Abstract
We have demonstrated recently that CICR (Ca2+-induced Ca2+ release) activity of RyR1 (ryanodine receptor 1) is held to a low level in mammalian skeletal muscle (‘suppression’ of the channel) and that this is largely caused by the interdomain interaction within RyR1 [Murayama, Oba, Kobayashi, Ikemoto and Ogawa (2005) Am. J. Physiol. Cell Physiol. 288, C1222–C1230]. To test the hypothesis that aberration of this suppression mechanism is involved in the development of channel dysfunctions in MH (malignant hyperthermia), we investigated properties of the RyR1 channels from normal and MHS (MH-susceptible) pig skeletal muscles with an Arg615→Cys mutation using [3H]ryanodine binding, single-channel recordings and SR (sarcoplasmic reticulum) Ca2+ release. The RyR1 channels from MHS muscle (RyR1MHS) showed enhanced CICR activity compared with those from the normal muscle (RyR1N), although there was little or no difference in the sensitivity to several ligands tested (Ca2+, Mg2+ and adenine nucleotide), nor in the FKBP12 (FK506-binding protein 12) regulation. DP4, a domain peptide matching the Leu2442–Pro2477 region of RyR1 which was reported to activate the Ca2+ channel by weakening the interdomain interaction, activated the RyR1N channel in a concentration-dependent manner, and the highest activity of the affected channel reached a level comparable with that of the RyR1MHS channel with no added peptide. The addition of DP4 to the RyR1MHS channel produced virtually no further effect on the channel activity. These results suggest that stimulation of the RyR1MHS channel caused by affected inter-domain interaction between regions 1 and 2 is an underlying mechanism for dysfunction of Ca2+ homoeostasis seen in the MH phenotype.
Keywords: calcium release channel, malignant hyperthermia, ryanodine receptor type 1, sarcoplasmic reticulum, skeletal muscle
Abbreviations: AMPPCP, β,γ-methylene adenosine triphosphate; CICR, Ca2+-induced Ca2+ release; DICR, depolarization-induced Ca2+ release; FKBP, FK506-binding protein; MH, malignant hyperthermia; MHS, MH-susceptible; Mopso, 3-(N-morpholino)-2-hydroxypropanesulfonic acid; Po, mean open probability; RyR, ryanodine receptor; SR, sarcoplasmic reticulum
INTRODUCTION
In skeletal muscle, RyR1 (type 1 ryanodine receptor) is a Ca2+ release channel of the SR (sarcoplasmic reticulum) and plays an important role in excitation–contraction coupling [1,2]. Ca2+ release through the RyR1 channel can be activated by two distinct modes: DICR (depolarization-induced Ca2+ release) and CICR (Ca2+-induced Ca2+ release). DICR is triggered by conformational change of the dihydropyridine receptor upon depolarization of the T tubular membrane [3,4]. CICR is a ligand-gated mode in which Ca2+ itself regulates the channel activity: micromolar or more concentrations of Ca2+ activate the channel, whereas millimolar Ca2+ concentrations inactivate it [5]. In addition, ‘gain’ is an important determinant for the CICR activity which determines the maximal activity under respective conditions independently of Ca2+-sensitivity [6,7]. The gain is affected by various CICR modulators, e.g. adenine nucleotides increase the gain, whereas procaine decreases it.
RyR1 is the major target for MH (malignant hyperthermia), an autosomal-dominant pharmacogenetic disorder triggered by volatile anaesthetics such as halothane, and to date more than 80 mutations have been identified in RyR1 of MH patients [8,9]. An enhanced CICR activity with higher sensitivity to caffeine or halothane was consistently reported in MH-mutated RyR1 [10]. Sensitization of DICR activity was also reported [11,12]. Thus it is widely accepted that MH mutations cause hyperactivation/hypersensitization of the Ca2+ release channel, resulting in abnormal Ca2+ homoeostasis in skeletal muscle. However, it remains to be elucidated how these mutations cause such dysfunctions in the Ca2+ release channel. Most known MH mutations are located in one of three ‘hot spots’, i.e. the N-terminal region (region 1, amino acids 35–614), the central region (region 2, amino acids 2163–2458) and the C-terminal transmembrane region (region 3, amino acids 3916–4973). Ikemoto and Yamamoto [13] have found that several short synthetic peptides corresponding to sequences within regions 1 and 2 of RyR1 activate the Ca2+ release channel. Among them, a 36-residue peptide corresponding to the Leu2442–Pro2477 region, designated DP4, potently activated RyR1 [14–16]. Importantly, introduction of an MH mutation in DP4 (DP4-mut, mimicking an Arg2458→Cys mutation) totally abolished these effects. Similar activation was induced by site-specific antibodies directed to the N-terminal (region 1) and the central (region 2) domains [17]. Further studies demonstrated that DP4 binds with the N-terminal portion of region 1 [18], and causes unzipping between regions 1 and 2 [19]. On the basis of these findings, it was hypothesized (i) that the two domains of RyR1 normally interact with each other to stabilize the closed state of the channel, (ii) that MH mutation in either domain weakens this interdomain interaction, resulting in destabilization of the channel, which erroneously increases channel activity, and (iii) that exogenous domain peptides (e.g. DP4) mimic the phenotype of MH mutation by competitively interfering with the interdomain interaction [13].
We have shown previously that [3H]ryanodine binding to RyR1 is significantly lower than that of RyR3 (type 3 ryanodine receptor), another isoform in skeletal muscle, suggesting that the CICR activity of RyR1 is ‘suppressed’ to a low level in skeletal muscle SR [20–22]. This suppression is attributed to a reduced gain of the CICR without significant changes in the sensitivity to known CICR ligands and drugs (e.g. Ca2+, Mg2+, adenine nucleotides and caffeine) [20,21]. The suppression effect is produced by two independent factors: protein-bound FKBP12 (FK506-binding protein 12) and the interdomain interaction within RyR1; the latter mechanism accounts for ∼70% of the suppression [21,22]. We claimed that suppression of the RyR1 channel is important in Ca2+ homoeostasis in skeletal muscle, and aberration in this mechanism thus causes dysfunctions of RyR1 channels as seen in some muscle diseases such as MH. However, this remains to be elucidated.
To test the hypothesis, in the present study, we investigated the properties of the RyR1 channel with the SR vesicles isolated from skeletal muscles of normal and MHS (MH-susceptible) pigs using [3H]ryanodine binding assays, single-channel current recordings and caffeine-induced Ca2+ release measurements. MHS pigs carry an Arg615→Cys mutation in the RyR1 and are known to be an excellent animal model for human MH [10]. Here we report that affecting the N-terminal and central domain interactions by the aforementioned domain peptide (DP4) produced a significant channel activation (i.e. removal of suppression) in the RyR1 from normal pigs (RyR1N), but it produced no further effect on the channel function of the RyR1 from MHS pigs (RyR1MHS). These results suggest that the suppression is largely impaired in RyR1MHS in comparison with RyR1N, and that this impaired suppression is caused primarily by the affected interdomain interaction within the RyR1MHS.
EXPERIMENTAL
Materials
Peptides (DP4 and DP4-mut) were synthesized on a synthesizer (Applied Biosystems model 431 A) employing Fmoc (fluoren-9-ylmethoxycarbonyl) as the α-amino-protecting group and purified by reverse-phase HPLC [14]. [3H]Ryanodine (50–60 Ci/mmol) was purchased from NEN Life Science Products. All other reagents were of analytical grade.
Animals and preparation of SR vesicles
All of the experiments were carried out in accordance with Juntendo University Ethics Committee guidelines. Homozygous MHS pigs for the RyR1 Arg615→Cys mutation and homozygous normal pigs were obtained by breeding heterozygous parent pigs that had been maintained in the Miyazaki Station, National Livestock Breeding Center (Miyazaki, Japan). Animals used for the experiments were 2-month-old littermates that had been tested for the presence of the normal and MHS RyR1 alleles. Animals were killed using an intravenous injection of pentobarbital overdose. Back muscles were excised immediately and frozen rapidly in liquid nitrogen. Crude SR vesicles were prepared from the muscle by the method of Murayama and Ogawa [23] and terminal cisterna-rich fractions were obtained by sucrose-density-gradient ultracentrifugation [21]. The vesicles were quickly frozen with liquid nitrogen, and stored at −80 °C until use.
[3H]Ryanodine binding
The [3H]ryanodine binding assay was carried out as described previously [22]. Briefly, the SR vesicles (100 μg of protein) were incubated with 8.5 nM [3H]ryanodine for 5 h at 25 °C in a 100 μl solution containing 0.17 M NaCl, 20 mM Mopso [3-(N-morpholino)-2-hydroxypropanesulfonic acid], pH 6.8, 2 mM dithiothreitol, 1 mM AMPPCP (β,γ-methylene adenosine triphosphate) and various concentrations of Ca2+ buffered with 10 mM EGTA (calculated using the value of 8.79×105 M−1 as the apparent binding constant for Ca2+ of EGTA [24]) unless otherwise indicated. The protein-bound [3H]ryanodine was separated by filtering through polyethyleneimine-treated Whatman GF/B filters. Non-specific radioactivity was determined in the presence of 20 μM unlabelled ryanodine. The [3H]ryanodine binding data (B) obtained under various assay conditions in the presence of a fixed concentration of [3H]ryanodine (8.5 nM throughout these experiments) were expressed in relative values to the maximal binding sites for the ligand (Bmax); thus B/Bmax reflected an apparent averaged activity of individual Ca2+ release channels. Bmax was determined on the Scatchard plot for [3H]ryanodine binding in a medium containing 1 M NaCl and various concentrations of [3H]ryanodine. Apparent dissociation constants for Ca2+ of the Ca2+-activation site (KA,Ca) and Ca2+-inactivation site (KI,Ca) and those for Mg2+ (KA,Mg and KI,Mg) were estimated according to the method of Murayama et al. [25].
Single-channel recordings
Single-channel recordings were carried out as described previously with CHAPS-solubilized SR protein as a material [26,27]. Lipid bilayers consisting of a mixture of L-α-phosphatidylethanolamine, L-α-phosphatidyl-L-serine and L-α-phosphatidylcholine (5:3:2 by weight) in n-decane (40 mg/ml) were formed across a hole of ∼250 μm in diameter in a polystyrene partition separating cis and trans chambers. Channel currents were recorded in symmetrical solutions of 250 mM caesium methanesulfonate buffered at pH 6.8 with 20 mM Hepes/Tris at the holding potential of −40 mV (cis). Experiments were carried out at 18–22 °C. Bilayers containing only a single channel were used for analysis. Channel currents amplified by an Axopatch 1D patch clamp amplifier (Axon Instruments) were filtered at 1 kHz using an eight-pole low-pass Bessel filter and collected at 5 kHz for analysis. Mean open probability (Po) was calculated from the records of duration >2 min by 50% threshold analysis using pClamp (version 6.0.4) software.
Ca2+ release measurements
Ca2+ release from the isolated SR vesicles was fluorometrically measured by monitoring free Ca2+ concentration in the solution [22]. Briefly, SR vesicles (80 μg) were incubated at 30 °C in a fluorimeter cuvette containing 400 μl of 0.17 M KCl, 20 mM Mopso, pH 6.8, 1 mM MgCl2, 5 mM potassium phosphate, 10 mM phosphocreatine, 2 units/ml of creatine kinase and 2 μM fura 2. Fluorescence was measured in a Hitachi F-4500 fluorescence spectrophotometer with wavelength settings of 340 and 380 nm for excitation (alternating) and 510 nm for emission. Active loading of the SR vesicles with Ca2+ was started by addition of 100 μM MgATP and 20 μM CaCl2. Free Ca2+ concentration in the cuvette declined with time and reached a steady state within 5 min. At this point, caffeine was added and the changes in fura 2 fluorescence were recorded. For some experiments, 30 μM DP4 was added before the start of Ca2+ loading. Calibration of fura 2 signals was carried out with 100–400 nM free Ca2+ buffered with 1 mM EGTA in the reaction medium.
Statistics
Data are given as means±S.E.M. for n repeated experiments. To determine the significance of the difference between mean values, Student's unpaired t test was applied.
RESULTS
Protein compositions and the RyR1 content in the SR vesicles isolated from normal and MHS pigs
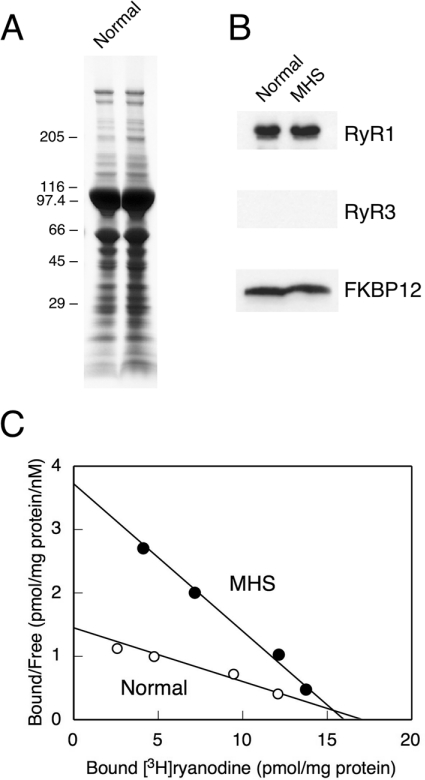
The protein compositions of SR vesicles isolated from normal and MHS pig skeletal muscles are shown in Figure 1. Both SR preparations show similar protein compositions in the Coomassie Brilliant Blue-stained SDS/polyacrylamide gel (Figure 1A). Western blots with the isoform-specific antibodies show that these SR preparations contain comparable amounts of RyR1 and FKBP12, an accessory protein of RyR1 (Figure 1B). There was no detectable RyR3 (Figure 1B). Fractions of active channel in the SR vesicles were determined by Scatchard plot analysis of [3H]ryanodine binding. The Bmax of the MHS vesicles (16.0±4.6 pmol/mg of protein) was comparable with that of the normal vesicles (17.1±1.1 pmol/mg of protein) (Figure 1C), indicating that the two SR preparations contain very similar amounts of active RyR1 channels. However, the Kd for [3H]ryanodine was 3-fold smaller (4.3±0.4 nM) in MHS SR than in normal SR (11.8±1.8 nM), indicating that the affinity of ryanodine binding is significantly higher in MHS pigs than in normal pigs.
Figure 1. Protein compositions and the RyR1 content in SR vesicles isolated from normal and MHS pigs.
(A) SR vesicles (30 μg) from normal (left) and MHS (right) pigs were subjected to SDS/PAGE and stained with Coomassie Brilliant Blue. The molecular markers (in kDa) are shown to the left of the gel. (B) Western blotting with antibodies against RyR1, RyR3 and FKBP12. Note that the normal and MHS SR preparations contain similar amounts of RyR1 and FKBP12, but no detectable RyR3. Data are representative of three experiments. (C) Scatchard plot analysis of [3H]ryanodine binding to SR vesicles. Assays were carried out as outlined in the Experimental section, except that 1 M NaCl instead of 0.17 M NaCl was used. Kd and Bmax values were 11.8±1.8 nM and 17.1±1.1 pmol/mg of protein and 4.3±0.4 nM and 16.0±4.6 pmol/mg of protein for normal and MHS pigs respectively. Results are means±S.E.M. for three experiments.
Removal of suppression in RyR1MHS as shown in the [3H]ryanodine binding assay
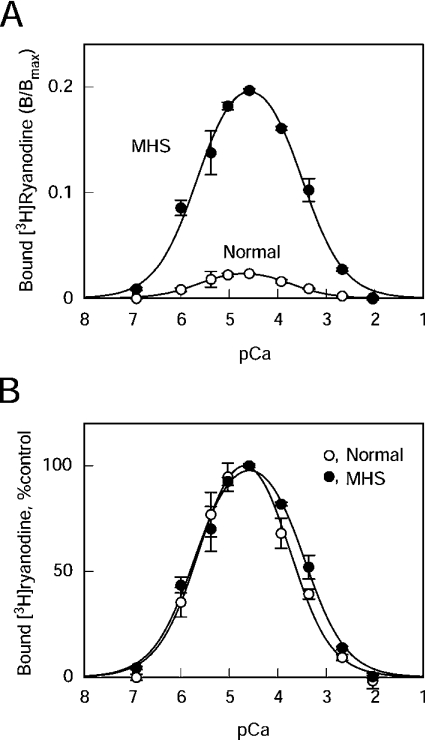
Figure 2 shows the Ca2+-dependence of the [3H]ryanodine binding to the SR vesicles from normal and MHS pig skeletal muscle. The activity is expressed as B/Bmax, in which the ryanodine binding values (B) were corrected for the maximum number of binding sites (Bmax), thus reflecting the apparent averaged activity of the individual channels (Figure 2A). The B/Bmax value of RyR1 in normal SR (RyR1N) at the optimum Ca2+ (0.023±0.002; n=3) was comparable with the values from bovine and rabbit skeletal muscle SR vesicles under similar conditions [21,22], suggesting that RyR1N is suppressed to the same extent as those of the other mammalian species. RyR1 in MHS SR (RyR1MHS) showed 8-fold greater magnitude of [3H]ryanodine binding than RyR1N at all Ca2+ concentrations examined (B/Bmax=0.18±0.02; n=3). As shown in the normalized plot (percentage of the peak value), both RyR1s show almost identical Ca2+-dependence in the activating range of Ca2+: the Kd values for Ca2+ of the Ca2+ activating site (KA,Ca) were 2.5±0.4 μM and 2.0±0.3 μM for RyR1N and RyR1MHS respectively (n=3). In the inhibitory concentration range, RyR1MHS showed 2-fold lower affinity than RyR1N: Kd values for Ca2+ of the Ca2+-inactivating site (KI,Ca) were 0.17±0.03 mM and 0.35±0.06 mM (n=3, P<0.05) for RyR1N and RyR1MHS respectively (Figure 2B).
Figure 2. Ca2+-dependence of the [3H]ryanodine binding activity of RyR1N and RyR1MHS.
[3H]Ryanodine binding was carried out as outlined in the Experimental section in 0.17 M NaCl, 20 mM Mopso, pH 6.8, 2 mM dithiothreitol, 1 mM AMPPCP and various free Ca2+ concentrations buffered with 10 mM EGTA. (A) Results are expressed as B/Bmax. RyR1MHS (●) showed 8-fold greater amplitude of ryanodine binding as compared with RyR1N (○). (B) Activity was normalized by respective peak values. Although Ca2+-sensitivity was not different for activation, RyR1MHS showed a slight reduction in Ca2+-sensitivity for inactivation (approx. 2-fold increase in IC50). Results are means±S.E.M. (n=3).
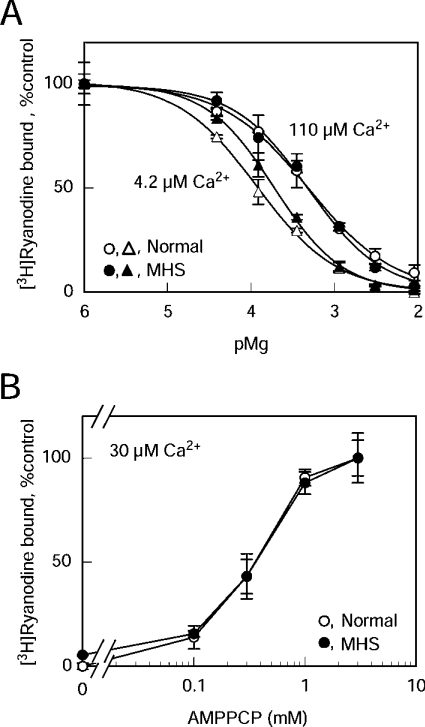
Mg2+ is known to inhibit the RyR1 channel by two distinct mechanisms: it acts as a competitive antagonist for the Ca2+-activating site and as an agonist for the Ca2+-inactivating site [25,28,29]. There was no substantial difference in Mg2+ inhibition between RyR1N and RyR1MHS at higher Ca2+ (110 μM) where Mg2+ acts exclusively as an agonist for the to Ca2+-inactivation site (Figure 3A, circles): Kd for Mg2+ of the Ca2+-inactivation site (KI,Mg) was estimated to be 0.26±0.04 mM and 0.30±0.06 mM for RyR1N and RyR1MHS respectively. On the other hand, there was a slight reduction in the Mg2+ inhibition of RyR1MHS at lower Ca2+ (4.2 μM) where the inhibitory effect of Mg2+ at the Ca2+-activation site becomes more involved (Figure 3A, triangles). Kd for Mg2+ of the Ca2+-activation site (KA,Mg) of RyR1MHS (0.40±0.02 mM; n=3) was significantly (P<0.05) greater than that of RyR1N (0.21±0.02 mM; n=3), indicating that Mg2+-sensitivity for the Ca2+-activation site is somehow reduced in RyR1MHS. There was no appreciable difference between RyR1N and RyR1MHS in the sensitivity to the agonist AMPPCP, a non-hydrolysable analogue of ATP: EC50 values were ∼0.3 mM for both SR preparations (Figure 3B). Taken together, these results indicate that the gain of the Ca2+-dependent activation of [3H]ryanodine binding is considerably increased in RyR1MHS without substantial alterations in sensitivity to the CICR ligands. Since the reduced gain of CICR activity is the sign of a suppressed Ca2+ channel [21,22], these results suggest that suppression is largely impaired in the RyR1MHS.
Figure 3. Effects of Mg2+ and AMPPCP on the [3H]ryanodine binding of RyR1N and RyR1MHS.
(A) Inhibition by Mg2+. Experiments were carried out as described in Figure 2 at 110 μM (circles) or 4.2 μM (triangles) Ca2+. RyR1N (open symbols) and RyR1MHS (closed symbols) show similar sensitivity to Mg2+ inhibition at 110 μM Ca2+, whereas RyR1MHS is less sensitive to Mg2+ than RyR1N at 4.2 μM Ca2+. The B/Bmax values in the absence of Mg2+ (corresponding to 100%) for RyR1N and RyR1MHS were 0.017 and 0.17 at 110 μM Ca2+ and 0.018 and 0.15 at 4.2 μM Ca2+ respectively. Results are means±S.E.M. (n=3). (B) Activation by AMPPCP. Experiments were carried out as described in Figure 2 at 30 μM Ca2+. The B/Bmax values in the presence of 3 mM AMPPCP (corresponding to 100%) for RyR1N and RyR1MHS were 0.016 and 0.17 respectively. Results are means±S.E.M. (n=3).
Unaltered regulation by FKBP12 in RyR1MHS
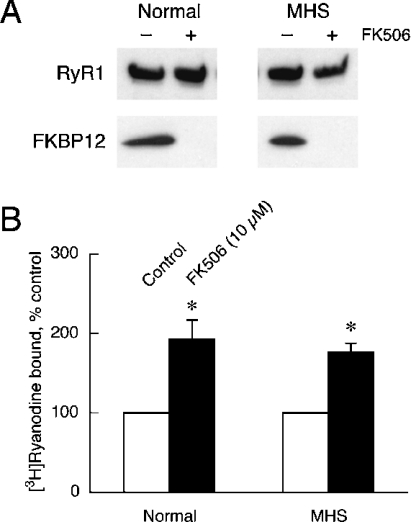
We showed previously that suppression of the RyR1 channel is exerted partly by FKBP12 and partly by some mechanisms involving the interdomain interaction [21,22]. FKBP12 is thought to stabilize a closed state of the RyR channel [30] and accounts for ∼30% of the suppression [21]. Therefore we investigated regulation of RyR1N and RyR1MHS by FKBP12. As shown in Figure 4(A), comparable amounts of FKBP12 were co-precipitated with RyR1N and RyR1MHS, indicating that the RyR1MHS retains capability to bind FKBP12. Furthermore, 10 μM FK506, which dissociated the bound FKBP12 from RyR1 (Figure 4A), increased the [3H]ryanodine binding of RyR1N and RyR1MHS to almost the same extent (1.9- and 1.8-fold for RyR1N and RyR1MHS respectively) (Figure 4B). Thus we can exclude the possibility that the FKBP12-mediated regulation might be involved in the channel stimulation seen in the RyR1MHS pigs.
Figure 4. Unaltered regulation by FKBP12 in RyR1MHS.
(A) Binding of FKBP12 to RyR1. CHAPS-solubilized SR proteins from normal (left-hand panel) and MHS (right-hand panel) pigs were immunoprecipitated with anti-RyR1 antibody in the presence (+) and absence (−) of 10 μM FK506. RyR1 and FKBP12 in the precipitate were detected by Western blotting. A similar amount of FKBP12 was co-precipitated with RyR1N and RyR1MHS. FK506 at 10 μM (+FK506) completely prevented the FKBP12 binding. (B) Effect of FK506 on [3H]ryanodine binding. Experiments were carried out as described in Figure 2 at 30 μM Ca2+ with (closed bars) and without (open bars) 10 μM FK506. The 100% values for RyR1N and RyR1MHS were 0.013 and 0.13 in B/Bmax respectively. Results are means±S.E.M. (n=3). *P<0.05.
Differential effects of domain peptides on RyR1N and RyR1MHS
The involvement of the interdomain interaction in the impaired suppression of RyR1MHS was examined using the domain peptide approach [14,22]. In the interdomain interaction hypothesis, the two domains (regions 1 and 2) of RyR1 should interact with each other to stabilize the closed state of the channel, and MH mutation in either domain should weaken the interaction, resulting in activation of the Ca2+ release channel. Similar activation can be produced by an exogenous domain peptide (e.g. DP4) which is a part of the mating domain and is reasonably hypothesized to competitively interfere with the interdomain interaction [13]. Thus, if the impaired suppression seen in the RyR1MHS is caused by the weakened interdomain interaction, we expect that the addition of such domain peptides will produce significant activation in RyR1N, but will produce little or no activation in RyR1MHS, because the interaction has already been weakened in the latter. The most effective way to test the physiological significance and specificity of the domain peptide is to introduce an MH mutation into the peptide (e.g. DP4-mut). The mutation should weaken or abolish the ability of the peptide to interfere with the interdomain interaction (cf. the hypothesis), and the mutated peptide has little or no activation effect [13].
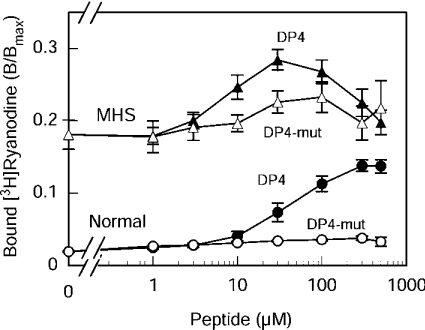
The results of this test are shown in Figure 5. As can be seen, DP4 increased the [3H]ryanodine binding to RyR1N in a concentration-dependent manner and reached a plateau at approx. 300 μM with the maximal enhancement of 7-fold (from 0.019±0.002 to 0.14±0.02; n=4). DP4-mut produced only slight activation (1.9-fold, from 0.019±0.002 to 0.036±0.003; n=3). These results are consistent with previous reports with rabbit [14] and bovine [22] skeletal muscle SR vesicles. However, the addition of DP4 to RyR1MHS produced only a very small increase of the ryanodine-binding activity with a peak value of 1.6-fold (from 0.18±0.02 to 0.28±0.03 at 30 μM; n=4), which is comparable with a negligible extent of activation by DP4-mut (1.3-fold, from 0.18±0.02 to 0.23±0.02 at 100 μM; n=3). Thus DP4 had only a little additional effect on RyR1MHS, in which the suppression had already been removed.
Figure 5. Effects of DP4 and DP4-mut on [3H]ryanodine binding to RyR1N and RyR1MHS.
Experiments were carried out as described in Figure 2 with 0–500 μM DP4 (closed symbols) or DP4-mut (open symbols) at 30 μM free Ca2+. Circles, RyR1N; triangles, RyR1MHS. Note that DP4 greatly activated RyR1N in a concentration-dependent manner, whereas it produced only small activation of RyR1MHS. DP4-mut had little activating effects. Results are means±S.E.M. (n=3–4).
Effects of domain peptides on single Ca2+ release channel currents of normal and MHS RyR1 channels
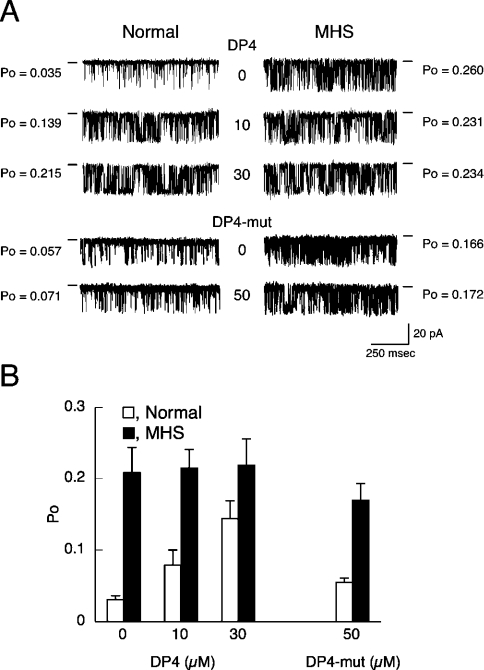
Single Ca2+ channel currents through the RyR1 were recorded in symmetrical solutions containing 250 mM caesium methane-sulfonate, 20 mM Hepes/Tris, pH 6.8, and 30 μM free Ca2+ at a holding potential of −40 mV (Figure 6). The RyR1N channel displayed low Po under control conditions and DP4 increased Po in a concentration-dependent manner; the Po value at 30 μM DP4 was approx. 6-fold higher than the control level (Figure 6A, left). In contrast, the Po of the RyR1MHS channel was already 7.4-fold higher than the control level of RyR1N even in the absence of DP4, and addition of DP4 (10 and 30 μM) produced virtually no further increase in Po (Figure 6A, right). A summary of these experiments is presented in Figure 6(B). The RyR1MHS channel showed nearly 7-fold higher Po (0.20±0.03; n=10) than RyR1N channel (0.030±0.006; n=10) in the absence of DP4, but the difference became smaller in the presence of 30 μM DP4 (Po=0.14±0.03 and 0.22±0.04 for RyR1N and RyR1MHS respectively; n=4). DP4-mut (50 μM) produced no activating effect on either RyR1N (Po=0.055±0.011; n=5) or RyR1MHS (Po=0.17±0.02; n=5) (Figure 6).
Figure 6. Single Ca2+ release channel currents through RyR1N and RyR1MHS and the effect of DP4.
Single Ca2+ channel currents through RyR1N and RyR1MHS were recorded at a holding potential of −40 mV (cis) in a symmetrical solution containing 250 mM caesium methanesulfonate buffered with 20 mM Hepes/Tris, pH 6.8, at 30 μM Ca2+. (A) Representative traces of the current recordings through RyR1N (left-hand panels) and RyR1MHS (right-hand panels) channels in the presence and absence of DP4 (upper six panels) or DP4-mut (lower four panels). The conductance level of the closed channel is shown by a short line on the side of each current recording. (B) Summary of the Po data collected in the presence of 0–30 μM DP4 or 50 μM DP4-mut. Results are means±S.E.M. (n=3–10).
Effects of domain peptides on caffeine-induced Ca2+ release from normal and MHS SR vesicles
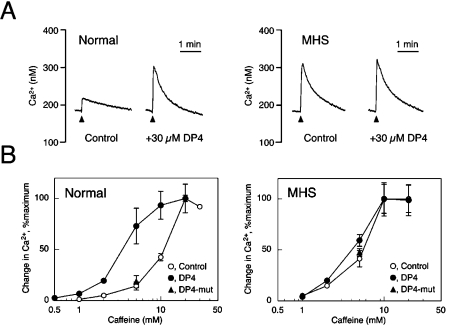
Ca2+ release from the isolated SR vesicles was fluorimetrically monitored using fura 2 as a fluorescent Ca2+ probe [22]. The SR vesicles were actively loaded with Ca2+ using MgATP in a solution containing 0.17 M KCl and 1 mM free Mg2+, and Ca2+ release was induced by caffeine. With normal SR vesicles, 5 mM caffeine induced a small Ca2+ release (Figure 7A, left-hand panel). Addition of 30 μM DP4 at the onset of Ca2+ loading greatly increased the subsequent Ca2+ release. With MHS SR vesicles, in contrast, Ca2+ release by 5 mM caffeine was greater than that with normal SR, but was not increased substantially by 30 μM DP4 (Figure 7A, right-hand panel). The Ca2+ release data obtained at various concentrations of caffeine are depicted in Figure 7(B). DP4 increased the apparent affinity of the normal SR to caffeine; the EC50 of caffeine was changed from ∼10 mM to ∼3 mM using 30 μM DP4 (Figure 7B, left-hand panel). This is consistent with the results from bovine SR vesicles [22]. In contrast, 30 μM DP4-mut had no effect on the caffeine-induced Ca2+ release (Figure 7B). In the MHS SR, the sensitivity of Ca2+ release to caffeine was much higher (EC50 ∼5 mM) than the normal SR, in agreement with a previous report [10] (Figure 7B, right-hand panel). The sensitivity, however, remained virtually unchanged by the addition of 30 μM DP4 or 30 μM DP4-mut.
Figure 7. Caffeine-induced Ca2+ release from the isolated SR vesicles and the effect of DP4.
Ca2+ release from the SR vesicles was fluorimetrically determined using fura 2 in a solution containing 0.17 M KCl, 20 mM Mopso, pH 6.8, and 1 mM MgCl2. The vesicles were actively loaded with Ca2+ by addition of 100 μM MgATP and 20 μM CaCl2, and then Ca2+ release was triggered by caffeine. (A) Representative traces of Ca2+ release induced by 5 mM caffeine (arrowhead) with (+30 μM DP4) or without added peptide (Control). DP4 was added to the solution just before the start of Ca2+ loading. Left, normal SR; right, MHS SR. (B) Caffeine-concentration-dependence of Ca2+ release in the presence (●) and absence (○) of 30 μM DP4. Left, normal SR; right, MHS SR. The change in Ca2+ concentration was normalized at the maximal values at each condition; 100% denotes 240, 180, 240 and 150 nM for Normal-Control, Normal-DP4, MHS-Control and MHS-DP4 respectively. Results are means±S.E.M. (n=3–6). Note that DP4 sensitized the normal SR to caffeine in releasing Ca2+, whereas it produced no sensitizing effect on the MHS SR. DP4-mut (30 μM) produced no effects on either SR (▲).
DISCUSSION
Our previous studies demonstrated that Ca2+-dependent [3H]ryanodine binding was much lower in RyR1 than in RyR3 in the SR without change in their Ca2+ dependences, which was referred to as suppression [20–22]. This finding would be along the same lines as the effect of an adenine nucleotide on CICR, where the agent increased the rate of CICR at any given Ca2+ concentration without major change in the Ca2+ sensitivity [29]. These findings indicate that occupation by Ca2+ of the Ca2+ sites may be a necessary but not a sufficient condition for Ca2+ release, and necessitate the introduction of the concept of gain or ‘attenuating coefficient’ besides the occupation of the Ca2+ sites. We proposed a hypothesis that the gain or the attenuating coefficient is mainly regulated by the interdomain interaction between region 1 and region 2 within RyR 1 [30]. Therefore we could paraphrase the previous results [30–32] as follows: the attenuating coefficient for RyR1 in the SR membrane is 1/7–1/8, whereas it is unity for RyR3. Using MHS pigs with the Arg615→Cys mutation in RyR1 in the present study, we showed that the attenuating factor was near to unity with the mutated RyR1 in the SR (Figure 2). We advanced further our hypothesis that aberration in the interdomain interaction may be the main causes of channel dysfunctions seen in RyR1-linked muscle diseases such as MH.
Normal suppression is impaired in the RyR1 channels of the MHS pig model
Extensive studies have been carried out on the MHS pig model, and a considerable amount of information has been accumulated in the literature concerning the altered properties of RyR1 in MH. It is widely recognized that there is a massive increase in the CICR activity in the RyR1MHS channel, as shown using various types of assay, such as Ca2+ release experiments with skinned fibres [33] or isolated SR vesicles [34], [3H]ryanodine binding assay [31,32] and single-channel recordings [35] (for a review, see also [10]). These results may be consistent with the hypothesis that defectiveness in the channel suppression mechanism is a causative mechanism of abnormal activation of the RyR1MHS channel. Some results, however, claimed a different mechanism: a reduced sensitivity to Ca2+/Mg2+ inhibition. This hypothesis was based on the finding that RyR1MHS is less sensitive to Ca2+ inactivation or Mg2+ inhibition than is RyR1N [36–40]. The extent of reduction in the Mg2+-sensitivity was in the range 1.5–3-fold in these studies. We also found in the present study that there is a 2-fold reduction in the apparent affinity for Ca2+ of the Ca2+-inactivation site (Figure 2B) and for Mg2+ of the Ca2+-activation site (Figure 3A). However, the magnitude of the change in the Ca2+- or Mg2+-sensitivity is too small to explain the increase in the CICR (8-fold) (Figure 2A). Thus it appears that the reduction in Ca2+/Mg2+-sensitivity may contribute little to the development of the pig MH phenotype.
However, there are controversial reports that no such increase in the CICR activity was observed in RyR1MHS channels of the pig model [37,40,41]. This reported claim is at least partly due to a higher pH used in these studies, as mentioned below. The dramatic effect of pH on normal and MHS RyR1 channels was demonstrated by Shomer et al. [35,41]. For instance, single RyR1MHS channels showed much larger Po (∼0.2) at optimum Ca2+ concentrations (∼10 μM) than the RyR1N channel (Po=∼0.05) at pH 6.8, whereas there was virtually no difference between the normal and MHS channels at pH 7.4 (Po=0.4 for both channels), indicating that the higher pH preferentially activated RyR1N. Balog et al. [40] reported almost the same peak [3H]ryanodine binding activity at pH 7.4, which contrasts with the previously reported results showing a significant difference between the normal and MHS channels at pH 7.0 [33,34] and the present data at pH 6.8. Thus it seems that higher pH (e.g. pH 7.4) preferentially activates the RyR1N channels; consequently the difference in the CICR activity between RyR1N and RyR1MHS channels becomes undetectable. We have found that such an alkaline pH as pH 7.4 lessened the magnitude of the suppression (T. Murayama, unpublished work). Considering that the intracellular pH in skeletal muscle is estimated to be ∼7.0 [42,43] and that metabolic acidosis precedes the muscle contracture in the episode of MH [10], it is quite likely that RyR1MHS channels are in the non-suppressed state unlike the suppressed RyR1N. The impaired suppression mechanism in the RyR1MHS channels will then cause erroneously enhanced CICR and cause dysfunction of Ca2+ homoeostasis in MHS pigs.
In human MH, more than 80 mutations have been found in the RyR1 gene [8,9]. An important question is then whether the conclusions deduced here from the particular pig MH mutation can be applied to the other MH mutations. With biopsied muscle fibres, Endo and colleagues [44,45] showed an abnormal enhancement of the CICR activity in MH patients at all of the Ca2+ concentrations examined. A similar observation was made in the [3H]ryanodine binding assay with SR vesicles from a MH patient carrying a Gly2434→Arg mutation [46]. Thus it appears that the impaired channel suppression is a general mechanism for the pig and human MH phenotype. Various studies of human MH mutations using cultured cells expressing the mutated RyR1 [12,47,48] have shown the increased sensitivity of MH mutants to caffeine or halothane. Yang et al. [12], in particular, reported that RyR1 channels carrying six human MH mutations show reduced Ca2+ inactivation and Mg2+ inhibition of [3H]ryanodine binding, as also observed in the present study. Unfortunately, only a limited amount of quantitative data are available about the extent of suppression of the channel and the CICR activity of these expressed mutants, probably owing to the limitation in the quantity of the expressed protein. However, the similarity in the phenotype of these expressed mutants suggests that all of these MH mutations share a common mechanism involved in their pathogenic process.
Mechanism of impaired suppression of the RyR1MHS channel
We have shown recently that the ‘suppression of CICR gain’ is controlled by two independent factors: the RyR1-bound FKBP12 and the tight interdomain interaction within RyR1; the latter mechanism accounts for ∼70% of the suppression [21,22]. In the present study, there was no appreciable change in the bound FKBP12 and its regulation in MHS pig skeletal muscle SR (Figure 4). This excludes the possibility that FKBP12 might be one of the major factors involved in the channel suppression mechanism. On the other hand, there is a clear difference in the effect of DP4 on RyR1N and RyR1MHS (Figures 5–7), indicative of the major contribution of the altered interdomain interaction to the mechanism of the impaired suppression in the RyR1MHS channel.
An increasing body of evidence supports the interdomain interaction hypothesis that the two domains of RyR1 harbouring many of the reported MH mutations, the N-terminal domain (region 1) and the central domain (region 2), interact with each other, and that tight interaction stabilizes the closed state of the Ca2+ channel and weakened interaction destabilizes the channel. Domain peptides that bind specifically to these domains produced channel destabilization effects as shown in several reports [14–17,22]. For example, DP4 that corresponds to the Leu2442–Pro2477 segment of region 2, binds with the N-terminal portion of region 1 [18]. This causes local conformational changes in RyR1 [19], unzips the interaction between regions 1 and 2 [19] and activates the Ca2+ release channel [15,16,22,49]. Furthermore, an antibody raised against DP4 was found to produce the same effects as DP4 (domain unzipping and channel activation) by reacting with its epitope located in region 2 [17]. These findings strongly support the hypothesis that the RyR Ca2+ channels are regulated in fact by mediation of the interaction between region 1 and region 2, and channel activation (i.e. removal of suppression or impaired suppression) by DP4 is caused by the effect of the peptide to interfere with the domain–domain interaction.
According to the interdomain-interaction hypothesis, MH mutation in either of these domains will weaken the interdomain interaction and will destabilize the channel. In fact, DP4-mut, a peptide carrying an Arg2458→Cys MH mutation in DP4, produced no activating effect on the RyR channel, presumably due to the reduced or lost ability to interfere with the domain–domain interaction [14,19]. However, this hypothesis has not yet been tested using an actual disease model. In the present study, we demonstrated that DP4 greatly activated RyR1N, but produced a little or no activation of RyR1MHS, as shown in the [3H]ryanodine binding assay (Figure 5), single-channel recordings (Figure 6) and Ca2+ release measurements (Figure 7). These findings well fit the hypothesis that the RyR1MHS channel is already activated by the weakened interdomain interaction. Furthermore, the level activated by DP4 in RyR1N was comparable with that in RyR1MHS without DP4, indicating that addition of DP4 to RyR1N could experimentally mimic the phenotype of RyR1MHS. The results of the present study provide the first direct evidence that the weakened interdomain interaction induced by a mutation in region 1 is the primary cause for the impaired suppression in MH.
It should also be noted that the effectiveness of DP4 varied with the type of measurements: more potent on single-channel recordings than in the [3H]ryanodine binding assay. The reason for this remains unclear, but it is suggested that the accessibility of the peptide to its reactive site may have varied depending on the assay conditions.
It is of interest to know whether affected interdomain interaction can explain the human MH phenotype. An Arg614→Cys mutation, equivalent to Arg615→Cys in MHS pigs, has also been found in humans [8]. A recent study with the domain peptides demonstrates that MH mutations within the DP4 peptide (Ile2453→Thr, Arg2454→Cys, Arg2458→Cys and Arg2458→His) reduce its activating effect on the RyR1 channels [50]. Thus the affected interdomain interaction might also be applicable to some human MH mutations. Several human MH mutations have also been found in the C-terminus region (region 3), where many mutations for CCD (central core disease) as well as MH are reported [51]. We tentatively propose that the information concerning the changes in the mode of interaction between region 1 and region 2 (e.g. weakening of the interaction) is transmitted to the transmembrane channel-forming segments by mediation of some regulatory domains, such as region 3. Both the functional and structural studies of the RyR1 channels with different MH mutations will help us to understand better the mechanism of dysfunction of Ca2+ release and the postulated involvement of the affected interdomain interaction in the mechanism of pathogenesis of human MH.
Acknowledgments
We thank Dr Renne C. Lu, Dr Paul Leavis, David Schrier and Elizabeth Gowell for synthesis and purification of the peptides. This work was supported in part by Grant-in-Aid for Scientific Research 17590227 (to T.M.) from JSPS (Japan Society for the Promotion of Science), and by NIH (National Institutes of Health) Grants AR 16922 (N.I.) from NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases), and HL072841 (N.I.) from NHLBI (National Heart, Lung, and Blood Institute).
References
- 1.Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa Y. Role of ryanodine receptors. Crit. Rev. Biochem. Mol. Biol. 1994;29:229–274. doi: 10.3109/10409239409083482. [DOI] [PubMed] [Google Scholar]
- 3.Rios E., Pizarro G. Voltage sensor of excitation–contraction coupling in skeletal muscle. Physiol. Rev. 1991;71:849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- 4.Schneider M. F. Control of calcium release in functioning skeletal muscle fibers. Annu. Rev. Physiol. 1994;56:463–484. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- 5.Endo M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa Y., Murayama T., Kurebayashi N. Ryanodine receptor isoforms of non-mammalian skeletal muscle. Front. Biosci. 2002;7:d1184–d1194. doi: 10.2741/A832. [DOI] [PubMed] [Google Scholar]
- 7.Murayama T., Ogawa Y. Roles of two ryanodine receptor isoforms coexisting in skeletal muscle. Trends Cardiovasc. Med. 2002;12:305–311. doi: 10.1016/s1050-1738(02)00179-2. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy T. V., Quane K. A., Lynch P. J. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum. Mutat. 2000;15:410–417. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Jurkat-Rott K., McCarthy T., Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve. 2000;23:4–17. doi: 10.1002/(sici)1097-4598(200001)23:1<4::aid-mus3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Mickelson J. R., Louis C. F. Malignant hyperthermia: excitation–contraction coupling, Ca2+ release channel, and cell Ca2+ regulation defects. Physiol. Rev. 1996;76:537–592. doi: 10.1152/physrev.1996.76.2.537. [DOI] [PubMed] [Google Scholar]
- 11.Melzer W., Dietze B. Malignant hyperthermia and excitation–contraction coupling. Acta Physiol. Scand. 2001;171:367–378. doi: 10.1046/j.1365-201x.2001.00840.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang T., Ta T. A., Pessah I. N., Allen P. D. Functional defects in six ryanodine receptor isoform-1 (RyR1) mutations associated with malignant hyperthermia and their impact on skeletal excitation–contraction coupling. J. Biol. Chem. 2003;278:25722–25730. doi: 10.1074/jbc.M302165200. [DOI] [PubMed] [Google Scholar]
- 13.Ikemoto N., Yamamoto T. Regulation of calcium release by interdomain interaction within ryanodine receptors. Front. Biosci. 2002;7:d671–d683. doi: 10.2741/A803. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T., El-Hayek R., Ikemoto N. Postulated role of interdomain interaction within the ryanodine receptor in Ca2+ channel regulation. J. Biol. Chem. 2000;275:11618–11625. doi: 10.1074/jbc.275.16.11618. [DOI] [PubMed] [Google Scholar]
- 15.Lamb G. D., Posterino G. S., Yamamoto T., Ikemoto N. Effects of a domain peptide of the ryanodine receptor on Ca2+ release in skinned skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 2001;281:C207–C214. doi: 10.1152/ajpcell.2001.281.1.C207. [DOI] [PubMed] [Google Scholar]
- 16.Shtifman A., Ward C. W., Yamamoto T., Wang J., Olbinski B., Valdivia H. H., Ikemoto N., Schneider M. F. Interdomain interactions within ryanodine receptors regulate Ca2+ spark frequency in skeletal muscle. J. Gen. Physiol. 2002;119:15–32. doi: 10.1085/jgp.119.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi S., Yamamoto T., Parness J., Ikemoto N. Antibody probe study of Ca2+ channel regulation by interdomain interaction within the ryanodine receptor. Biochem. J. 2004;380:561–569. doi: 10.1042/BJ20040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi S., Bannister M. L., Gangopadhyay J. P., Hamada T., Parness J., Ikemoto N. Dantrolene stabilizes domain interactions within the ryanodine receptor. J. Biol. Chem. 2005;280:6580–6587. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T., Ikemoto N. Spectroscopic monitoring of local conformational changes during the intramolecular domain–domain interaction of the ryanodine receptor. Biochemistry. 2002;41:1492–1501. doi: 10.1021/bi015581z. [DOI] [PubMed] [Google Scholar]
- 20.Murayama T., Ogawa Y. Selectively suppressed Ca2+-induced Ca2+ release activity of α-ryanodine receptor (α-RyR) in frog skeletal muscle sarcoplasmic reticulum: potential distinct modes in Ca2+ release between α- and β-RyR. J. Biol. Chem. 2001;276:2953–2960. doi: 10.1074/jbc.M005809200. [DOI] [PubMed] [Google Scholar]
- 21.Murayama T., Ogawa Y. RyR1 exhibits lower gain of CICR activity than RyR3 in the SR: evidence for selective stabilization of RyR1 channel. Am. J. Physiol. Cell Physiol. 2004;287:C36–C45. doi: 10.1152/ajpcell.00395.2003. [DOI] [PubMed] [Google Scholar]
- 22.Murayama T., Oba T., Kobayashi S., Ikemoto N., Ogawa Y. Postulated role of interdomain interactions within the type 1 ryanodine receptor in the low gain of Ca2+-induced Ca2+ release activity of mammalian skeletal muscle sarcoplasmic reticulum. Am. J. Physiol. Cell Physiol. 2005;288:C1222–C1230. doi: 10.1152/ajpcell.00415.2004. [DOI] [PubMed] [Google Scholar]
- 23.Murayama T., Ogawa Y. Characterization of type 3 ryanodine receptor (RyR3) of sarcoplasmic reticulum from rabbit skeletal muscles. J. Biol. Chem. 1997;272:24030–24037. doi: 10.1074/jbc.272.38.24030. [DOI] [PubMed] [Google Scholar]
- 24.Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid with calcium around neutral pH. J. Biochem. 1980;87:1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- 25.Murayama T., Kurebayashi N., Ogawa Y. Role of Mg2+ in Ca2+-induced Ca2+ release through ryanodine receptors of frog skeletal muscle: modulations by adenine nucleotides and caffeine. Biophys. J. 2000;78:1810–1824. doi: 10.1016/S0006-3495(00)76731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oba T., Murayama T., Ogawa Y. Redox states of type 1 ryanodine receptor alter Ca2+ release channel response to modulators. Am. J. Physiol. Cell Physiol. 2002;282:C684–C692. doi: 10.1152/ajpcell.01273.2000. [DOI] [PubMed] [Google Scholar]
- 27.Oba T., Maeno Y. Acetaldehyde alters Ca2+-release channel gating and muscle contraction in a dose-dependent manner. Am. J. Physiol. Cell Physiol. 2004;286:C1188–C1194. doi: 10.1152/ajpcell.00388.2003. [DOI] [PubMed] [Google Scholar]
- 28.Laver D. R., Baynes T. M., Dulhunty A. F. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J. Membr. Biol. 1997;156:213–229. doi: 10.1007/s002329900202. [DOI] [PubMed] [Google Scholar]
- 29.Meissner G., Rios E., Tripathy A., Pasek D. A. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J. Biol. Chem. 1997;272:1628–1638. doi: 10.1074/jbc.272.3.1628. [DOI] [PubMed] [Google Scholar]
- 30.Marks A. R. Cellular functions of immunophilins. Physiol. Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 31.Mickelson J. R., Gallant E. M., Litterer L. A., Johnson K. M., Rempel W. E., Louis C. F. Abnormal sarcoplasmic reticulum ryanodine receptor in malignant hyperthermia. J. Biol. Chem. 1988;263:9310–9315. [PubMed] [Google Scholar]
- 32.Hawkes M. J., Nelson T. E., Hamilton S. L. [3H]Ryanodine as a probe of changes in the functional state of the Ca2+-release channel in malignant hyperthermia. J. Biol. Chem. 1992;267:6702–6709. [PubMed] [Google Scholar]
- 33.Ohta T., Endo M., Nakano T., Morohoshi Y., Wanikawa K., Ohga A. Ca2+-induced Ca2+ release in malignant hyperthermia-susceptible pig skeletal muscle. Am. J. Physiol. 1989;256:C358–C367. doi: 10.1152/ajpcell.1989.256.2.C358. [DOI] [PubMed] [Google Scholar]
- 34.Carrier L., Villaz M., Dupont Y. Abnormal rapid Ca2+ release from sarcoplasmic reticulum of malignant hyperthermia susceptible pigs. Biochim. Biophys. Acta. 1991;1064:175–183. doi: 10.1016/0005-2736(91)90299-n. [DOI] [PubMed] [Google Scholar]
- 35.Shomer N. H., Mickelson J. R., Louis C. F. Caffeine stimulation of malignant hyperthermia-susceptible sarcoplasmic reticulum Ca2+ release channel. Am. J. Physiol. 1994;267:C1253–C1261. doi: 10.1152/ajpcell.1994.267.5.C1253. [DOI] [PubMed] [Google Scholar]
- 36.Mickelson J. R., Litterer L. A., Jacobson B. A., Louis C. F. Stimulation and inhibition of [3H]ryanodine binding to sarcoplasmic reticulum from malignant hyperthermia susceptible pigs. Arch. Biochem. Biophys. 1990;278:251–257. doi: 10.1016/0003-9861(90)90255-w. [DOI] [PubMed] [Google Scholar]
- 37.Fill M., Coronado R., Mickelson J. R., Vilven J., Ma J. J., Jacobson B. A., Louis C. F. Abnormal ryanodine receptor channels in malignant hyperthermia. Biophys. J. 1990;57:471–475. doi: 10.1016/S0006-3495(90)82563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laver D. R., Owen V. J., Junankar P. R., Taske N. L., Dulhunty A. F., Lamb G. D. Reduced inhibitory effect of Mg2+ on ryanodine receptor-Ca2+ release channels in malignant hyperthermia. Biophys. J. 1997;73:1913–1924. doi: 10.1016/S0006-3495(97)78222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen V. J., Taske N. L., Lamb G. D. Reduced Mg2+ inhibition of Ca2+ release in muscle fibers of pigs susceptible to malignant hyperthermia. Am. J. Physiol. 1997;272:C203–C211. doi: 10.1152/ajpcell.1997.272.1.C203. [DOI] [PubMed] [Google Scholar]
- 40.Balog E. M., Fruen B. R., Shomer N. H., Louis C. F. Divergent effects of the malignant hyperthermia-susceptible Arg615→Cys mutation on the Ca2+ and Mg2+ dependence of the RyR1. Biophys. J. 2001;81:2050–2058. doi: 10.1016/S0006-3495(01)75854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shomer N. H., Louis C. F., Fill M., Litterer L. A., Mickelson J. R. Reconstitution of abnormalities in the malignant hyperthermia-susceptible pig ryanodine receptor. Am. J. Physiol. 1993;264:C125–C135. doi: 10.1152/ajpcell.1993.264.1.C125. [DOI] [PubMed] [Google Scholar]
- 42.Bittar E. E. Washington, DC: Butterworth Inc.; 1964. Cell pH. [Google Scholar]
- 43.Hagberg H., Larsson S., Haljamae H. A new design of double-barrelled microelectrodes for intracellular pH-measurement in vivo. Acta Physiol. Scand. 1983;118:149–153. doi: 10.1111/j.1748-1716.1983.tb07255.x. [DOI] [PubMed] [Google Scholar]
- 44.Endo M., Yagi S., Ishizuka T., Horiuti K., Koga Y., Amaha K. Changes in the Ca-induced Ca release mechanism in the sarcoplasmic reticulum of the muscle from a patient with malignant hyperthermia. Biomed. Res. 1983;4:83–92. [Google Scholar]
- 45.Kawana Y., Iino M., Horiuti K., Matsumura N., Ohta T., Matsui K., Endo M. Acceleration in calcium-induced calcium release in the biopsied muscle fibers from patients with malignant hyperthermia. Biomed. Res. 1992;13:287–297. [Google Scholar]
- 46.Richter M., Schleithoff L., Deufel T., Lehmann-Horn F., Herrmann-Frank A. Functional characterization of a distinct ryanodine receptor mutation in human malignant hyperthermia-susceptible muscle. J. Biol. Chem. 1997;272:5256–5260. doi: 10.1074/jbc.272.8.5256. [DOI] [PubMed] [Google Scholar]
- 47.Tong J., Oyamada H., Demaurex N., Grinstein S., McCarthy T. V., MacLennan D. H. Caffeine and halothane sensitivity of intracellular Ca2+ release is altered by 15 calcium release channel (ryanodine receptor) mutations associated with malignant hyperthermia and/or central core disease. J. Biol. Chem. 1997;272:26332–26339. doi: 10.1074/jbc.272.42.26332. [DOI] [PubMed] [Google Scholar]
- 48.Dirksen R. T., Avila G. Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1. Biophys. J. 2004;87:3193–3204. doi: 10.1529/biophysj.104.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto T., Ikemoto N. Peptide probe study of the critical regulatory domain of the cardiac ryanodine receptor. Biochem. Biophys. Res. Commun. 2002;291:1102–1108. doi: 10.1006/bbrc.2002.6569. [DOI] [PubMed] [Google Scholar]
- 50.Bannister M. L., Hamada T., Murayama T., Harvey P. J., Casarotto M. G., Dulhunty A. F., Ikemoto N. Malignant hyperthermia mutation sites in the Leu2442–Pro2477 (DP4) region of RyR1 are clustered in a structurally and functionally definable area. Biochem. J. 2007;401:333–339. doi: 10.1042/BJ20060902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dirksen R. T., Avila G. Altered ryanodine receptor function in central core disease: leaky or uncoupled Ca2+ release channels? Trends Cardiovasc. Med. 2002;12:189–197. doi: 10.1016/s1050-1738(02)00163-9. [DOI] [PubMed] [Google Scholar]