Abstract
The first cases of infection caused by avian metapneumoviruses (aMPVs) were described in turkeys with respiratory disease in South Africa during 1978. The causative agent was isolated and identified as a pneumovirus in 1986. aMPVs have been detected in domestic nonpoultry species in Europe, but tests for the detection of these viruses are not available in the United States. To begin to understand the potential role of domestic ducks and geese and wild waterfowl in the epidemiology of aMPV, we have developed and evaluated a blocking enzyme-linked immunosorbent assay (bELISA) for the detection of aMPV type C (aMPV-C)-specific antibodies. This assay method overcomes the species-specific platform of indirect ELISAs to allow detection of aMPV-C-specific antibodies from potentially any avian species. The bELISA was initially tested with experimental turkey serum samples, and the results were found to correlate with those of virus neutralization assays and indirect enzyme-linked immunosorbent assay (iELISA). One thousand serum samples from turkey flocks in Minnesota were evaluated by our bELISA, and the level of agreement of the results of the bELISA and those of the iELISA was 94.9%. In addition, we were able to show that the bELISA could detect aMPV-C-specific antibodies from experimentally infected ducks, indicating its usefulness for the screening of serum samples from multiple avian species. This is the first diagnostic assay for the detection of aMPV-C-specific antibodies from multiple avian species in the United States.
Avian metapneumoviruses (aMPVs) belong to the family Paramyxoviridae, subfamily Pneumovirinae, and genus Metapneumovirus (19). aMPVs cause an acute upper respiratory disease characterized by coughing, nasal discharge, tracheal rales, foamy conjunctivitis, and sinusitis that have been reported principally in turkeys; but cases have been identified in chickens, ducks, pheasants, and guinea fowl (3, 7, 9, 21). The first cases were described in turkeys in South Africa in 1978, and the causative agent was isolated and identified as a pneumovirus in 1986 in Europe (4, 14, 17). Since its initial identification in Europe, the virus has spread throughout most of Europe, Japan, and South America (1, 7, 25). In 1997, the first aMPV was isolated from commercial turkeys in Colorado with a respiratory disease, and this strain was found to differ from previous aMPV isolates (20; R. K. Edson, Proc. 101st Annu. Meet. U.S. Anim. Health Assoc., p. 471-472, 1997).
Identification of aMPV infection in turkey flocks routinely involves serology, reverse transcriptase PCR, and virus isolation assays (7, 10). Reverse transcriptase PCR and virus isolation are generally labor-intensive, expensive, and dependent on the duration of virus replication in the animal, which usually ends before clinical signs develop (16). Serologic evidence of infection is present long after infection (7). The ability to use serum to determine present or past infection increases the possibility of discovering whether birds have been exposed to aMPV, while serum can be used for serologic testing, which is to easy to perform and inexpensive.
aMPVs have been tentatively designated type A, B, C, or D on the basis of virus neutralization and sequence analysis (6, 7, 15). Type A and B viruses are found in Europe, Japan, and South and Central America; type D is found in France; and type C is found only in the United States (1, 3, 7, 25; Edson, Proc. 101st Annu. Meet. U.S. Animal Health Assoc., p. 471-472, 1997). Due to differences in the amino acid sequences among the different types, serologic tests do not cross-react among all subtypes (7).
Many enzyme-linked immunosorbent assays (ELISAs) have been developed for the detection of antibodies to aMPV. The ELISAs for aMPV type C (aMPV-C) available in the United States use whole virus prepared from lysed cell culture as an antigen and depend on anti-turkey or anti-chicken secondary antibodies for virus detection (5). On the basis of the results of these assays, aMPV infections in the United States are detected only in Minnesota. In 1999, 37% of turkey flocks in Minnesota were positive for aMPV antibodies by ELISA, while 48.7% were positive in 2000 (5, 12). Additionally, Gulati et al. (12, 13) have developed two recombinant ELISAs using the matrix or nucleocapsid protein as the antigen for the detection of antibodies to aMPV-C. Although these ELISAs are sensitive and specific, they can detect antibodies only in samples from turkeys and chickens.
aMPV has been reported in farm-reared pheasants, ducks, and guinea fowl outside of the United States (7, 9, 24, 27). Much of the research in the United States has focused on turkey and wild birds, while little attention has been focused on farm-raised ducks and geese. The presence of the virus in experimentally infected ducks and the recent isolation of aMPV from wild geese demonstrate that these birds may also harbor the virus (21, 22). A quick and inexpensive test is needed to determine the infection status of domestic geese and ducks. Such a test could also be used to determine the infection status of other poultry or wild birds. To resolve this shortfall and to better understand the epidemiology of aMPV, we developed a blocking ELISA (bELISA) for the detection of aMPV-C-specific antibodies in a broader range of hosts.
MATERIALS AND METHODS
Virus purification.
The aMPV-C isolate used in this study, aMPV/turkey/Colorado/97 (aMPV/CO), was obtained from the National Veterinary Service Laboratory, Ames, Iowa. The virus was originally isolated from commercial turkeys in Colorado with respiratory signs (18). Vero cells were inoculated with aMPV/CO and frozen at −70°C at 72 h postinfection. Infected cells were subjected to three rounds of freezing-thawing. The virus was inactivated by treatment with 0.1% β-propiolactone (Sigma, St. Louis, Mo.) at room temperature for 2 h, followed by refrigeration overnight. Cell debris was removed by clarification by centrifugation at 10,000 × g for 30 min at 4°C. The virus was pelleted by ultracentrifugation at 80,000 × g for 2 h and resuspended in 2 M Tris buffer (pH 8.8) and then centrifuged (76,000 × g for 2 h) through a sucrose cushion of 20 and 60% (wt/vol). The band was collected and diluted in Tris buffer, overlaid on a sucrose gradient (20 to 70%), and centrifuged at 76,000 × g for 2 h (11). The virus band was collected and resuspended in a 1:6 volume of Tris buffer and pelleted as described above. The purified virus was rinsed to remove the sucrose and resuspended in Tris buffer to a concentration of approximately 1 mg/ml. The protein concentration was determined with the BCA Protein Assay kit (Pierce, Rockford, Ill.). This purified product was used as antigen for antibody production and as antigen for both the indirect ELISA (iELISA) and the bELISA.
aMPV antibody production.
The Polyclonal Antibody Production Service, University of Georgia, Athens, Ga., prepared the aMPV-C antiserum. New Zealand White rabbits received 0.5 ml (225 μg) of inactivated purified aMPV-C antigen with Freund's complete adjuvant. Subsequent immunizations consisting of Freund's incomplete adjuvant and 225 μg of antigen were given at 21 and 58 days. Rabbits were bled at 91 days after the initial immunization. Immunoglobulins were purified from total serum proteins by using a T-Gel Purification kit (Pierce). The purified rabbit polyclonal antibody samples were then directly conjugated to horseradish peroxidase (HRP) by using an EZ-Link Activated Peroxidase Antibody Labeling kit (Pierce). The rabbit aMPV antibody conjugated to HRP has been tested by ELISA and Western blotting analysis and does not react with noninfected Vero cells or other avian viruses.
Serum samples. (i) Turkey hyperimmune serum.
Eleven 4-week-old specific-pathogen-free Small Beltsville White turkeys from an in-house flock received 1 ml of β-propiolactone-inactivated aMPV/CO (40 μg) in an oil emulsion as a vaccine or a sham control preparation by subcutaneous injection in the back of the neck. The turkeys were vaccinated again at 2 weeks and were bled at 28 days after the initial inoculation.
(ii) Turkey convalescent-phase serum.
Turkeys were obtained from British United Turkeys of America (Lewisburg, W. Va.). At 2 weeks of age, the turkey poults were inoculated via the intranasal route with live aMPV/CO (104.5 50% tissue culture infective doses [TCID50s] per ml), Escherichia coli (107 CFU/ml), or Newcastle disease virus (NDV; 105 50% egg infectious doses/ml) or were sham inoculated as described previously (26). Serum samples were collected at days 0, 8, and 14 postinoculation.
(iii) Field serum samples.
A total of 1,000 individually labeled turkey serum samples were obtained from the Minnesota Poultry Testing Laboratory (MPTL), Willmar, Minn. The serum samples were individually labeled and tested by both the bELISA and the diagnostic iELISA (described below).
Experimental infection of Pekin ducks.
Specific-pathogen-free Pekin duck eggs were obtained from Cornell University, Ithaca, N.Y. Once the ducks hatched, the ducks were housed in stainless steel isolation cabinets in negative-pressure isolation rooms within a biosafety level 3 agriculture facility at Southeast Poultry Research Laboratory, Athens, Ga. The ducks were inoculated at 2 weeks of age with 103.4 TCID50s of aMPV/CO in a 0.2-ml dose via the intranasal route. Serum samples were taken at 0 and 14 days postinoculation.
VN assay.
The virus neutralization (VN) assay was preformed in replicates of four. Each serum sample was assayed on Vero cells in a 96-well flat-bottom tissue culture plate. Test serum, diluted 1:2 to 1:512, was added to 100 TCID50s of aMPV/CO, and the mixture was incubated for 30 min at 37°C. After incubation, 50 μl was inoculated onto Vero cell monolayers, and the mixture was incubated for 30 min. This was followed by the addition of 150 μl of minimum essential medium with 3% fetal bovine serum. The cell monolayers were then monitored for cytopathic effects (23).
iELISA.
Two iELISAs were used in this study. An iELISA was developed at the Southeast Poultry Research Laboratory to test experimental samples as described previously (26). Briefly, ELISA plates were coated with 2 μg of purified aMPV/CO per ml and incubated overnight. After blocking of the plates, test serum was added at a starting dilution of 1:25, followed by the addition of fivefold dilutions. An HRP-conjugated goat anti-turkey immunoglobulin G (IgG) antibody (Southern Biotech, Birmingham, Ala.) was added to detect bound antibodies. Serum samples with optical densities (OD) of 3 standard deviations above the ODs for the negative controls were considered positive for aMPV antibodies. Each plate contained multiple positive and negative serum samples to serve as controls. The second iELISA used in this study was the diagnostic iELISA presently used at the Minnesota Poultry Testing Laboratory (5).
bELISA.
For the bELISA, Immulon 4HBX (Dynex, Chantilly, Va.) plates were coated with 2 μg of purified aMPV/CO per ml and incubated overnight at 4°C in 0.1 M bicarbonate coating buffer (pH 9.6). The plates were blocked with 1% polyvinylpyrrolidone for 1 h at room temperature (Sigma). The test serum sample was added at a 1:5 dilution, and the plate was incubated for 1 h at room temperature. The plates were washed with phosphate-buffered saline-Tween 20 (0.05%). After the plate was washed, HRP-conjugated rabbit aMPV/CO antibody was added, and antibodies were detected by using an o-phenylenediamine dihydrochloride substrate. Substrate development was stopped by the addition of 2 M sulfuric acid, and the OD at 490 nm was determined. Serum samples with ODs 3 standard deviations below the ODs for the negative controls were considered positive. The cutoff value of 3 standard deviations (99.7%) was chosen to increase the stringency of the test and to help eliminate potential false-positive results. Each plate contained multiple positive and negative serum samples as controls. Noninoculated Vero cells and serum samples infected with avian influenza virus (AIV), NDV, and bovine respiratory syncytial virus were included as negative controls. Optimal antigen and rabbit aMPV/CO-HRP antibody concentrations were determined by standard checkerboard titration (8).
Western blotting analysis.
Purified aMPV/CO was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 10% polyacrylamide gel (Bio-Rad, Hercules, Calif.) under reducing conditions and transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline with 0.01% Tween 20 (TBST) for 1 h (2). Serum samples were added at a 1:50 dilution and incubated for 1 h at room temperature or 4°C overnight. The membrane was washed in TBST, incubated with goat anti-turkey IgG-HRP or goat anti-rabbit IgG (Southern Biotech), and detected with enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, N.J.).
Statistics.
Results from the iELISA and the bELISA were tested for correlation by using the Pearson product moment correlation with personal computer-based software (SigmaStat; Jandel Scientific, San Rafael, Calif.).
RESULTS
Experimental turkey samples. (i) Turkey hyperimmune serum.
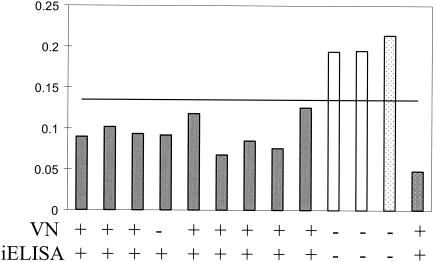
Serum samples from the 11 sham-vaccinated and aMPV-C-vaccinated turkeys were tested for aMPV antibodies by a VN assay and iELISA. Sera from eight of the nine aMPV-C-vaccinated turkeys were found to have neutralizing antibodies to aMPV/CO, as detected by the VN assay (Fig. 1). Sera from all nine of the aMPV-C-vaccinated turkeys had detectible antibodies to aMPV/CO, as determined by both the iELISA and the bELISA. No aMPV-C-specific antibodies were detected in the sera from the two sham-vaccinated turkeys by any of the assays.
FIG. 1.
Antibody titers from turkey hyperimmunne serum. The graph shows the ODs from the bELISA. Values below 0.143 represent positivity for antibodies to aMPV. Gray bars, vaccinated turkeys; white bars, control turkeys; dotted bars, positive (gray) and negative (white) controls. The results of the VN assay and the iELISA are presented below the graph. Serum samples were taken 4 weeks after initial vaccination.
(ii) Turkey convalescent-phase serum.
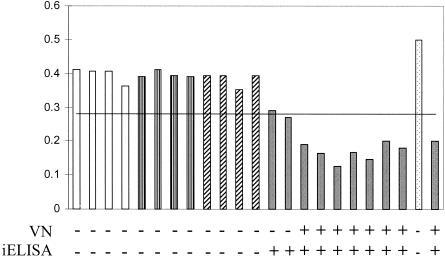
Turkeys from British United Turkeys of America inoculated with live aMPV/CO all developed antibodies to the virus by 15 days postinoculation (Fig. 2). Serum samples from the turkeys inoculated with E. coli, AIV, and NDV showed no activity by the VN assay and were negative by both the iELISA and the bELISA. Although some of the serum samples from the aMPV/CO-inoculated turkeys taken at 8 days postinoculation did have detectable antibodies by the iELISA, neither the bELISA nor the VN assay detected antibodies (data not shown). All aMPV-C-infected birds had detectable antibodies by 14 days postinoculation (Fig. 2).
FIG. 2.
Antibody titers in convalescent-phase turkey serum samples. The graph shows the ODs from the bELISA. Values below 0.284 represent positivity for antibodies to aMPV. White bars, controls; bars with vertical stripes, E. coli-infected serum samples; bars with diagonal stripes, NDV-infected serum samples; gray bars, aMPV-infected serum samples; dotted bars, positive (gray) and negative (white) controls. The results of the VN assay and the iELISA are presented below the graph. Serum samples were taken at 14 days postinoculation.
Field turkey samples. (i) Comparison of ELISAs.
A total of 1,000 serum samples collected from turkeys in the field in Minnesota in 2001 were tested by the diagnostic iELISA and the bELISA (Table 1). Of the 1,000 turkey serum samples tested, 235 were positive for aMPV-C-specific antibodies by both our bELISA and the iELISA, while 714 samples were negative by both tests. A total of 280 turkey serum samples were positive and 720 serum samples were negative by the diagnostic iELISA, while 244 serum samples were positive and 756 serum samples were negative by the bELISA. When the results for the individual samples were compared, the results for 51 of the serum samples were not in agreement, resulting in a 94.9% agreement between the two ELISAs, which is significant (R = 0.871; P < 2.1 × 10−310).
TABLE 1.
Comparison of results of bELISA to those of diagnostic iELISA for 1,000 turkey field serum samples
| iELISA result | No. (%) of serum samples with the following result by bELISA:
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 235 | 42 | 280 |
| Negative | 9 | 714 | 720 |
| Total | 244 | 756 | 51a (5.1) |
Of 100 serum samples evaluated.
(ii) Western blotting analysis.
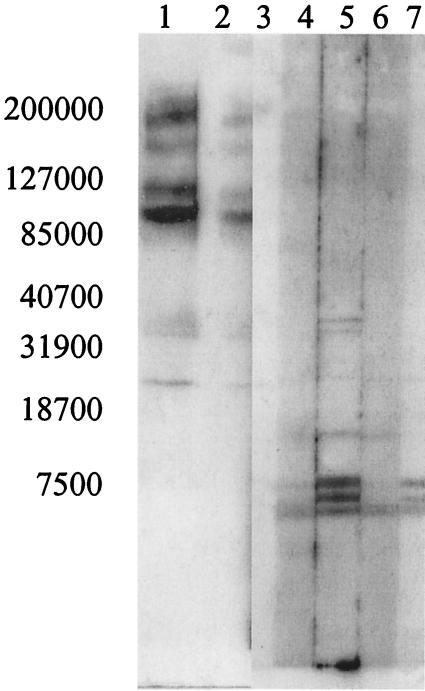
The 51 samples with discrepant results from the direct comparison were further analyzed by Western blotting (Fig. 3). Western blots were used to determine the presence or absence of aMPV-C-specific antibodies in the serum samples with discrepant results. Lanes with bands specific for aMPV-C proteins were considered positive for aMPV-C-specific antibodies. Western blotting analysis determined that 27 of the 50 serum samples tested were positive for aMPV-C-specific antibodies and 23 were negative. One serum sample did not have a sufficient volume for the Western blotting analysis. Three samples had false-positive results and 24 samples had false-negative results by the bELISA, as determined from the results of Western blotting (Table 2). Multiple banding patterns were noted in the field serum samples, indicating that the turkeys have various immunological responses to individual viral proteins (Fig. 3).
FIG. 3.
Western blot of field turkey serum samples. Purified aMPV/CO antigen was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Serum samples were added at a 1:50 dilution and detected with a goat anti-turkey antibody. The Western blot demonstrates the different banding patterns seen for field turkey serum samples with discrepant results between the iELISA and the bELISA (lanes 2, 4, 5, 6, and 7), a positive control (lane 1), and a negative control (lane 3). The numbers on the left are molecular masses (in kilodaltons).
TABLE 2.
Analysis of turkey field serum samples for which the results of in the bELISA and the iELISA disagreeda
| bELISA result | No. (%) of serum samples with the following result by Western blotting:
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 3 | 3 | 6 |
| Negative | 24 | 20 | 44 |
| Total | 27 | 23 | 27b (54) |
The results of Western blotting analysis were compared to those of the bELISA.
Of 50 serum samples tested.
Experimental infection of Pekin ducks.
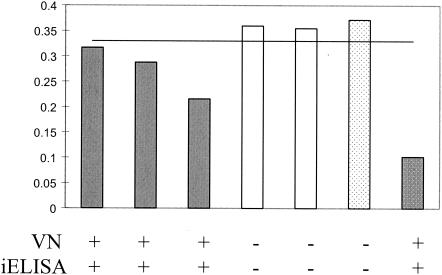
Serum samples from experimentally infected Pekin ducks were tested for the presence of aMPV-C-specific antibodies by a VN assay. The serum samples were then tested by the bELISA. The sera from all inoculated ducks had detectable antibodies to aMPV/CO by 15 days postinoculation, while the sera from the control ducks remained negative throughout the experiment (Fig. 4). The clinical signs of aMPV-C infection observed in the ducks inoculated with live aMPV/CO were limited to increased mucus in the sinus cavity, observed at 2 to 6 days postinoculation (data not shown). The bELISA was able to detect all of the aMPV-C-positive duck serum samples, as confirmed by VN assays (Fig. 4).
FIG. 4.
Antibody titers in experimentally infected ducks. The graph shows the ODs from the bELISA. Values below 0.34 represent positivity for antibodies to aMPV. Gray bars, vaccinated turkeys; white bars, control turkeys; dotted bars, positive (gray) and negative (white) controls. The results of the VN assay and the iELISA are presented below the graph. Serum samples were taken at 14 days postinoculation.
DISCUSSION
Since the appearance of aMPV in the United States in 1997, researchers have speculated that wild birds were a reservoir, harboring and disseminating the virus. Shin et al. (22) detected viral nucleic acids in wild birds captured on or near farms with aMPV-C-infected turkeys in Minnesota. Although the origin of the virus is unknown, their research indicates that the viruses found in turkeys can be isolated from wild birds. This was also demonstrated by Shin et al. (21), in which experimentally inoculated ducks had signs of aMPV-C infection. They detected virus in oral swab specimens and neutralizing antibodies, indicating that aMPV-C can replicate in ducks. The experimental findings from the present study also demonstrate that ducks are able to replicate the virus, as determined by virus reisolation from choanal swab specimens (data not shown), the observation of increased amounts of mucus in the sinus cavities (data not shown), and the production of a measurable antibody response (Fig. 4). In order to more thoroughly identify and understand potential reservoirs of aMPV, such as farm-reared geese and ducks, as well as wild waterfowl, we developed a bELISA that can be used to detect antibodies to aMPV-C regardless of the host species.
Our bELISA was tested and optimized with sera from different experimentally infected turkeys (Figs. 1 and 2). The bELISA did not detect any antibodies in NDV-, AIV-, or E. coli-infected turkeys. The aMPV-C-specific antibody status of the experimental serum samples was first determined by VN assays and an iELISA. All serum samples from infected birds that were positive by VN assays were also found to be positive by the bELISA. The iELISA was able to detect virus in serum samples that were collected from experimentally infected birds at 8 days postinoculation but in which virus was not detected by the bELISA or VN assay. By day 14, antibodies were detected by all assays. This was not surprising, since bELISAs are generally less sensitive than iELISAs (8). To compensate for the reduced sensitivity of the bELISA, we were able to reduce the dilution of the test serum from 1:25 to 1:5 without increasing the background. Even more concentrated serum samples can be tested by the bELISA with minimal increases in background; however, we chose a dilution of 1:5 due to the limited total volume for some samples. When the bELISA was tested for specificity, it did not recognize serum antibodies to AIV, NDV, or bovine respiratory syncytial virus.
After the bELISA was used to test the experimental serum samples, the bELISA was used to analyze 1,000 field turkey serum samples collected in 2001 at the Minnesota Poultry Testing Laboratory. The results of the bELISA and the diagnostic iELISA had a high level of agreement (94.9%). When the results of the two tests were compared, the results for only 51 of the 1,000 samples tested differed. The serum samples whose results differed between the two ELISAs were analyzed by Western blotting. Of these, 27 were positive and 23 were negative for aMPV antibodies. When the results of the bELISA were compared with those of the diagnostic iELISA and Western blotting, the bELISA was able to correctly identify the presence or absence of aMPV antibodies in 97.3% of the samples, demonstrating a high level of sensitivity comparable to that of the diagnostic assay used at present. The bELISA did have a slight increase in the rate of false-negative results compared with the rate for the diagnostic iELISA, but the bELISA has the advantage of being able to test samples from multiple avian species.
To demonstrate that this assay can effectively and accurately detect aMPV-C-specific antibodies from non-turkey serum samples, we used the bELISA to test serum from experimentally infected Pekin ducks. We found that the bELISA was just as effective at detecting aMPV-C-specific antibodies from duck sera as it was at detecting aMPV-C-specific antibodies from turkey sera (Fig. 1, 2, and 4). The bELISA was slightly less sensitive than the iELISA at detecting aMPV-C-specific antibodies, but it was more sensitive than the VN assay. The ability of the bELISA to detect aMPV-C-specific antibodies in both turkeys and ducks, along with its demonstrated specificity, supports the potential usefulness of the bELISA for the detection of aMPV-C-specific antibodies in any avian serum sample. Because aMPV continues to cause problems in the United States, research is needed to determine the extent of aMPV infection in domestic poultry, domestic geese, and ducks, as well as the extent of infection in wild birds.
Acknowledgments
We acknowledge the efforts of Cam Green and the Polyclonal Antibody Production Service, University of Georgia, for assistance with aMPV-C-specific antibody production and labeling. We also acknowledge Lucinda Dahlberg, Minnesota Poultry Testing Laboratory, for efforts with the collection, organization, and identification of field serum samples.
This research was supported by USDA/ARS CRIS project 6612-32000-022-00D.
REFERENCES
- 1.Alexander, D. J. 1997. Newcastle disease and other avian Paramyxoviridae infections, p. 541-569. In B. W. Calnek, H. J. Barnes, C. W. Beard, L. R. McDougall, and Y. M. Saif (ed.), Diseases of poultry. Iowa State University Press, Ames.
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhh. 1997. Current protocols in molecular biology. John Wiley & Sons Ltd., West Sussex, England.
- 3.Bayon-Auboyer, M. H., C. Arnauld, D. Toquin, and N. Eterradossi. 2000. Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subgroup. J. Gen. Virol. 81:2723-2733. [DOI] [PubMed] [Google Scholar]
- 4.Buys, S. B., and J. H. Du Preez. 1980. A preliminary report on the isolation of a virus causing sinusitis in turkeys in South Africa and attempts to attenuate the virus. Turkeys 28:36. [Google Scholar]
- 5.Chiang, S., A. M. Dar, S. M. Goyal, M. A. Sheikh, J. C. Pedersen, B. Panigrahy, D. Senne, D. A. Halvorson, K. V. Nagaraja, and V. Kapur. 2000. A modified enzyme-linked immunosorbent assay for the detection of avian pneumovirus antibodies. J. Vet. Diagn. Investig. 12:381-384. [DOI] [PubMed] [Google Scholar]
- 6.Collins, M. S., R. E. Gough, and D. J. Alexander. 1993. Antigenic differentiation of avian pneumovirus isolates using polyclonal antisera and mouse monoclonal antibodies. Avian Pathol. 22:469-479. [DOI] [PubMed] [Google Scholar]
- 7.Cook, J. K. 2000. Avian pneumovirus infections of turkeys and chickens. Vet. J. 160:118-125. [DOI] [PubMed] [Google Scholar]
- 8.Crowther, J. R. 2001. Theoretical considerations, p. 115-152. In The ELISA guidebook. Humana Press, Totowa, N.J.
- 9.Gough, R. E., M. S. Collins, W. J. Cox, and N. J. Chettle. 1988. Experimental infection of turkeys, chickens, ducks, geese, guinea fowl, pheasants and pigeons with turkey rhinotracheitis virus. Vet. Rec. 123:58-59. [DOI] [PubMed] [Google Scholar]
- 10.Goyal, S. M., S. J. Chiang, A. M. Dar, K. V. Nagaraja, D. P. Shaw, D. A. Halvorson, and V. Kapur. 2000. Isolation of avian pneumovirus from an outbreak of respiratory illness in Minnesota turkeys. J. Vet. Diagn. Investig. 12:166-168. [DOI] [PubMed] [Google Scholar]
- 11.Guerin, J. L., J. Gelfi, L. Dubois, A. Vuillaume, C. Boucraut-Baralon, and J. L. Pingret. 2000. A novel polyomavirus (goose hemorrhagic polyomavirus) is the agent of hemorrhagic nephritis enteritis of geese. J. Virol. 74:4523-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulati, B. R., K. T. Cameron, B. S. Seal, S. M. Goyal, D. A. Halvorson, and M. K. Njenga. 2000. Development of a highly sensitive and specific enzyme-linked immunosorbent assay based on recombinant matrix protein for detection of avian pneumovirus antibodies. J. Clin. Microbiol. 38:4010-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulati, B. R., S. Munir, D. P. Patnayak, S. M. Goyal, and V. Kapur. 2001. Detection of antibodies to U. S. isolates of avian pneumovirus by a recombinant nucleocapsid protein-based sandwich enzyme-linked immunosorbent assay. J. Clin. Microbiol. 39:2967-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, R. C., C. Baxter-Jones, G. P. Wilding, and D. F. Kelly. 1986. Demonstration of a candidate virus for turkey rhinotracheitis in experimentally inoculated turkeys. Vet. Rec. 119:599-600. [PubMed] [Google Scholar]
- 15.Juhasz, K., and A. J. Easton. 1994. Extensive sequence variation in the attachment (G) protein gene of avian pneumovirus: evidence for two distinct subgroups. J. Gen. Virol. 75(Pt 11):2873-2880. [DOI] [PubMed] [Google Scholar]
- 16.Mase, M., S. Asahi, K. Imai, K. Nakamura, and S. Yamaguchi. 1996. Detection of turkey rhinotracheitis virus from chickens with swollen head syndrome by reverse transcriptase-polymerase chain reaction (RT- PCR). J. Vet. Med. Sci. 58:359-361. [DOI] [PubMed] [Google Scholar]
- 17.McDougall, J. S., and J. K. Cook. 1986. Turkey rhinotracheitis: preliminary investigations. Vet. Rec. 118:206-207. [DOI] [PubMed] [Google Scholar]
- 18.Panigrahy, B., D. A. Senne, J. C. Pedersen, T. Gidlewski, and R. K. Edson. 2000. Experimental and serologic observations on avian pneumovirus (APV/turkey/Colorado/97) infection in turkeys. Avian Dis. 44:17-22. [PubMed] [Google Scholar]
- 19.Pringle, C. R. 1999. Virus taxonomy—1999. The universal system of virus taxonomy, updated to include the new proposals ratified by the International Committee on Taxonomy of Viruses during 1998. Arch. Virol. 144:421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seal, B. S. 1998. Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains. Virus Res. 58:45-52. [DOI] [PubMed] [Google Scholar]
- 21.Shin, H. J., M. K. Njenga, D. A. Halvorson, D. P. Shaw, and K. V. Nagaraja. 2001. Susceptibility of ducks to avian pneumovirus of turkey origin. Am. J. Vet. Res. 62:991-994. [DOI] [PubMed] [Google Scholar]
- 22.Shin, H. J., M. K. Njenga, B. McComb, D. A. Halvorson, and K. V. Nagaraja. 2000. Avian pneumovirus (APV) RNA from wild and sentinel birds in the United States has genetic homology with RNA from APV isolates from domestic turkeys. J. Clin. Microbiol. 38:4282-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thayer, S., and C. W. Beard. 1998. Serologic procedures, p. 255-266. In D. E. Swayne, J. R. Glisson, M. W. Jackwood, J. E. Pearson, and W. M. Reed (ed.), A laboratory manual for the isolation and identification of avian pathogens. American Association of Avian Pathologists, Kennett Square, Pa.
- 24.Toquin, D., M. H. Bayon-Auboyer, N. Eterradossi, V. Jestin, and H. Morin. 1999. Isolation of a pneumovirus from a Muscovy duck. Vet. Rec. 145:680. [PubMed] [Google Scholar]
- 25.Toro, H., H. Hidalgo, M. Ibanez, and H. M. Hafez. 1998. Serologic evidence of pneumovirus in Chile. Avian Dis. 42:815-817. [PubMed] [Google Scholar]
- 26.Turpin, E. A., L. E. Perkins, and D. E. Swayne. 2002. Experimental infection of turkeys with avian pneumovirus and either Newcastle disease virus or Escherichia coli. Avian Dis. 46:412-422. [DOI] [PubMed] [Google Scholar]
- 27.Welchman, D. B., J. M. Bradbury, D. Cavanagh, and N. J. Aebischer. 2002. Infectious agents associated with respiratory disease in pheasants. Vet. Rec. 150:658-664. [DOI] [PubMed] [Google Scholar]