magnetic resonance imaging; NPV, negative predictive value; PPV, positive predictive value.
LR‐positive test, sensitivity/(1−specificity).
LR‐negative test, (1−sensitivity)/specificity.
Discussion
In this cohort of patients with IBP of short duration as the main inclusion criterion, a remarkably high proportion classified for SpA according to three different criteria sets. Half of the patients fulfilled all three criteria sets, and an additional fifth fulfilled at least two criteria sets. The explanation may be that most patients were referred by rheumatologists, and that we screened for IBP in patients with an SpA‐related disease (psoriasis, uveitis and IBD) and in a population of relatives with ankylosing spondylitis or SpA. Undoubtedly, the prevalence of SpA in our cohort will not reflect the prevalence of SpA in individuals with IBP from the general population.
Fourteen patients already fulfilled the modified New York criteria for ankylosing spondylitis. Feldtkeller et al5 reported a long delay between start of symptoms and a diagnosis of ankylosing spondylitis. Active case finding based on IBP in combination with SpA feature(s) may importantly reduce the time interval between the start of symptoms and a diagnosis of ankylosing spondylitis in at least some patients with ankylosing spondylitis.
The proportion of women in this SpA cohort seems high (62%) in light of the male predominance in cohorts with established ankylosing spondylitis, but is in line with sex rates in other studies on patients with IBP of short duration.13,14,15 One explanation may be that, unlike ankylosing spondylitis, SpA is a disease that occurs more often in women than in men. Another explanation is that these women in fact do not have “true SpA”, and that the specificity of SpA criteria is too low. The observation that these women are often HLA‐B27 negative, without inflammation on MRI, adds to the validity of the second explanation. As a consequence, we found a lower rate of HLA‐B27‐positive patients (46%) as compared with those with ankylosing spondylitis. Bollow et al14 found 69% of their patients with SpA positive for HLA‐B27, Puhakka et al15 found a prevalence of 63% and Hanly et al13 reported 50%. The data described above are consistent with the hypothesis that HLA‐B27‐positive male patients with axial SpA are more likely to develop ankylosing spondylitis. This hypothesis can be tested in the follow‐up of this cohort.
The ESPAC cohort is an appropriate cohort to investigate the diagnostic performance of the Berlin diagnostic algorithm for axial SpA. We had to define an external criterion, as a gold standard for a diagnosis of ankylosing spondylitis in an early, preradiographic phase is lacking. We assumed the fulfilment of both the ESSG criteria and the Amor criteria as a sufficiently robust substitute for a gold standard. Interestingly, and despite an important overlap, there were five patients who fulfilled the Amor criteria and did not fulfil the ESSG criteria. The major discrepancy between both criteria sets includes the contribution of asymmetrical oligoarthritis to a classification according to Amor. Because our aim was to test the performance of the Berlin criteria for axial SpA (not peripheral SpA), we believed that we should exclude those patients who fulfilled only the Amor (but not the ESSG) classification criteria because of arthritis. Such an approach may be criticised. The distinction between axial and peripheral SpA is artificial, and there may be an important overlap. Both the ESSG and Amor variables for SpA were developed as classification criteria and we have used them in a diagnostic setting, which may lead to underperformance.16 Also, testing one criteria set against a combination of other criteria sets with an overlap in items has a danger of circularity. With our artificial construct as a gold standard, the performance of the Berlin algorithm in our study was moderate. The yield improved after conversion of the order of HLA‐B27 and MRI. The advantage of MRI, unlike HLA‐B27 testing, is that MRI directly visualises the inflammatory process. Limitations are that interpretation of MRI abnormalities is observer dependent, that MRI does not yield a clear positive or negative result, and that it is more expensive.
A noteworthy finding in this study is that it clearly shows the relationship between HLA‐B27 and sacroiliac‐joint inflammation (table 3). Of the patients with sacroiliac inflammation, 73% were positive for HLA‐B27, compared with only 33% of the patients without inflammation. Conversely, among the HLA‐B27‐positive patients, half of the patients showed sacroiliac inflammation on MRI, compared with one sixth of HLA‐B27‐negative patients. There was a male predominance in the group with inflammation, and more patients with sacroiliac inflammation on MRI responded to NSAIDs. This “inflammatory group”, resembling a group of patients with established ankylosing spondylitis, may ultimately develop definite ankylosing spondylitis.
A limitation of our study is that we did not analyse spinal inflammation. Recent data showed a high proportion of patients with ankylosing spondylitis and patients with inflammation of the spine in the thoracic segment.17 We also did not test the usefulness of our recent observation that the assessment of structural changes on conventional radiography in combination with inflammation on MRI is most sensitive for detecting abnormalities in sacroiliitis.11
We intend to investigate prospectively which patients in our cohort will develop ankylosing spondylitis over time, and which algorithm is most useful to predict such a course.
Supplementary Material
Abbreviations
ESSG - European Spondylarthropathy Study Group
HLA‐B27 - human leucocyte antigen B27
IBD - inflammatory bowel disease
IBP - inflammatory back pain
MRI - magnetic resonance imaging
NSAID - non‐steroidal anti‐inflammatory drugs
SpA - spondyloarthritis
Footnotes
Funding: This study was partly supported by a grant from the Dutch Arthritis Association.
References
- 1.Moll J M, Johnson G, Wright V. Psoriatic arthritis: a unique family. Rheumatol Rehabil 197413154–157. [DOI] [PubMed] [Google Scholar]
- 2.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A.et al The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991341218–1227. [DOI] [PubMed] [Google Scholar]
- 3.Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic 19905785–89. [PubMed] [Google Scholar]
- 4.van der Linden S, Valkenburg H A, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 198427361–368. [DOI] [PubMed] [Google Scholar]
- 5.Feldtkeller E, Bruckel J, Khan M A. Scientific contributions of ankylosing spondylitis patient advocacy groups. Curr Opin Rheumatol 200012239–247. [DOI] [PubMed] [Google Scholar]
- 6.Rudwaleit M, Khan M A, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum 2005521000–1008. [DOI] [PubMed] [Google Scholar]
- 7.Calin A, Porta J, Fries J F, Schurman D J. Clinical history as a screening test for ankylosing spondylitis. JAMA 19772372613–2614. [PubMed] [Google Scholar]
- 8.Garrett S, Jenkinson T, Kennedy L G, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994212286–2291. [PubMed] [Google Scholar]
- 9.Doward L C, Spoorenberg A, Cook S A, Whalley D, Helliwell P S, Kay L J.et al Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 20036220–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin A, Garrett S, Whitelock H, Kennedy L G, J O H, Mallorie P.et al A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994212281–2285. [PubMed] [Google Scholar]
- 11.Heuft‐Dorenbosch L, Landewe R, Weijers R, Wanders A, Houben H, van der Linden S.et al Combining information obtained from MRI and conventional radiographs in order to detect sacroiliitis in patients with recent‐onset inflammatory back pain. Ann Rheum Dis 2005641703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heuft‐Dorenbosch L, Weijers R, Landewe R, van der Linden S, van der Heijde D. Magnetic resonance imaging changes of sacroiliac joints in patients with recent‐onset inflammatory back pain: inter‐reader reliability and prevalence of abnormalities. Arthritis Res Ther 20058R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanly J G, Mitchell M J, Barnes D C, MacMillan L. Early recognition of sacroiliitis by magnetic resonance imaging and single photon emission computed tomography. J Rheumatol 1994212088–2095. [PubMed] [Google Scholar]
- 14.Bollow M, Braun J, Hamm B, Eggens U, Schilling A, Konig H.et al Early sacroiliitis in patients with spondyloarthropathy: evaluation with dynamic gadolinium‐enhanced MR imaging. Radiology 1995194529–536. [DOI] [PubMed] [Google Scholar]
- 15.Puhakka K B, Jurik A G, Schiottz‐Christensen B, Hansen G V, Egund N, Christiansen J V.et al Magnetic resonance imaging of sacroiliitis in early seronegative spondylarthropathy. Abnormalities correlated to clinical and laboratory findings. Rheumatology (Oxford) 200443234–237. [DOI] [PubMed] [Google Scholar]
- 16.Inman R D. Clinical stratification in the spondylarthropathies: E. pluribus unum or ex uno plures? Arthritis Rheum 200145475–477. [DOI] [PubMed] [Google Scholar]
- 17.Baraliakos X, Hermann K G, Landewe R, Listing J, Golder W, Brandt J.et al Assessment of acute spinal inflammation in patients with ankylosing spondylitis by magnetic resonance imaging: a comparison between contrast enhanced T1 and short tau inversion recovery (STIR) sequences. Ann Rheum Dis 2005641141–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.