Abstract
Background
Autoantibodies to the 20S proteasome represent an unspecific but common serological phenomenon in patients with systemic autoimmune diseases. Interestingly, a high prevalence of these antibodies have been observed in patients with connective tissue diseases, where anti‐nuclear antibodies (ANAs) serve as an important diagnostic screening test.
Objective
To disclose interference of anti‐proteasome antibodies with known ANA patterns.
Methods
Anti‐proteasome antibodies were isolated for comprehensive immunofluorescence analyses. The immunofluorescence pattern of human anti‐proteasome antibodies was compared with a panel of monoclonal and polyclonal reference antibodies, and colocalisation was analysed using confocal microscopy.
Results
Anti‐proteasome antibodies clearly contributed to the ANA patterns of their respective serum samples from patients with different rheumatic disorders. In addition to the nuclear pattern, proteasomal staining was also detectable in the cytoplasm, at the endoplasmic reticulum and perinuclear regions showing features overlapping with other known autoantibodies such as those to mitochondria. The specificity of anti‐proteasome antibodies was proved by competition experiments and by colocalisation with monoclonal reference antibodies in confocal microscopy.
Conclusion
In ANA diagnostics, interference of anti‐proteasome antibodies will have to be taken into account, especially in the differentiation of anti‐cytoplasmatic autoantibodies.
Antibodies to nuclear and cytoplasmatic components are a central diagnostic tool in connective tissue diseases, with increasing evidence of pathogenic importance.1 Using a standard screening method, they are commonly detected by indirect immunofluorescence (IIF) on HEp‐2 cells or organ samples. Previously, a common humoral autoimmune response to proteasomes was shown in patients with autoimmune myositis, systemic lupus erythematosus and primary Sjögren's syndrome, and occasionally in other systemic autoimmune diseases.2,3 The targets for these autoantibodies are predominantly the α‐subunits of the 20S proteasomes, especially C9 (α3), although autoantibodies reactive to the catalytic β‐subunits have also been detected.4 In this context, important questions arose about the characterisation of the anti‐proteasome antibodies and potential interference with a known pattern in IIF.
The 20S proteasome is the major proteinase complex of the intracellular, non‐lysosomal, ATP‐dependent protein degradation.5,6 As an essential protease found in all eukaryotic organisms as well as in archaebacteriae and encoded by highly preserved genes, it is responsible for the ubiquitin‐dependent protein degradation and the rapid turnover of transcription factors.7 Moreover, it is the main source for the generation of peptides bound by major histocompatibility complex class I complexes for presentation to cytotoxic CD8+ T cells.8,9 Proteasomes are activated by protein complexes that bind to the outer rings of α‐subunits. In this context, binding of PA28 induces opening of the entrance and exit gates of the proteasome and stimulates the hydrolysis of peptides.10 Remarkably, the interaction between 20S proteasomes and PA28 is efficiently blocked by human anti‐proteasome antibodies.23 Distinct peptides generated by proteasomes are transported into the lumen of the endoplasmic reticulum via transporter molecules associated with antigen processing (TAP) where they undergo trimming and bind to newly generated major histocompatibility complex class I precursor molecules.11 In this way, they contribute to the differentiation between self and non‐self.
The intracellular localisation of proteasomes is complex, depending on the tissue type and the metabolic state of the cell.12 In living cells, proteasomes are highly mobile in the cytoplasm with intermediate association with the endoplasmic reticulum or the cytoskeleton, whereas they are absent from mitochondria.13 Moreover, the nuclear membrane represents a transport barrier, allowing unidirectional transport into the nucleus.14 In HeLa cells and HEK 293 cells, colocalisation has been shown with the centrosomal marker γ‐tubulin.15 Associations with cytoskeletal structures such as vimentin, cytokeratin, actin and desmin have also been described.16,17
By using affinity‐purified human anti‐proteasome antibodies, this study investigated whether a reproducible proteasomal staining pattern exists. With relevance to screening of anti‐nuclear antibodies (ANAs) on HEp‐2 cells, we point out similarities in proteasomal patterns and patterns produced by antibodies against other defined cell structures and proteins, respectively.
Patients and methods
Patients
Serum samples from eight anti‐proteasome antibody‐positive patients and one anti‐proteasome antibody‐negative patient were investigated by IIF: three patients with systemic lupus erythematosus fulfilling the 1982 revised American College of Rheumatology criteria,18 three patients with dermatomyositis/polymyositis classified according to Bohan and Peter,19,20 one patient with primary Sjögren's syndrome diagnosed according to Vitali et al21 and one patient with undifferentiated connective tissue disease. Patients' sera were screened for anti‐proteasome antibodies by ELISA and immunoblotting. The serum samples were obtained from patients of the Department of Rheumatology and Clinical Immunology, Charité‐Universitätsmedizin Berlin, Berlin, Germany, after approval of the local ethics committee (table 1).
Table 1 Anti‐proteasome antibodies and autoantibody profiles of the patients investigated by indirect immunofluorescence.
| Patient number | Age/sex | Disease | ANA titre | Autoantibody profile | Anti‐proteasome antibodies | ||
|---|---|---|---|---|---|---|---|
| ELISA (U) | IB | ||||||
| 1 | 30/F | Myositis | 1:2560 | U1‐RNP/Sm, Ro, La | 95 | ND | |
| 2 | 38/F | Myositis | 1:2560 | U1‐RNP/Sm, Jo1, Histone, Ribosome P | 58 | ND | |
| 3 | 42/M | SLE | 1:2560 | U1‐RNP/Sm, Ro, La | >100 | ND | |
| 4 | 53/F | pSS | 1:2560 | Ro, La | 16 | Positive | |
| 5 | 48/F | UCTD | 1:1280 | U1‐RNP/Sm, Ro, La | 84 | Positive | |
| 6 | 34/F | SLE | 1:640 | Nucleosome | >100 | ND | |
| 7 | 44/F | Myositis | 1:160 | Negative | 68 | Positive | |
| 8 | 54/F | SLE | 1:2560 | dsDNA, Ro, La, Sm | 5.5 | Positive | |
ANA, anti‐nuclear antibody; F, female; IB, immunoblotting; M, male, ND, not done; pSS, primary Sjögren's syndrome; SLE, systemic lupus erythematosus; UCTD, undifferentiated connective tissue disease; U1‐RNP, U1‐ribonucleoprotein.
Autoantibody profiles
The 20S proteasome was isolated from human erythrocytes following standard procedures as previously described.22 Anti‐proteasome antibodies were detected by ELISA and immunoblotting. In ELISA, microtitre plates were incubated with 4 μg/ml of purified proteasome solution in carbonate buffer, pH 9.5, for 24 h at 4°C. Patients' sera were diluted 1:100 in phosphate‐buffered saline (PBS)–0.1% Tween 20, pH 7.2. As a secondary antibody, a goat‐anti‐human‐peroxidase‐labelled antibody was used in PBS–0.1% Tween 20, pH 7.2. Bound antibodies were visualised with tetramethyl benzidine solution. Standard curves were established by using a strong reactive anti‐proteasome serum from a patient with polymyositis from the Department of Rheumatology and Clinical Immunology, Charité‐Universitätsmedizin Berlin. The cut‐off level for anti‐proteasomal antibodies (20 U) was calculated from 80 healthy people. Immunoblotting was carried out as previously described.4
ANA and anti‐proteasome antibody patterns were detected in the human larynx epithelioma cancer cell line HEp‐2 (Bios Gmbh, Gräfelfing, Germany) by IIF using a fluorescein isothiocyanate (FITC)‐labelled secondary anti‐human immunoglobin (Ig)G‐Fc antibody (The Binding Site, Birmingham, UK). The dilution of sera was started at 1:80
Isolation of anti‐proteasome antibodies
The anti‐proteasome antibody fraction from all patients was enriched from serum and plasma samples in three steps: (1) isolation of the globulin fraction by ammonium sulphate precipitation (40% saturation); (2) isolation of IgG fraction by adsorption on to protein G–Sepharose (Amersham Pharmacia Biotech, Freiburg, Germany) according to manufacturer's instructions (binding/washing buffer: PBS, pH 7; 10 mM EDTA; elution buffer: 0.1 M glycine/HCl, pH 3); and (3) isolation of anti‐proteasome antibodies by affinity chromatography on proteasome–Sepharose. The fraction of total IgG was dialysed against binding buffer (PBS, pH 7, 0.1% Tween 20), and the proteasome‐specific antibodies were bound in batch by gentle shaking overnight in the cold room (2 ml gel in 10 ml binding buffer at a protein concentration of 1 g/l). Thereafter, the gel was placed in a column (FPLC, Fast Protein Liquid Chromatography) for removing unbound material after elution of adsorbed antibodies (elution buffer: 0.1 M glycine/HCl, pH 3). The eluate was neutralised, equilibrated in PBS containing 4% bovine serum albumin by ultrafiltration, and stored in a frozen state. Isolated antibodies were pure IgG as deduced by separation on sodium dodecyl sulfate‐15% polyacrylamide gel electrophoresis (SDS‐PAGE) (not shown). The specificity of affinity purified anti‐proteasome antibodies was verified by ELISA and immunoblotting using MOLT‐4 cell extracts (Human acute lymphoblastic leukemia cell line), 20S proteasomes and PA28 as antigens.25 Moreover, antibodies were tested against purified 20S proteasomes in two‐dimensional PAGE western blots, and the targeted subunits were determined. No cross reactivity of the eluted antibodies against other autoantigens was observed.
Preparation of proteasome–Sepharose
The 20S proteasome was isolated from human erythrocytes following standard procedures.23 The native 20S proteasomes from human erythrocytes were equilibrated in TEAD buffer (20 mM TRIS/HCl, pH 7.5, 1 mM EDTA, 1 mM sodium azide, 1 mM dithiothreitol containing 0.8 M guanidinium chloride to obtain an affinity matrix stable at the acidic elution conditions. These conditions caused partial dissociation of the 20S proteasome in a subunit mixture with an apparent molecular mass of 45 kDa. From this mixture, the main fraction was separated by gel filtration on Sephacryl S‐200 in the same buffer. After dialysis in coupling buffer (0.1 M carbonate, pH 8.2, 0.1% Tween 20, 0.8 M guanidinium chloride), the subunit mixture was immobilised on BrCN‐activated Sepharose (Amersham Pharmacia Biotech) according to the manufacturer's standard procedure (6 ml protein solution at 0.8 g/l concentration was incubated with 6 ml swollen gel).
Immunofluorescence
To determine the anti‐proteasome pattern, HEp‐2 cells (human larynx carcinoma cell line; Bios), Saos‐2 cells (human osteogenic sarcoma cell line) and HeLa‐cells (human cervix epitheloid carcinoma cell line) were used. Cultivated cells were immobilised, fixed and permeabilised as previously described.22 Cells were blocked with 1% bovine serum albumin for 3 min before incubation. The fixed cells were incubated with the purified antibodies at a concentration of 50 μg/μl for 60 min. After being washed, the cells were incubated with a secondary rabbit anti‐human IgG‐Fc FITC (The Binding Site) or tetramethylrhodamine isothiocyanate‐labelled (tetramethylrhodamine isothiocyanate) antibodies (Biogenes, Berlin, Germany) in dilutions ranging from 1:200 to 1:500.
Reference antibodies in immunofluorescence
The monoclonal antibodies against native proteasomes HP810 and HP903 (FITC) were generated according to standard procedures (Biogenes),24 and used in a dilution of 1:80 to 1:160. Both monoclonal antibodies HP810 and HP903 recognise native 20S proteasome, and express no reactivity against denaturated proteasomal subunits in SDS‐PAGE. Therefore, determination of the target subunit is not possible by immunoblotting. The specificity of the monoclonal antibodies was tested by immunoprecipitation of proteasomes from [35S]‐methionine‐labelled cells (not shown). The monoclonal mouse antibody to proteasomal subunit C2 (α6) was a gift from K Tanaka (The Tokyo Metropolitan Institute of Medical Science, Bunkyo‐Ku, Japan). The polyclonal anti‐proteasome rabbit antibody MP3 has been previously described.4
For colocalisation analysis, antibodies to different marker proteins were first incubated with HEp‐2 cells (Bios): polyclonal rabbit antibody to the endoplasmic reticulum‐specific proteins TAP1 and TAP2 (BIOCOR, Yardley, Pennsylvania, USA), a monoclonal mouse antibody to the mitochondrial heat shock protein hsp60 (Stressgen Biotechnologies, Victoria, Canada), a human anti‐mitochondrial antibody (AMA‐M2) positive serum and a purified human anti‐proteasomal antibody. After a washing step, the corresponding secondary antibodies were incubated: porcine anti‐rabbit IgG‐Fc antibody TRITC (Dako, Glostrup, Denmark) used in a dilution of 1:20, anti‐mouse IgG‐Fc antibody FITC in a dilution of 1:100, anti‐mouse IgG‐Fc Cy3 in a dilution of 1:200 or rabbit anti‐human IgG‐Fc TRITC (Biogenes) in a dilution of 1:500. Unspecific background reactivities of the secondary antibodies were excluded. After the next washing step, double stains were performed using direct FITC‐labelled monoclonal antibodies to native proteasomes HP903 (Biogenes) in a dilution of 1:160.
Competition of the antibodies
20S proteasomes from human erythrocytes were added to the affinity‐purified antibodies from human sera and to the reference antibody (polyclonal anti‐proteasome rabbit antibody MP3) in a calculated molar ratio of 2:1 (6 μg of proteasome on 1 μg of IgG). After incubation for 1 h, the samples were centrifuged for 10 min at 12 000 rpm. HEp‐2 cells were incubated with the depleted supernatants and with the same antibody solutions without competition.
The staining was carried out according to the procedures described for determination of the anti‐proteasomal pattern by immunofluorescence. The emission of fluorescein was measured in a standard field, on each cell stain in several sections. The average residual emission was compared with the reference stain to calculate a factor of inhibition. The optical appearance of the cells incubated with competing antibodies was compared with the pattern of the referring antibody.
Results
Determination of anti‐proteasome fluorescence pattern
The purified human anti‐proteasome antibodies were used in standard IIF on HEp‐2 cells (fig 1A,C). The nuclear stain appeared as a fine speckled pattern with fluctuating intensity in the nucleus, sparing the nucleoli. The cytoplasmic pattern showed filament stains and, to a varying extent, fine speckles extending around the nucleus and throughout the cell in relation to the nuclear envelope. Although all purified anti‐proteasomal antibodies showed the same characteristics, the ANA pattern of these sera differed remarkably, with the exception of patient 7 (fig 1B, D). The images were compared with stains of a monoclonal anti‐proteasome antibody (fig 1E) and with a polyclonal anti‐proteasome antibody (fig 1F). On HEp‐2 cells, all human, mouse and rabbit‐derived anti‐proteasome antibodies showed similar characteristics. Stains on Saos‐2 and HeLa cells showed cell cycle‐dependent nuclear staining with varying intensity and constant fine speckles in the cytoplasm, whereas cytoskeletal structures were less apparent (not shown).
Figure 1 Immunofluorescence patterns of anti‐proteasome antibodies on HEp‐2 cells. Representative stains of purified anti‐proteasome antibodies from patients 3 and 2 (A,C) compared with their corresponding anti‐nuclear antibody (ANA) pattern (B,D). Patterns of monoclonal mouse anti‐proteasomal C2 (α6) antibody (E) and polyclonal rabbit anti‐proteasome antibody MP3 on HEp‐2 cells (F) are shown as reference.
Surprisingly, obvious similarities were identified between the cytoplasmic pattern of anti‐proteasomal antibodies and the filamentous pattern of anti‐mitochondrial antibodies (AMA) on HEp‐2 cells. To clarify this observation, double staining was performed by using affinity‐purified anti‐proteasome antibodies as well as in comparison with an AMA‐M2‐positive human serum and a monoclonal antibody against the mitochondrial heat shock protein hsp60 (fig 2). As a result, a similar distribution was confirmed in the extranuclear compartment. However, the appearance of the proteasome was less granular and rather fine speckled as compared with the AMA pattern. Furthermore, double staining was also performed with antibodies against the endoplasmic reticulum‐specific proteins, TAP1 and TAP2, revealing a partial colocalisation as expected (fig 2).
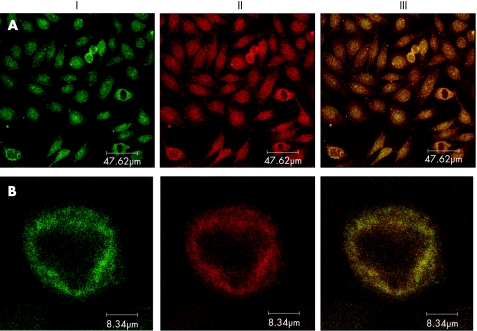
Figure 2 Double stains by directly fluorescein isothiocyanate‐labelled anti‐proteasome antibody HP903 (I) with tetrarhodamine isothiocyanate or Cy3‐labelled antibodies against (II) various cell structures (anti‐TAP1/TAP2 antibody, anti‐AMA M2 serum, anti‐hsp60 antibody and human affinity purified anti‐proteasomal antibody) and the superimposition of both stains (III) on HEp‐2‐cells.
Specificity of anti‐proteasome antibodies
Double staining was performed with anti‐proteasome antibodies of different binding properties to verify the specificity of anti‐proteosome antibodies. This made individual staining characteristics of the different antibodies more obvious. As expected, double stains with the monoclonal mouse antibodies HP810 and HP903 showed perfectly overlapping patterns (not shown). Double stains with the monoclonal mouse antibody HP903 and the polyclonal rabbit antibody MP3 showed a colocalisation as well, but the polyclonal rabbit antibody stained an additional filamentous cytoplasmatic structure. By using conventional immunofluorescence (fig 2) and moreover confocal microscopy for enhanced resolution (fig 3), double stains of affinity‐purified human anti‐proteasome antibodies (II) and monoclonal anti‐proteasome antibodies (I) showed a clear colocalising pattern.
Figure 3 Double stains in confocal microscopy (DM IRE2, Leica, Germany) by direct fluorescein isothiocyanate‐labelled anti‐proteasome antibody HP903 (I) and tetrarhodamine isothiocyanate‐labelled human affinity‐purified anti‐proteasomal antibody (II) were superimposed (III) on HEp‐2 cells; as an overview (A) and on the single‐cell magnification (B).
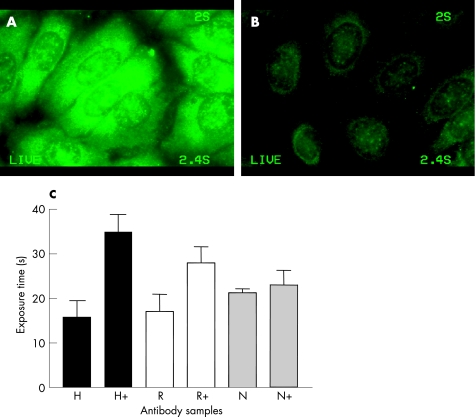
To exclude an unspecific cross‐reaction of the anti‐proteasome antibodies, competition experiments were performed with intact 20S proteasomes before staining. The respective antibodies with and without antigen depletion were used in the same dilution (fig. 4). Pre‐incubation with the antigen resulted in a clear reduction of emission of purified human anti‐proteasome antibodies, with an average of 46% (fig 4B). The reference polyclonal and monoclonal (R) antibodies showed an average reduction of 38% and the negative control (N) of 7% (fig 4C).
Figure 4 Specific competition of purified human anti‐proteasome antibodies in indirect immunofluorescence on HEp‐2 cells. Purified anti‐proteasome antibody and a fluorescein isothiocyanate‐labelled anti‐human secondary antibody (A) in comparison with the same antibodies in the same concentrations blocked by pre‐incubation with native 20S proteasomes (B). (C) Photometric analysis of all examined human (H) and reference anti‐proteasome antibodies (R) showed a similar degree of inhibition of the blocked (+) in comparison with non‐blocked antibodies according to a prolonged exposure time for equal emission. The negative control (N) showed virtually no change in luminescence.
Discussion
In this study, anti‐proteasome autoantibodies were purified by affinity from sera and plasma samples of patients with different systemic autoimmune disorders. As shown by immunofluorescence experiments, anti‐proteasomal antibodies represent a novel identified specificity of ANA. The specificity of these antibodies was confirmed by comparing the immunofluorescence patterns with reference antibodies and by competition experiments with isolated native 20S proteasomes. Moreover, colocalisations for the pattern of human anti‐proteasome and reference antibodies was shown using confocal microscopy.
Anti‐proteasome antibodies were used for detailed immune fluorescence analyses to extend interpretation of known ANA patterns and to evaluate interference. As a result, the patterns obtained by affinity‐purified antibodies were different from those of complete patient sera with the exception of patient 7, because only that serum contained no other autoantibodies. In our opinion, it was not possible to reliably identify a specific pattern of anti‐proteasome autoantibodies by IIF, owing to the complex distribution and colocalisation of the antigen.26 Therefore, to confirm anti‐proteasome reactivity, additional analyses are necessary, for instance, by using ELISA or immunoblotting. Of particular importance for the ANA diagnostics is the fact that anti‐proteasome antibodies interfere to some extent with other known autoantibody patterns in IIF on HEp‐2 cells (especially in the cytoplasm, for instance, against endoplasmic reticulum or mitochondria).
We confirmed the cytoplasm, the outside of the endoplasmic reticulum and of the nuclear envelope, and the nucleus to be potential localisation sites of proteasomes. Other subcellular structures could not be defined owing to optical limits. A nucleolar fluorescence was observed only with the monoclonal antibodies HP810, HP903 and anti‐C2. By contrast, the human affinity‐purified anti‐proteasome antibodies showed no or only a weak nucleolar background fluorescence. The various cells, cultured and fixed according to the normal standard protocols, showed different staining features in the nucleus and cytoplasm depending on cell growth and nutritional conditions. This suggests that the distribution of proteasomes is dependent on the cell type and cell cycle, where it may reflect changes in cell metabolism. Colocalising stains showed that the relative affinity towards nuclear and cytosolic proteasomes seems to vary between different anti‐proteasome antibodies, possibly owing to different affinities towards the proteasomes, different structural properties of proteasomes—that is, different subtypes26—or different interactions with regulatory proteins in the nucleus and cytoplasm. This possibly indicates different subsets of proteasomes in a single cell corresponding to the isolated proteasome subtypes.23
In this study, we showed that anti‐proteasome autoantibodies have to be taken into account in the screening of patients' sera in ANA diagnostics. Anti‐proteasome antibodies largely contribute to the overall autoantibody spectrum, especially in patients with connective tissue diseases.2,3,4 Their presence seems to be an epiphenomenon of an autoimmune or inflammatory process, probably induced by a breakdown of the tolerance barrier owing to greatly increased levels of circulating antigens.24 However, because of the specific interaction, the antibodies could take part in the clearance of the released proteasomes in vivo. In this context, it still remains uncertain whether anti‐proteasome antibodies are involved in the pathogenesis of autoimmune diseases or not.
Acknowledgements
We thank K Tanaka (The Tokyo Metropolitan Institute of Medical Science, Bunkyo‐Ku, Japan) for the generous gift of the monoclonal anti‐C2 antibody and Peter M Kloetzel (Institute of Biochemistry, Charité‐Universitätsmedizin Berlin, Berlin, Germany) for providing laboratory space and for his support.
Abbreviations
ANA - anti‐nuclear antibody
FITC - fluorescein isothiocyanate
IIF - indirect immunofluorescence
PA28 - proteasome activator
PAGE - polyacrylamide gel electrophoresis
TAP - transporter associated with antigen processing
Footnotes
Funding: This study was supported by the Kompetenznetz‐Rheuma C2.3 of the German Ministry of Education and Science, and by grants from Deutsche Forschungsgemeinschaft (SFB421/B13 and DFG Ku1261).
Competing interests: None declared.
References
- 1.Casiano C A, Tan E M. Recent developments in the understanding of antinuclear autoantibodies. Int Arch Allergy Immunol 1996111308–313. [DOI] [PubMed] [Google Scholar]
- 2.Arribas J, Luz Rodriguez M, Alvarez Do Forno R, Castano J G. Autoantibodies against the multicatalytic proteinase in patients with systemic lupus erythematosus. J Exp Med 1991173423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feist E, Dörner T, Kuckelkorn U, Schmidtke G, Micheel B, Hiepe F.et al The proteasome α‐type subunit C9 is a primary target of autoantibodies in sera of patients with myositis and systemic lupus erythematosus. J Exp Med 19961841313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feist E, Kuckelkorn U, Dörner T, Dönitz H, Scheffler S, Hiepe F.et al Autoantibodies in primary Sjögren's syndrome are directed against proteasomal subunits of the α‐ and ß‐type. Arthritis Rheum 199942697–702. [DOI] [PubMed] [Google Scholar]
- 5.Rivett‐AJ Proteasomes: multicatalytic proteinase complexes. Biochem J 19932911–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rechsteiner‐M, Hoffman‐L, Dubiel‐W The multicatalytic and 26 S proteases. J Biol Chem 19932686065–6068. [PubMed] [Google Scholar]
- 7.Coux O, Tanaka K, Goldberg A L. Structure and function of the 20S and 26S proteasomes. Annu Rev Biochem 199665801–847. [DOI] [PubMed] [Google Scholar]
- 8.Seemüller E, Lupas A, Stock D, Löwe J, Huber R, Baumeister W. Proteasome from Thermoplasma acidophilum: a threonine protease. Science 1995268579–582. [DOI] [PubMed] [Google Scholar]
- 9.Rammensee H G, Falk K, Rötzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol 199311213–244. [DOI] [PubMed] [Google Scholar]
- 10.Rechsteiner M, Hill C P. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol 20051527–33. [DOI] [PubMed] [Google Scholar]
- 11.Groettrup M, Soza A, Kuckelkorn U, Kloetzel P M. Peptide antigen production by the proteasome: complexity provides efficiency. Immunol Today 199617429–435. [DOI] [PubMed] [Google Scholar]
- 12.Machiels B M, Henfling M E, Broers J L, Hendil K B, Ramekers F C. Changes in immunocytochemical detectability of proteasome epitopes depending on cell growth and fixation conditions of lung cancer cell lines. Eur J Cell Biol 199566282–292. [PubMed] [Google Scholar]
- 13.Palmer A, Rivett A J, Thomson S, Hendil K B, Butcher G W, Fuertes G.et al Subpopulations of proteasomes in rat liver nuclei, microsomes and cytosol. Biochem J 1996316401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reits E, Benham A M, Plougastel B, Neefjes J, Trowsdale J. Dynamics of proteasome distribution in living cells. EMBOJ 1997166087–6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wigley C, Fabunmi R P, Lee M G, Marino C R, Muallem S, DeMartino G .et al Dynamic association of oroteasomal machinery with the centrosome. J Cell Biol 1999145481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arcangeletti C, Olink‐Coux A, Minisini R, Huesca M, Chezzi C, Scherrer K. Patterns of cytodistribuation of prosomal antigens on vimentin and cytokeratin networks of monkey kidney cells. Eur J Cell Biol 199259464–476. [PubMed] [Google Scholar]
- 17.DeConto F, Missorini S, Arcangeletti C, Pinardi F, Montarras D, Pinset C.et al Prosome cytodistribution relative to desmin and actin filaments in dividing C2.7 myoblasts and during myotube formation in vitro. Exp Cell Res 197923399–117. [DOI] [PubMed] [Google Scholar]
- 18.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 19.Bohan A, Peter J B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975292344–347. [DOI] [PubMed] [Google Scholar]
- 20.Bohan A, Peter J B. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975292403–407. [DOI] [PubMed] [Google Scholar]
- 21.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos H M, Alexander E L, Carsons S E.et al European study group on classification criteria for Sjogren's syndrome. classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American‐European consensus group. Ann Rheum Dis 200261554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuckelkorn U, Knuehl C, Boes‐Fabian B, Drung I, Kloetzel P M. The effect of heat shock on 20S/26S proteasomes. Biol Chem 20003811017–1023. [DOI] [PubMed] [Google Scholar]
- 23.Dahlmann B, Ruppert T, Kuehn L, Merforth S, Kloetzel P M. Different proteasome subtypes in a single tissue exhibit different enzymatic properties. J Mol Biol 2000303643–653. [DOI] [PubMed] [Google Scholar]
- 24.Egerer K, Kuckelkorn U, Rudolph P E, Ruckert J C, Dorner T, Burmester G R.et al Circulating proteasomes are markers of cell damage and immunologic activity in autoimmune diseases. J Rheumatol 2002292045–2052. [PubMed] [Google Scholar]
- 25.Brychcy M, Kuckelkorn U, Hausdorf1 G, Egerer K, Kloetzel P‐M, Burmester G‐R, et al. Anti‐20S proteasome autoantibodies inhibit the proteasome stimulation by proteasome activator (PA28). Arthritis Rheum 200654 pp 2175-83 Erratum in: Arthritis Rheum 2006542702. [DOI] [PubMed] [Google Scholar]
- 26.Dino Rockel T, von Mikecz A. Proteasome‐dependent processing of nuclear proteins is correlated with their subnuclear localization. J Struct Biol 2002140189–199. [DOI] [PubMed] [Google Scholar]