Abstract
Background and objective
Since the original description of psoriatic arthritis (PsA) subgroups by Moll and Wright, there has been some discrepancy in the precise prevalence of the different subgroups and in particular the proportion of patients with polyarthritis. The higher prevalence of the polyarthritis subgroup may be due to the inclusion of patients with seronegative rheumatoid arthritis with coincidental psoriasis. The classification of psoriatic arthritis (CASPAR) study database provided an opportunity to examine this question.
Methods
The CASPAR study collected clinical, radiological and laboratory data on 588 patients with physician‐diagnosed PsA and 525 controls with other inflammatory arthritis, 70% of whom had rheumatoid arthritis. Patients with PsA were divided into two groups: polyarthritis and non‐polyarthritis (which included the Moll and Wright subgroups of spinal disease, distal interphalangeal predominant and arthritis mutilans) and were compared with patients with rheumatoid arthritis. Comparisons were made between all three groups and, if a significant difference occurred, between the two groups with PsA.
Results
The three groups differed significantly with regard to all clinical and laboratory variables except duration of disease. Significant differences were also found between the two groups of PsA in terms of age, sex, total number of involved joints, disability score and symmetry. However, no differences were found between the groups of patients with PsA in terms of seropositivity for rheumatoid factor and antibodies to cyclic citrullinated peptide, enthesitis, and spinal pain and stiffness. Further, dactylitis was commonly seen in patients with PsA (57% in the polyarticular group and 45% in non‐polyarticular group), and uncommonly found in patients with rheumatoid arthritis (5%). With the exception of entheseal changes, syndesmophytes and osteolysis, typical radiological features of PsA could not be used to distinguish between the PsA subgroups.
Conclusions
The evidence suggests that the changing prevalence of the polyarticular subgroup of PsA is not because doctors include patients with seronegative rheumatoid arthritis with coincidental psoriasis.
Psoriatic arthritis (PsA) is an inflammatory arthritis associated with psoriasis. Most patients are classified using the criteria of Moll and Wright:1 an inflammatory arthritis (peripheral arthritis, sacroiliitis or spondylitis), the presence of psoriasis and the (usual) absence of serological tests for rheumatoid factor. Although Wright originally suggested that most of the patients with PsA had a polyarticular pattern,2 later studies by Moll and Wright described the most frequent clinical pattern as an asymmetrical oligoarthritis.1 However, most of the published series in the past 20 years have reported polyarthritis as the most frequent subgroup, at about 60%.3,4,5,6,7,8 The reason for this discrepancy is not entirely clear, although it is unlikely that the disease has changed since the description given by Moll and Wright. It is more likely that Moll and Wright were using more specific, but unstated, criteria to identify their patients.9 Later authors, unaware of this, may have interpreted the Moll and Wright criteria meticulously—resulting in the inclusion of a higher percentage of patients with seronegative symmetrical polyarthritis and without any of the other specific features that are thought to characterise PsA. Thus, it is possible that some of the patients included in the later series have seronegative rheumatoid arthritis with coincidental psoriasis.10
The situation is confounded by factors such as the precise method for ascertaining joint involvement—joint involvement may be much more extensive if tender and swollen joints are both counted or if imaging modalities such as ultrasound are used. It must also be recognised that the disease pattern in an individual patient will change over time both with evolution of the disease7 and with treatment.11 It is possible that Moll and Wright included patients with earlier disease than those in the latter series, but this is unlikely (JMH Moll, personal communication, 2004).
More specific criteria for classifying PsA have been proposed, but none, with the exception of Fournié et al,12 was derived statistically from patient‐derived data. It was for this reason that the classification of psoriatic arthritis (CASPAR) study was established: to examine the performance characteristics of existing criteria for classifying PsA and to develop new criteria. The CASPAR study collected clinical, radiological and laboratory data on 588 patients with physician diagnosed PsA and 536 controls with other inflammatory arthritis, 70% of whom had rheumatoid arthritis.13 The CASPAR study also provided an opportunity to examine the hypothesis that patients with seronegative rheumatoid arthritis are included in case series of PsA. The aim of our study was, therefore, to examine the clinical, laboratory and radiological features of the subgroups of PsA and to compare these with patients diagnosed with rheumatoid arthritis.
Methods
Ethical approval for this study was obtained at each of the contributing study sites. Consecutive clinic attenders with physician‐diagnosed PsA were enrolled into the study by 30 rheumatology clinics in 13 countries. Controls were the next clinic attenders with inflammatory arthritis. It was prespecified that at least 50% of controls were to have rheumatoid arthritis to reflect the disease distribution seen in normal rheumatological practice. Data were recorded on standardised forms and included demographics (ethnicity, sex and age), psoriasis (whether currently evident or previously observed, nail involvement), onset of skin disease and arthritis, dactylitis, chest wall pain, diffuse enthesis pain, inflammatory heel pain, clinical sacroiliitis, swollen and tender joint examination findings, family history of psoriasis, inflammatory spinal pain (lumbar, thoracic and cervical), rheumatoid factor, raised acute‐phase reactant, rheumatoid nodules, spinal mobility measures and joint counts (using manikins recording 78 tender, swollen and damaged joints: collaborators were requested to complete the tender and swollen counts using historical data to record, in effect, whether the joint had ever been involved). Information on approximately 50 variables was collected.
Sera and whole‐blood DNA extracts were submitted to the central coordinating centre. Antibodies to cyclic citrullinated peptide were determined by ELISA using a second‐generation commercially available kit (QUANTA Lite IgG ELISA, A Menarini Diagnostics, http://www.menarini.com). A cut‐off of 20 U/ml was used to define positivity. Radiographs of the hands, feet, pelvis, lumbar spine and cervical spine were obtained, unless films were available within the previous 12 months. Hard copy films or digitised images were submitted to the central coordinating centre and read by two observers in tandem, blinded to the physician diagnosis. Radiological features were identified using the definitions developed by an earlier observer reliability study.14
Variables used for analysis and statistical approach
Data were recoded on a standardised proforma and sent to the coordinating site where they were entered into an SPSS database. Accuracy of data entry was checked on a random sample of 10% of cases and the appropriateness of the diagnostic label was checked on a further sample of 10% by a quality control committee. Subgroup definitions were as follows:
Oligoarthritis/polyarthritis: Using a cut‐off of five involved joints (⩾5 involved joints defined as polyarthritis).
Distal interphalangeal joint predominant: >50% of the total joint count were distal interphalangeal joints (fingers and toes included).
Arthritis mutilans: This was a stand‐alone variable in the case record form completed by the observer at the time of the examination.
Spine predominant: Inflammatory spinal pain (in any of three areas—lumbar, thoracic and cervical), reduced spinal mobility with reference to normative values,15 and radiographic sacroiliitis (at least grade 2 unilateral sacroilitis). A further category of isolated sacroiliitis was included. Unfortunately, radiological data for the spine were missing for 39% of the patients.
Symmetry: >50% of joints (grouping small joins of hands and feet) involved as matched pairs.16
Derived subgroups were compared using clinical, radiological and laboratory variables and compared with appropriate statistics.
Results
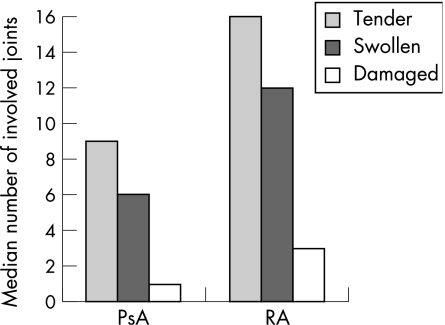
Data were collected prospectively from 588 consecutive clinic attenders with PsA and 536 controls, as described earlier. Controls had rheumatoid arthritis (n = 384), ankylosing spondylitis (n = 72), undifferentiated arthritis (n = 38), connective tissue disorders (n = 14) and other diseases (n = 28). The disease duration for patients with PsA was 12.5 years and for those with rheumatoid arthritis was 12.4 years. Patients with PsA were younger and more likely to be men than those with rheumatoid arthritis (men age 50.3 v 58.0 years; male sex 52% v 29%, respectively). Figure 1 gives the median number of tender, swollen and damaged joints for patients with psoriatic arthritis and those with rheumatoid arthritis.
Figure 1 Tender, swollen and damaged joint counts in patients with psoriatic arthritis (PsA) and rheumatoid arthritis (RA).
In 39% of participants, the recommendation to record the presence of swollen, tender and damaged joints on the basis of past and present involvement had been followed. In 26% patients, the joints were recorded as they were on the day of the examination; data were unavailable for the other respondents. According to this schema, total joint counts differed significantly (counts being higher for the “ever” group), but there were no differences in the proportion of respondents recording “ever” for each of the PsA and rheumatoid arthritis groups (p = 0.001). Further analysis therefore disregarded this potential bias assuming that discrepancies in joint counts applied equally to both rheumatoid arthritis and PsA groups.
Table 1 gives the Moll and Wright subgroups for PsA and rheumatoid arthritis. Polyarthritis, largely symmetrical (82%), was the predominant subgroup in both PsA and rheumatoid arthritis groups. One patient with rheumatoid arthritis and 23 with PsA did not have any peripheral joint involvement recorded on the case record form.
Table 1 Moll and Wright subgroups applied to the current (classification of psoriatic arthritis) dataset.
| PsA, n = 588 | RA, n = 384 | |||
|---|---|---|---|---|
| n | % | n | % | |
| Not defined | 19 | 3 | 1 | <1 |
| Arthritis mutilans | 16 | 3 | 5 | 1 |
| DIPJ predominant | 23 | 4 | 0 | NA |
| Oligoarthritis | 76 | 13 | 20 | 5 |
| Polyarthritis | 372 | 63 | 358 | 93 |
| Spinal involvement | 82 | 14 | 0 | NA |
DIPJ, distal interphalangeal joint; NA, not applicable; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
PsA and rheumatoid arthritis groups were divided to facilitate further analysis. Patients were divided into two groups: polyarticular (⩾5 involved joints) and others (hereafter called non‐polyarticular). Those with distal interphalangeal predominant arthritis and any spinal involvement (including isolated sacroiliitis) were grouped, along with oligoarticular cases, in the non‐polyarticular group. Patients with arthritis mutilans were included in the polyarticular group as they had >5 affected joints. The four groups were polyarticular PsA (n = 388), polyarticular rheumatoid arthritis (n = 363), non‐polyarticular PsA (n = 200) and non‐polyarticular rheumatoid arthritis (n = 21). As the non‐polyarticular rheumatoid arthritis group was small and not of primary interest, this group was not further analysed. Tables 2 and 3 compare the other three groups.
Table 2 Clinical and laboratory features of the present groups.
| Polyarticular PsA, n = 388 | Polyarticular RA, n = 363 | Non‐polyarticular PsA, n = 200 | Test statistic for all three groups | p Value | Test statistic for polyarticular PsA v non‐polyarticular PsA | p Value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 51.5 | 58.3 | 47.9 | F = 48.3 | <0.001 | NA | 0.005* |
| Sex | m = 183; f = 205 | m = 103; f = 260 | m = 123; f = 77 | χ2 = 62.4 | <0.001 | χ2 = 10.9 | 0.001 |
| Duration of arthritis (years) | 13.0 | 12.5 | 11.5 | F = 1.61 | NS | NA | NA |
| Duration of psoriasis (years)† | 20.0 | 17.8 | 18.4 | F = 1.19 | NS | NA | NA |
| Total number of involved joints (median, range) | 21 | 23 | 4 | KW χ2 = 180.2 | <0.001 | U = 15 714 | <0.001 |
| HAQ | 0.90 | 0.89 | 0.68 | F = 0.83 | NS | NA | NA |
| Symmetry number‡ | 0.67 | 0.86 | 0.50 | KW χ2 = 152.3 | <0.001 | U = 23 710 | <0.001 |
| RF positive, n (%) | 22 (6) | 274 (76) | 5 (3) | χ2 = 522.3 | <0.001 | χ2 = 3.0 | NS |
| CCP positive, n (%)§ | 15 (7) | 163 (71) | 11 (10) | χ2 = 245.1 | <0.001 | χ2 = 0.9 | NS |
| Dactylitis, n (%) | 220 (57) | 17 (5) | 89 (45) | χ2 = 246.9 | <0.001 | χ2 = 11.8 | 0.008 |
| Enthesitis, n (%) | 209 (54) | 53 (15) | 103 (52) | χ2 = 145.1 | <0.001 | χ2 = 0.5 | NS |
| Spinal pain and stiffness, n (%) | 148 (38) | 83 (23) | 81 (41) | χ2 = 32.4 | <0.001 | χ2 = 2.4 | NS |
CCP, cyclic citrullinated peptide; f, female; F, F ratio of one‐way analysis of variance; HAQ, Health Assessment Questionnaire; KW, Kruskall–Wallis; m, male; NA, not applicable; NS, not significant; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RF, rheumatoid factor; T, independent samples t test statistic; U, Mann–Whitney U statistic.
*Tukey's retrospective analysis.
†14 patients with rheumatoid arthritis also had psoriasis.
‡Ratio of number of matched joint pairs to total number of joints (range 0–1).
§CCP available for only 222 patients with polyarticular PsA, 229 with polyarticular RA and 112 patients with non‐polyarticular PsA.
Table 3 Radiological features of the patient groups.
| Polyarticular PsA n = 388, n (%) | Polyarticular RA n = 366, n (%) | Non‐polyarticular PsA n = 200, n (%) | Test statistic for all three groups | p Value | Test statistic for polyarticular PsA v non‐polyarticular PsA | p Value | |
|---|---|---|---|---|---|---|---|
| Marginal syndoesmophytes* | 5 (1) | 1 (<1) | 16 (8) | χ2 = 41.5 | <0.001 | χ2 = 21.70 | <0.001 |
| Non‐marginal syndesmophytes* | 25 (6) | 16 (4) | 22 (11) | χ2 = 13.1 | 0.01 | χ2 = 7.73 | 0.021 |
| Entheseal erosion† | 23 (6) | 21 (6) | 10 (5) | χ2 = 2.48 | NS | χ2 = 1.02 | NS |
| Entheseal new bone formation† | 49 (13) | 35 (10) | 29 (15) | χ2 = 5.23 | NS | χ2 = 1.11 | NS |
| Distal interphalangeal erosion | 82 (21) | 40 (11) | 40 (20) | χ2 = 16.40 | 0.003 | χ2 = 1.38 | NS |
| Juxta‐articular new bone | 59 (15) | 15 (4) | 37 (19) | χ2 = 35.52 | <0.001 | χ2 = 2.91 | NS |
| Osteolysis‡ | 42 (11) | 34 (9) | 23 (12) | χ2 = 2.40 | NS | χ2 = 1.54 | NS |
| Tuft osteolysis | 14 (4) | 0 | 8 (4) | χ2 = 15.69 | 0.003 | χ2 = 1.48 | NS |
| Ankylosis‡ | 38 (10) | 11 (3) | 23 (12) | χ2 = 19.49 | 0.001 | χ2 = 2.02 | NS |
| Ray involvement§ | 18 (5) | 6 (2) | 13 (7) | χ2 = 10.90 | 0.028 | χ2 = 1.99 | NS |
NS, not significant; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
*Marginal and non‐marginal syndesmophytes in any spinal segment.
†Entheseal erosion and new bone formation at the inferior and posterior aspects of calcaneum, tibial and patellar sites and pelvis.
‡Osteolysis and ankylosis at any of the small joints of hands and feet.
§Ray involvement indicates the combined involvement of metacarpophalangeal, proximal interphalangeal and distal interphalangeal joint of same digit.
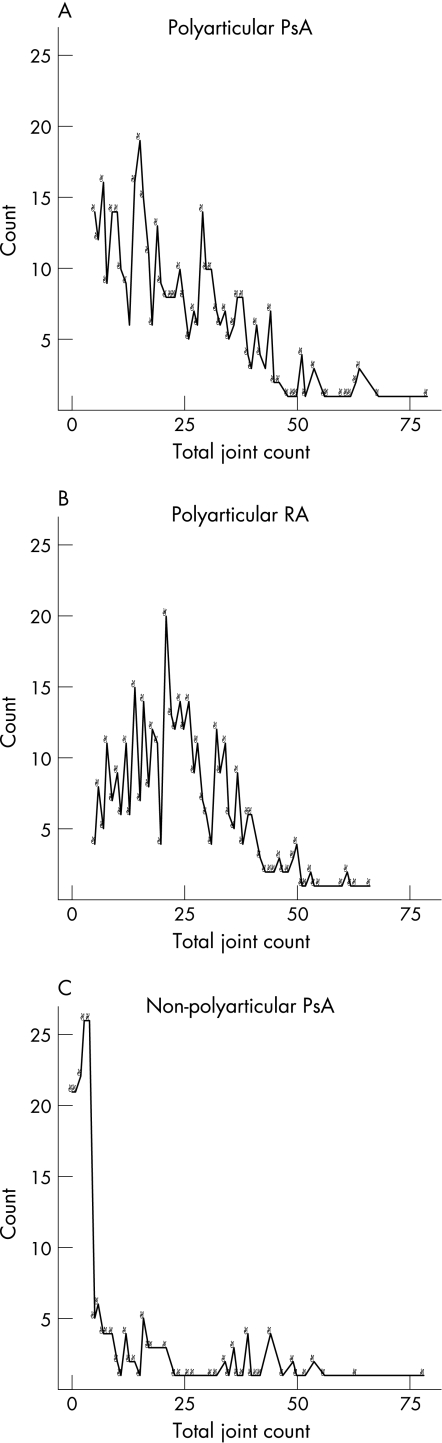
Table 2 compares the three groups using clinical and laboratory variables. As would be expected, the non‐polyarticular group has a higher percentage of men, lower joint count, lower symmetry score and lower disability scores. More importantly, variables that would be expected to differentiate PsA from rheumatoid arthritis consistently show that the polyarticular PsA group more closely resembles the non‐polyarticular PsA group than the rheumatoid arthritis group. The distribution of joint counts shows that the mode for polyarticular PsA is 15, for polyarticular rheumatoid arthritis 21 and for non‐polyarticular PsA 3 (fig 2).
Figure 2 Distribution of joint counts in the three groups: (A) polyarticular psoriatic arthritis (PsA), (B) polyarticular rheumatoid arthritis (RA) and (C) non‐polyarticular PsA. Count (on the y axis) is the number of patients and the x axis represents the total joint count.
Table 3 shows the radiological features of the three patient groups. Sacroiliitis is not shown as this was specifically excluded from the polyarticular subgroup. Marginal syndesmophytes were, not unexpectedly, seen more often in the non‐polyarticular PsA group. Non‐marginal syndesmophytes, although seen more often in the non‐polyarticular PsA, were also seen in the other two groups, which probably reflects the difficulty in distinguishing this as a feature of PsA from diffuse idiopathic skeletal hyperostosis. No differences in the entheseal features were seen among the three groups. Peripheral small‐joint abnormalities, apart from osteolysis at any digital joint, again consistently favoured the association between the two groups with PsA.
Discussion
The doctors contributing patients to the CASPAR database all had an acknowledged special interest in PsA and were distributed worldwide. The strength of this database rests in this heterogeneity and mitigates against any local or regional bias in the diagnosis and classification of PsA. Although most of the contributors were probably using the Moll and Wright criteria to identify their patients with PsA, a small percentage of patients were seropositive, and the most common subgroup had polyarthritis, mainly symmetrical. Although this is consistent with contamination by cases of rheumatoid arthritis, the results of this study suggest otherwise: the polyarthritis subgroup has more in common with the non‐polyarticular group of PsA than with rheumatoid arthritis.
Of course, coincidental cases of psoriasis and rheumatoid arthritis exist, and this was reflected in the CASPAR database (14 had psoriasis and were diagnosed as having rheumatoid arthritis), but doctors use other clinical features to help diagnose individual cases: the presence of inflammatory spinal symptoms, dactylitis and enthesitis help to “push” the patient into the PsA group, and the presence of nodules and extra‐articular features suggest a diagnosis of rheumatoid arthritis. Often, the clinician also relies on immunology (the presence of rheumatoid factor) and radiology, although many of the features used to distinguish rheumatoid arthritis and PsA do not occur in early disease. Diagnosis at the individual patient level is a matter different from case classification, which is for the purposes of epidemiology and clinical trials. As yet, criteria for the diagnosis of PsA at an individual level have not been developed. In fact, there is continued doubt about the ability of “experts” to agree on the diagnosis of PsA in “grey” cases, as shown in a recent “paper” exercise.17 A common difficult distinction is the patient with seronegative, symmetrical polyarthritis and psoriasis where features such as those mentioned above are not evident.18
Is there any other purpose in wishing to distinguish PsA from rheumatoid arthritis? Does it matter in terms of treatment and outcome? Recent developments would support the concept of PsA as a disease distinct from rheumatoid arthritis both on synovial histopathology19,20 and using imaging techniques.21 In synovial histopathology, major differences have been found between the synovium in rheumatoid arthritis and PsA. In rheumatoid arthritis, highly specific intracellular citrullinated peptides and monoclonal antibody 12A were seen with increased lining layer thickness, whereas in PsA, there was greater vascularity, increased CD163 and intracellular adhesion molecule 1, and a higher number of polymorphonuclear cells. No histopathological differences were seen between oligoarticular and polyarticular PsA. With newer imaging techniques, both ultrasound and magnetic resonance imaging have shown more abnormalities in the periarticular structures in PsA compared with rheumatoid arthritis, implicating more entheseal pathology. It seems unlikely therefore that we are studying a condition where otherwise typical rheumatoid arthritis is modified by the presence of psoriasis. On the other hand, many of the currently available treatments used are equally efficacious, including the new anti‐tumour necrosis factor drugs. However, as a better understanding of the pathophysiology of these diseases emerges, different treatment targets may become more relevant. Further work is needed to establish the nosology and natural history of these two common inflammatory joint diseases, with particular reference to adverse prognostic factors, which will govern not the class of drug used but the aggressiveness of the treatment chosen.
Although our study has confirmed the high proportion of the polyarticular subgroups compared with Moll and Wright criteria, the paradox might be explained on the basis of taxonomy. Later series have adopted tighter definitions to classify the subgroups, whereas Moll and Wright were rather vague in their descriptions. The desire to place patients into subgroups has occasioned some debate,5,22 but it could be said that there is no useful purpose served by distinguishing the different patterns of peripheral joint involvement and that articular disease in PsA should be classified as either axial or peripheral.23 The development of new classification criteria for PsA—the main aim of the CASPAR study—is perhaps more important, which will result in the inclusion of more homogeneous populations of patients in future studies in addition to more comparable prognostic and immunopathological data between centres.
In summary, our study has confirmed the clinical distinction between PsA and rheumatoid arthritis and has found no evidence to support the notion that patients with seronegative rheumatoid arthritis are being classified as having PsA.
Abbreviations
CASPAR - classification of psoriatic arthritis
PsA - psoriatic arthritis
Footnotes
Funding: The following organisations provided financial support for this project: European League Against Rheumatism, Barnsley District NHS Trust, Groote Schuur Hospital (Cape Town), Department of Medical Sciences (University Hospital, Uppsala), Krembil Foundation, St Vincent's University Hospital Radiology Department (Dublin), Inkosi Albert Luthuli Central Hospital (Durban), El Ayachi Hospital (Morocco), National Psoriasis Foundation (USA), The Foundation for Scientific Research of the Belgian Society of Rhumatology, Arthritis New Zealand.
Competing interests: None.
Contributors: PSH and GP (Airedale General Hospital, Keighley, UK) performed the radiographic assessment. The anti‐CCP tests were performed by Dr Neil McHugh and Pat Owen (Royal National Hospital for Rheumatic Diseases, Bath, UK).
Ethical approval: Ethical approval for this study was obtained at each of the 32 contributing sites.
References
- 1.Moll J M H, Wright V. Psoriatic arthritis. Sem Arthritis Rheum 1973351–78. [DOI] [PubMed] [Google Scholar]
- 2.Wright V. Rheumatism and psoriasis: a re‐evaluation. Am J Med 195927454–462. [DOI] [PubMed] [Google Scholar]
- 3.Gladman D D, Shuckett R, Russell M L, Thorne J C, Schachter R K. Psoriatic arthritis (PSA)—an analysis of 220 patients. Q J Med 1987238127–141. [PubMed] [Google Scholar]
- 4.Biondi O C, Scarpa R, Pucino A, Oriente P. Psoriasis and psoriatic arthritis. Dermatological and rheumatological co‐operative clinical report. Acta Dermato‐Venereol 1989146(Suppl)69–71. [PubMed] [Google Scholar]
- 5.Helliwell P, Marchesoni A, Peters M, Barker M, Wright V. A re‐evaluation of the osteoarticular manifestations of psoriasis [see comments]. Br J Rheumatol 199130339–345. [DOI] [PubMed] [Google Scholar]
- 6.Alonso J C T, Perez A R, Castrillo J M A, Garcia J B, Noriega J L R, Larrea C L. Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br J Rheumatol 199130245–250. [DOI] [PubMed] [Google Scholar]
- 7.Jones S M, Armas J B, Cohen M G, Lovell C R, Evison G, McHugh N J. Psoriatic arthritis: outcome of disease subsets and relationship of joint disease to nail and skin disease. Br J Rheumatol 199433834–839. [DOI] [PubMed] [Google Scholar]
- 8.Veale D, Rogers S, Fitzgerald O. Classification of clinical subsets in psoriatic arthritis [see comments]. Br J Rheumatol 199433133–138. [DOI] [PubMed] [Google Scholar]
- 9.Helliwell P S. Relationship of psoriatic arthritis with other spondyloarthropathies. Curr Opin Rheum 200416344–349. [DOI] [PubMed] [Google Scholar]
- 10.Helliwell P S, Taylor W J. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis 200564(Suppl II)ii3–ii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane D, Stafford L, Bresnihan B, Fitzgerald O. A classification study of clinical subsets in an inception cohort of early psoriatic peripheral arthritis—‘Dip or not Dip revisited'. Rheumatology 2003421469–1476. [DOI] [PubMed] [Google Scholar]
- 12.Fournie B, Crognier L, Arnaud C, Zabraniecki L, Lascaux‐Lefebvre V, Marc V.et al Proposed classification criteria of psoriatic arthritis: a preliminary study in 260 patients. Rev Rheum (Engl Edn) 199966446–456. [PubMed] [Google Scholar]
- 13.Taylor W J, Gladman D D, Helliwell P S, Marchesoni A, Mease P, Mielants H.et al Classification criteria for psoriatic arthritis: results from the CASPAR study group. Arthritis Rheum 2006542665–2673. [DOI] [PubMed] [Google Scholar]
- 14.Taylor W J, Porter G G, Helliwell P S. Operational definitions and observer reliability of the plain radiographic features of psoriatic arthritis. J Rheumatol 2003302645–2658. [PubMed] [Google Scholar]
- 15.Moll J M H, Wright V. Normal range of spinal mobility: an objective clinical study. Ann Rheum Dis 197130381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helliwell P S, Hetthen J, Sokoll K, Green M, Marchesoni A, Lubrano E.et al Joint symmetry in early and late rheumatoid and psoriatic arthritis—comparison with a mathematical model. Arthritis Rheum 200043865–871. [DOI] [PubMed] [Google Scholar]
- 17.Symmons D P, Lunt M, Watkins G, Helliwell P S, Jones S M, McHugh N J.et al Developing classification criteria for peripheral joint psoriatic arthritis. Step I. Establishing whether the rheumatologists opinion on the diagnosis can be used as the “gold standard”. J Rheumatol 200633552–557. [PubMed] [Google Scholar]
- 18.Palazzi C, Olivieri I, Petricca A, Salvarani C. Rheumatoid arthritis or psoriatic arthritic symmetrical polyarthritis? A difficult differential diagnosis. Clin Exp Rheumatol 2002203–4. [PubMed] [Google Scholar]
- 19.Baeten D, Kruithof E, De Rycke L, Vandooren B, Wyns B, Boullart L.et al Diagnostic classification of spondylarthropathy and rheumatoid arthritis by synovial histopathology: a prospective study in 154 consecutive patients. Arthritis Rheum 2004502931–2941. [DOI] [PubMed] [Google Scholar]
- 20.Kruithof E, Baeten D, De Rycke L, Vandooren B, Foell D, Roth J.et al Synovial histopathology of psoriatic arthritis, both oligo‐ and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. [see comments]. Arthritis Res Ther 20057R569–R580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGonagle D, Conaghan P, Emery P. Psoriatic arthritis: a unified concept 20 years on. Arthritis Rheum 1999421080–1086. [DOI] [PubMed] [Google Scholar]
- 22.Veale D, Fitzgerald O. Psoriatic arthritis—“DIP or not DIP? That is the question” [letter; comment]. Br J Rheumatol 199231430–431. [DOI] [PubMed] [Google Scholar]
- 23.Taylor W J, Zmierczak H G, Helliwell P S. Problems with the definition of axial and peripheral disease patterns in psoriatic arthritis. J Rheumatol 200532974–977. [PubMed] [Google Scholar]