Psoriatic arthritis (PsA) shares features with the spondyloarthropathy (SpA) concept and with rheumatoid arthritis. Treatment of PsA should target the skin, the SpA‐like features and the rheumatoid arthritis‐like features of the disease. Effects of treatment can be measured by one index covering different axes at once or by evaluating each axis separately.1 In this analysis, we evaluated different composite indices that have been validated in rheumatoid arthritis and compared them with the Psoriatic Arthritis Response Criteria (PsARC).2
The study population consisted of 18 patients with PsA, previously enrolled in a randomised monocentre double‐blind placebo‐controlled study evaluating effect of infliximab in patients with SpA,3 of whom nine received placebo. Patients were evaluated at baseline and at weeks 1, 2, 6, 8 and 12, which included the evaluation of the single components included in the PsARC, the Disease Activity Score using 28 joint counts (DAS28)4 and the DAS response (http://www.das‐score.nl). A modified American College of Rheumatology (mACR) response was calculated by using the Bath Ankylosing Spondylitis Functional Index or the Dougados Functional Index instead of the Health Assessment Questionnaire.4,5,6
Although only 18 patients were included in our study, this was enough to assess the values of the different outcome measures in patients with PsA, as reflected by the considerable differences between groups after treatment. The results of the mACR response were identical whether the Bath Ankylosing Spondylitis Functional Index or the Dougados Functional Index was used. PsARC, mACR20 and DAS28 responses were similarly effective in distinguishing between the two treatment groups (table 1). The mACR and the DAS28 responses can be used as a dichotomous (response yes or no) variable, and also as ordinal variables (0/20/50/70 response for the mACR response and no/moderate/good response for the DAS28). The evaluation of such ordinal categories by a special measure of association (such as the ordinal γ statistic7) adds statistical power (table 1).
Table 1 Baseline characteristics of patients' and comparison of the different outcome measures evaluating the effects of treatment.
| Placebo | Infliximab | Difference between groups | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years)* | 47 (39–63) | 48 (30–65) | NS |
| Men/women† | 4:5 | 5:4 | NS |
| Disease duration (years)* | 5 (3–26) | 5 (1–18) | NS |
| Patient's global assessment VAS* | 47 (24–97) | 66 (42–84) | NS |
| Physician's global assessment VAS* | 65 (51–77) | 65 (60–85) | NS |
| 66 swollen joint count* | 8 (1–17) | 9 (3–14) | NS |
| 28 swollen joint count* | 4 (1–7) | 5 (1–9) | NS |
| 28 tender joint count* | 5 (1–10) | 4 (1–8) | NS |
| ESR (mm/at the end of the first hour)* | 8 (4–43) | 11 (1–26) | NS |
| CRP (mg/dl)* | 0.8 (0.27–1.51) | 0.96 (0–2.41) | NS |
| DAS28 ‡ | 3.78 (1.16) | 4.32 (1.21) | NS |
| HLA B27 positive/negative† | 3/6 | 2/7 | NS |
| Effects of treatment | |||
| mACR no/20/50/70 responders† | 8/0/1/0 | 0/3/2/3 | γ = 0.861 ±0.142 p = 0.002§ |
| mACR20 responders (no/yes)† | 8/1 | 1/8 | OR 28 (95% CI 2.1 to 379) p = 0.015¶ |
| No/moderate/good DAS28 response† | 7/2/0 | 0/1/7 | γ = 0.944 ±0.067 p = 0.001§ |
| Moderate DAS28 response (no/yes)† | 7/2 | 0/8 | OR 28 (95%CI: 2.1 to379) p = 0.015¶ |
| PsARC response: (no/yes)† | 7/2 | 0/9 | p = 0.002¶ |
| Remaining DAS28 score at week 12* | 3.91 (1.48) | 2.06 (0.84) | p = 0.007** |
| Difference in DAS28 score between baseline and week 12* | +0.127 (0.84) p = NS†† | −2.26 (1.33) p = 0.001†† | |
| Area under the curve | −0.95 | −29.31 | p = 0.006‡‡ |
CRP, C reactive protein; ESR, erythrocyte sedimentation rate; HLA, human leucocyte antigen; NS, not significant; VAS, visual analogue scale.
*Values are the median (range); †Values are number of patients in each category; ‡Values are the mean (SD); §Exact γ statistic; ¶Fisher's exact test; **t test; ††Paired sample t test; ‡‡Mann–Whitney U test;
DAS28 as a continuous variable can be evaluated by measuring changes over time or by comparing the remaining disease activity at week 12 between the two treatment arms (table 1). The effect size8 and standardised response mean9 of DAS28 were 1.9 and 1.7, respectively in the infliximab treated group; similar results were observed in patients with PsA and in those with rheumatoid arthritis (personal observations). Profiles over time can be evaluated by comparing areas under the curve (table 1) or by linear mixed‐models analysis. Mixed‐models analysis can correct for small differences in baseline conditions between groups.8 We therefore fitted a general linear mixed model with DAS28 as the outcome variable resulting in a significant effect of the treatment over time, reflected by a significant treatment effect at week 2 (mean = −1.15 v baseline; p = 0.016), week 6 (mean = −2.12 v baseline; p<0.001) and week 12 (mean = −2.39 v baseline; p<0.001), resulting in a highly significant global treatment effect (Fisher's exact test p<0.001).
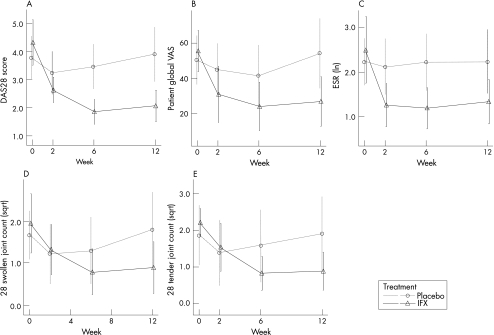
DAS28 and other variables, such as tender or swollen joint counts, can be used as continuous outcome measures, evaluated at different time points. However, as shown in fig 1, DAS28 can distinguish more efficiently between the two treatment groups than among each of its four components alone (less overlap of error bars) or the 66/68 joint counts. This also suggests that the reduction in the number of evaluable joints by the use of a 28‐joint count does not affect the efficacy of the DAS28.
Figure 1 Plots of the Disease Activity Score for the 28‐joint count (DAS28) score and different single measures. (A) Evolution of the DAS28 over time. (B–E) Evolution of the components of the DAS28 over time. (F,G) Evolution of 66 and 68 swollen and tender joint counts over time. There is more overlap of the error bars of the individual components and the 66 and 68 swollen joint counts than the DAS28. ESR, erythrocyte sedimentation rate at first hour; ln, natural logarithm; sqrt, squared root. Error bars show mean (2 SE).
In conclusion, we evaluated different outcome measures and statistical methods related rheumatoid arthritis to measure effects of treatment in patients with PsA. We showed that the different response scores are equally efficient. Also, the DAS28, as a measure of absolute disease activity, can be used as a powerful tool to evaluate effects of treatment in patients with PsA.
Acknowledgements
BVC was supported by a concerted action grant GOA 2001/12051501 from Ghent University, Belgium. This study was supported by a grant from Centocor and Schering Plough. We thank Mrs Ilse Ghyselinck for her assistance with the data collection.
Footnotes
Competing interests: None declared.
References
- 1.Gladman D D, Helliwell P, Mease P J, Nash P, Ritchlin C, Taylor W. Assessment of patients with psoriatic arthritis: a review of currently available measures. Arthritis Rheum 20045024–35. [DOI] [PubMed] [Google Scholar]
- 2.Clegg D O, Reda D J, Mejias E, Cannon G W, Weisman M H, Taylor T.et al Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 1996392013–2020. [DOI] [PubMed] [Google Scholar]
- 3.Van Den Bosch F, Kruithof E, Baeten D, Herssens A, de Keyser F, Mielants H.et al Randomized double‐blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum 200246755–765. [DOI] [PubMed] [Google Scholar]
- 4.Dougados M, Gueguen A, Nakache J P, Nguyen M, Mery C, Amor B. Evaluation of a functional index and an articular index in ankylosing spondylitis. J Rheumatol 198815302–307. [PubMed] [Google Scholar]
- 5.Calin A, Garrett S, Whitelock H, Kennedy L G, O'Hea J, Mallorie P.et al A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994212281–2285. [PubMed] [Google Scholar]
- 6.Felson D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C.et al American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. [DOI] [PubMed] [Google Scholar]
- 7.Agresti A. Ordinal measure of association: gamma. In: Balding D, et al. Categorical data analysis. New Jersey: Wiley and Sons, 200258–59.
- 8.Fitzmaurice T M, Laird N, Ware J. Adjusment for baseline response. In: Balding D, et al. Applied longitudinal analysis. New Jersey, USA: Wiley, 2004122–133.
- 9.Verhoeven A C, Boers M, van Der Linden S. Responsiveness of the core set, response criteria, and utilities in early rheumatoid arthritis. Ann Rheum Dis 200059966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]