Abstract
The mitochondrion is a major organelle contributing to energy metabolism but also a main site of ROS (reactive oxygen species) production. LPS (lipopolysaccharide)-induced ROS signalling is a critical event in macrophage activation. In the present paper we report that part of LPS-mediated ROS signalling comes from mitochondria inside a signal amplification loop that enhances MAPK (mitogen-activated protein kinase) activation. More precisely, we have identified the inner mitochondrial membrane UCP2 (uncoupling protein 2) as a physiological brake on ROS signalling. Stimulation of murine bone marrow-derived macrophages by LPS quickly down-regulated UCP2 through the JNK (c-Jun N-terminal kinase) and p38 pathways. UCP2 down-regulation was shown to be necessary to increase mitochondrial ROS production in order to potentiate MAPK activation. Consistent with this, UCP2-deficient macrophages exhibit an enhanced inflammatory state characterized by increased nitric oxide production and elevated migration ability. Additionally, we found that the absence of UCP2 renders macrophages more resistant to nitric oxide-induced apoptosis.
Keywords: lipopolysaccharide, mitochondria, mitogen-activated protein kinase (MAPK), reactive oxygen species (ROS), signalling, uncoupling protein 2 (UCP2)
Abbreviations: ATF-2, activating transcription factor-2; DMNQ, 2,3-dimethoxy-1,4-naphthoquinone; ERK, extracellular-signal-regulated kinase; HUVEC, human umbilical-vein endothelial cells; IFNγ, interferon γ; IκB, inhibitor of κB; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; Mnk-1, MAPK-interacting kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB; NO, nitric oxide; ROS, reactive oxygen species; SNAP, S-nitroso-N-acetyl-DL-penicillamine; TNF-α, tumour necrosis factor α; TLR, Toll-like receptor; UCP2, uncoupling protein 2
INTRODUCTION
Innate immunity, the host's first line of defense against pathogens, relies on many cells such as neutrophils and macrophages. Activation of macrophages finally induces production of NO (nitric oxide), cytokines and ROS (reactive oxygen species) as prime tools of antimicrobial function [1]. Innate activation of macrophages occurs with the recognition of microbial products [2,3] such as LPS (lipopolysaccharide) [1–4]. LPS stimulation triggers signalling pathways leading to activation of the three members of the MAPK (mitogen-activated protein kinase) family [3,5], which are ERK (extracellular-signal-regulated kinase), JNK (c-Jun N-terminal kinase) and p38 [6–9], and also to activation of the nuclear transcription factor NF-κB (nuclear factor κB) [2–3]. Furthermore, LPS induces an early ROS production, which constitutes a primordial signalling pathway potentiating MAPK activation [10–12]. Both NADPH oxidase [13–15] and mitochondria [12,16,17] are associated with this ROS signalling.
UCP2 (uncoupling protein 2) is a carrier protein located in the inner mitochondrial membrane [18] which negatively regulates ROS production [19–21]. Several lines of evidence underline its role in immunity. First, UCP2 is expressed in immune cells such as phagocytes and lymphocytes [22]. Secondly, Ucp2−/− mice are more resistant to a Toxoplasma gondii or Listeria monocytogenes infection than Ucp2+/+ mice [20,22]. Thirdly, the development of unstable atherosclerotic plaques is greater in a Ucp2−/− mouse model of atherosclerosis [23]. Finally, transgenic mice overexpressing UCP2 show a reduced inflammatory response following LPS treatment [24].
The aim of the present study was (i) to provide mechanistic evidence to explain the immune phenotypes of Ucp2−/− and Ucp2+/+ macrophages; (ii) to demonstrate the involvement of mitochondria in LPS-induced ROS signalling; and (iii) to identify the mitochondrial protein UCP2 as a physiological brake on this phenomenon. Therefore we propose a signal amplification loop model in which UCP2 is down-regulated in response to LPS through the JNK and p38 pathways. This down-regulation increases mitochondrial ROS production, which in turn enhances MAPK activity in order to enable the macrophage response.
EXPERIMENTAL
Reagents
LPS from Escherichia coli (serotype 0111:B4), SNAP (S-nitroso-N-acetyl-DL-penicillamine), DMNQ (2,3-dimethoxy-1,4-naphthoquinone), SP600125, SB202190, PD98059, rapamycin, rotenone and antimycin A were purchased from Sigma–Aldrich. Recombinant murine IFNγ (interferon γ) was from PBL. MCP-1 (monocyte chemoattractant protein-1) and IL (interleukin)-10 were from R&D Systems. Synthetic double-stranded RNA [p(I:C)] was from Amersham Pharmacia.
Animals and macrophage culture
Studies on mice were performed in agreement with the institutional CNRS guidelines defined by the European Community guiding principles and by the French decree No. 87/848 of October 19, 1987. Authorization to perform animal experiments was given by the French Ministry of Agriculture, Fisheries and Food (A92580 issued February 2 1994 and 92-148 issued May 14, 2002). Ucp2−/− and Ucp2+/+ littermate mice raised in a C57BL/6J genetic background were maintained under a 12 h light, 12 h dark schedule, with food and water ad libitum. Bone marrow-derived macrophages were obtained as previously described [25]. Briefly, mice were killed, femurs were dissected and bone marrow tissue was flushed with RPMI 1640 medium. Cell aggregates were dispersed by passing through a 25-gauge needle. Cells were cultured in RPMI 1640 with glutamax containing 10% foetal calf serum and 15% L929 cell-conditioned medium as a source of macrophage colony-stimulating factor. Following 7 days of culture, macrophages were obtained as an adherent cell population and were synchronized by an overnight macrophage colony-stimulating factor deprivation. Macrophages were trypsinized and plated at 4×106 cells/ml, except when otherwise stated. When Ucp2−/− and Ucp2+/+ macrophage responses were compared, macrophages were cultured in fresh medium 2 h prior to any experiment.
Endothelial cell culture and macrophage transmigration
HUVEC (human umbilical-vein endothelial cells) were cultured using the endothelial cell growth medium system from Clonetics (Cambrex Bio Science) containing 2% foetal calf serum according to the manufacturer's protocol. HUVEC were used in the fourth passage for experiments. Transmigration of macrophages through a confluent monolayer of HUVEC was analysed upon colorimetric labelling of the transmigrated macrophages using the QCM™ 24-well collagen-based cell invasion assay (Chemicon®). Briefly, 5×104 HUVEC were seeded into invasion chamber inserts of the assay and were grown to confluence. Afterwards, 5×105 macrophages were added to the confluent endothelial monolayer and cells were incubated overnight with or without MCP-1 (10 ng/ml) and/or IFNγ (100 units/ml). The number of transmigrated macrophages was analysed photometrically (λ=550 nm) after cell staining according to the manufacturer's protocol.
Western blot analysis
Whole-cell extracts were obtained by cell lysis with Roti-load (Carl Roth). Mitochondrial protein extracts were loaded on to polyacrylamide gels, separated by electrophoresis and transferred onto nitrocellulose membranes as previously described [26]. Western blots were hybridized with an anti-UCP2 antibody (hUCP2-605 at 1/2000 dilution) followed by a rabbit anti-IgG (at 1/3000 dilution) or an anti-porin antibody (Molecular Probes; 1/4000 dilution) followed by a mouse anti-IgG (Sigma Aldrich; 1/3000 dilution). The amount of UCP2 was normalized by using antibodies against porin. iNOS (inducible NO synthase), IκB (inhibitor of κB) and β-actin were detected with a rabbit polyclonal antibody from Sigma, Cell Signaling Technology and Santa Cruz Biotechnology respectively. Direct recording of the chemiluminescence (ECL kit, Amersham Pharmacia Biotech) was performed using the CCD camera of a GeneGnome analyser and quantification was achieved using Genetool software (Ozyme).
Determination of MAPK and ATF-2 (activating transcription factor-2) phosphorylation
p38, ERK and ATF-2 phosphorylation was determined using Pathscan Sandwich ELISA kits specific to phosphorylated forms of p38, ERK1/2 and ATF-2 (Cell Signaling Technology) according to the manufacturer's protocol.
Determination of NF-κB activity
NF-κB DNA binding activity in nuclei of Ucp2−/− and Ucp2+/+ macrophages was determined using a TransAM NF-κB kit (Active Motif) according to the manufacturer's protocol.
Cytokine measurement and NO production
Macrophages were treated with LPS for 24 h. Culture medium was collected and centrifuged for 5 min at 1000 g at 20 °C to remove any detached cells. Cytokine [IL-6, TNF-α (tumour necrosis factor α) and MCP-1] production was assayed by ELISA using the R&D systems kit according to the manufacturer's instructions. NO production was estimated by measuring nitrite in the macrophage medium. Samples were incubated with an equal volume of Griess reagent and absorbance at 550 nm was measured. Macrophages (0.15×106) were cultured in RPMI 1640 without Phenol Red to avoid interference with Griess absorbance. The nitrite concentration was obtained by comparison to a standard curve.
Mitochondrial ROS production
Mitochondrial ROS production was estimated by analysis of aconitase and fumarase activities. Non-stimulated or LPS-stimulated macrophages were resuspended in isolation buffer [320 mM sucrose, 1 mM EGTA, 10 mM Tris/Base, 0.2% BSA (pH 7.4)] with protease inhibitors (1 μg/ml pepstatin, 4 μg/ml aprotinin, 2 μg/ml leupeptin and 5 μg/ml bestatin) and homogenized in a Dounce homogenizer. Unbroken cells and nuclei were removed by centrifugation for 10 min at 750 g at 4 °C. The supernatant was centrifuged for 20 min at 12000 g at 4 °C and mitochondria were resuspended in isolation buffer then lysed in 0.2% Triton X-100. Enzymatic activities were measured as previously described [27] by following the absorbance increase at 240 nm for 20 min in appropriate medium (30 mM sodium isocitrate, 50 mM Tris/HCl, 0.6 mM MnCl2 at pH 7.4 for aconitase, and 50 mM sodium L-malate, 50 mM sodium phosphate at pH 7.4 for fumarase). The linear rate allowed evaluation of enzymatic activities. The fumarase/aconitase ratio was expressed as the ratio of respective rates of absorbance increase.
Measurement of apoptosis
Apoptosis was quantified using an ELISA technique (Cell Death Detection ELISA Plus, Roche) directed against cytoplasmic histone-associated DNA fragments. The loss of plasma membrane asymmetry was assayed by annexin-V and propidium iodide staining with the Annexin V-FITC Apoptosis Detection Kit I (Pharmingen).
Statistical analysis
Values are expressed as means±S.E.M. and n represents the number of independent experiments. Comparisons among the different groups were assessed by one-way ANOVA. The level of significance (P value) was *P<0.05, **P<0.01 and ***P<0.005.
RESULTS
Ucp2−/− macrophages exhibit an increased inflammatory response
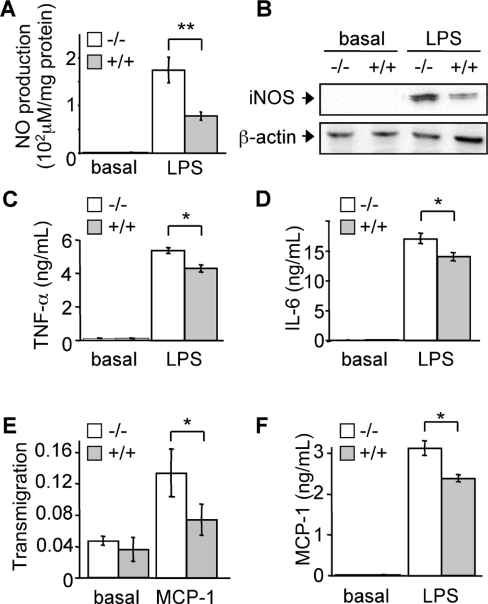
To determine the involvement of UCP2 in immunity and more precisely in macrophages, we used primary bone marrow-derived macrophage cultures. The suitability of our cellular model was first checked by analysing known phenotypes. To this end, we examined the inhibitory role of UCP2 in NO and cytokine production in response to LPS [21,28]. When cells were stimulated with LPS for 24 h, we observed a 2.1-fold higher NO production (Figure 1A) and a 2.5-fold higher iNOS protein expression (Figure 1B) in Ucp2−/− macrophages compared with Ucp2+/+ macrophages. At the same time, TNF-α (Figure 1C) and IL-6 (Figure 1D) releases were significantly higher in Ucp2−/− macrophages. Our data strongly agree with recent reports [21,28], proving the accuracy of our cellular model for further investigations.
Figure 1. Ucp2−/− macrophages exhibit an increased inflammatory response.
(A–D) Macrophages were stimulated with LPS (1 μg/ml) or left unstimulated for 24 h. NO production (A) was determined (n=4) and iNOS protein (B) was quantified by Western blot analysis. The amount of β-actin served as a loading control. Western blots represent the results of one experiment representative of four independent experiments. TNF-α (C) and IL-6 (D) secretions of macrophages were measured by ELISA (n=3). (E) Transmigration of macrophages (A550 nm) through an endothelial cell monolayer in the presence or absence of MCP-1 was determined as described in the Experimental procedures section (n=3). (F) MCP-1 release of macrophages stimulated with LPS (1 μg/ml) or unstimulated for 24 h was measured by ELISA (n=3). *P<0.05, **P<0.01.
We then looked for other immune phenotypes of Ucp2−/− macrophages. A pivotal event in the inflammatory response is transmigration of immune cells through the endothelial barrier. Therefore we studied the ability of macrophages to migrate upon stimulation by the chemokine MCP-1. Interestingly, transmigration of Ucp2−/− macrophages through a confluent monolayer of endothelial cells (HUVEC) was 2-fold higher than that of the Ucp2+/+ macrophages (Figure 1E). Also, MCP-1 release of Ucp2−/− macrophages was higher than that of Ucp2+/+ macrophages after 24 h of LPS stimulation (Figure 1F), indicating a better ability of Ucp2−/− macrophages to recruit immune cells and a better ability to migrate.
LPS-induced mitochondrial ROS production is controlled by UCP2
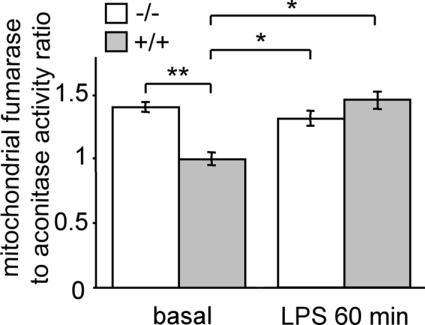
Since UCP2 is described as a regulator of ROS production [20,21], the mitochondrial ROS generation of macrophages following LPS stimulation was investigated. We used an assay of the fumarase/aconitase ratio to study this as this is a specific measurement of the imbalance between mitochondrial ROS production and antioxidant defense rather than an assay of overall cellular ROS production. Mitochondrial aconitase, an enzyme of the Krebs cycle, is a target of mitochondrial ROS because of its Fe-S centre [29,30] whereas fumarase, another Krebs cycle enzyme, is ROS-insensitive [31]. The ratio of fumarase/aconitase activities provides physiologically relevant information since it tests the activity of a known mitochondrial ROS target [31,32]. Under basal conditions, mitochondrial ROS production in Ucp2−/− macrophages was higher than in Ucp2+/+ macrophages (Figure 2). Macrophage stimulation with LPS for 1 h increased the mitochondrial ROS production of Ucp2+/+ macrophages (Figure 2). In contrast, ROS production by Ucp2−/− mitochondria was not modified upon LPS stimulation. As a result, once the macrophage was activated, the ROS level of LPS-treated Ucp2+/+ macrophage mitochondria reached that of Ucp2−/− macrophage mitochondria (Figure 2). This result demonstrates that LPS stimulation suppresses the difference in mitochondrial ROS production between Ucp2−/− and Ucp2+/+ macrophages and suggests an early role for UCP2 in macrophage activation.
Figure 2. LPS-induced mitochondrial ROS production is controlled by UCP2.
Macrophages were stimulated with LPS (1 μg/ml) or unstimulated for 60 min. Mitochondrial ROS production was measured as the ratio of mitochondrial fumarase/aconitase activities as described in the Experimental procedures section (n=4). *P<0.05, **P<0.01.
Macrophage activation by LPS leads to early down-regulation of UCP2
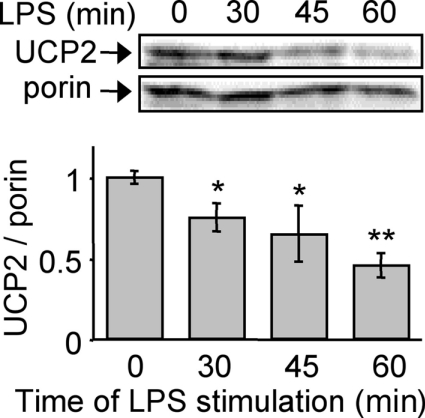
We next investigated the reason why the difference in mitochondrial ROS production was not maintained after 1 h of LPS treatment. UCP2 protein levels began to decrease 30 min after LPS treatment (Figure 3), reached its nadir (50% decrease) after 1 h of LPS treatment and remained low until 48 h (results not shown). Furthermore, in the present conditions, the UCP2 mRNA level was not affected by short LPS stimulation (results not shown), suggesting a decrease in the translation of UCP2 or an increase of the rate of UCP2 degradation. The former hypothesis is in agreement with a half-life of UCP2 of 30 min (S. Rousset, unpublished work). Following 1 h LPS stimulation, Ucp2+/+ macrophages exhibited a negligible UCP2 level and are comparable with Ucp2−/− macrophages. Finally, these results highlight a role for UCP2 in the early phase of macrophage activation.
Figure 3. Macrophage activation by LPS leads to early down-regulation of UCP2.
Mitochondrial fractions were prepared prior to immunoblotting. Immunoblot analysis of UCP2 protein after LPS treatment (1 μg/ml). The amount of porin served as a loading control. Western blots represent the results of one experiment representative of four independent experiments. *P<0.05, **P<0.01.
Ucp2−/− macrophages show a stronger activation of p38 and ERK in response to LPS
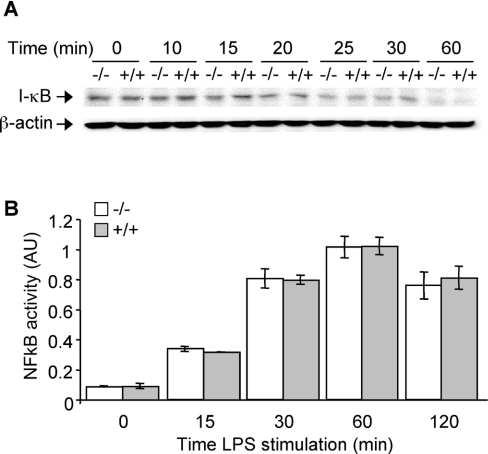
The quick down-regulation of UCP2 protein levels led us to investigate any concurrent changes in signalling pathways. LPS-induced cell activation depends on MAPK and NF-κB activation [2–3,5]. We investigated the kinetics of the activation of these signalling pathways in response to LPS. The kinetics of IκB degradation or of NF-κB DNA binding activity were similar in Ucp2−/− and Ucp2+/+ macrophages (Figures 4A and 4B), therefore excluding the influence of the NF-κB signalling pathway.
Figure 4. The NF-κB signalling pathway is not modified in Ucp2−/− macrophages.
Macrophages were stimulated with LPS (1 μg/ml) for the indicated time periods. (A) IκB degradation was analysed by Western blot. The amount of β-actin served as a loading control. Western blots represent the results of one experiment representative of three independent experiments. (B) NF-κB DNA binding activity in nuclei of macrophages stimulated with LPS (1 μg/ml) or unstimulated was analysed as described in the Experimental section (n=2). AU, arbitrary units.
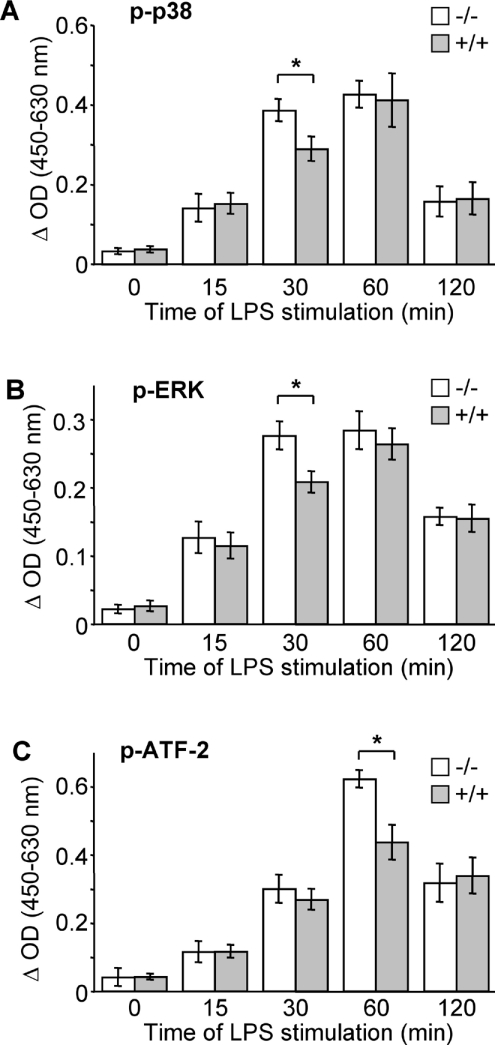
We next analysed MAPK activation by studying the phosphorylation of p38, ERK and JNK. In Ucp2+/+ macrophages, p38 and ERK phosphoryation was maximal 60 min after LPS stimulation, whereas in Ucp2−/− macrophages phosphorylation was maximal only 30 min after LPS stimulation (Figures 5A and 5B). As LPS induced mitochondrial ROS production, the differences were not maintained after 60 min of treatment. No difference in JNK activation was observed between Ucp2−/− and Ucp2+/+ macrophages (results not shown).
Figure 5. LPS-induced p38, ERK and ATF-2 phosphorylation is higher in Ucp2−/− macrophages.
Macrophages were stimulated with LPS (1 μg/ml) for the indicated time periods. (A) Phosphorylation of p38 (p-p38), (B) phosphorylation of ERK (p-ERK), and (C) phosphorylation of ATF-2 (p-ATF-2), was determined by an ELISA-based method as described in the Experimental section (n=3). *P<0.05.
To confirm that the enhanced activation of the p38 pathway had physiologic consequences, we analysed a downstream target of p38 named ATF-2. ATF-2 is a transcription factor implicated in iNOS induction in response to LPS [33]. The phosphorylation level of ATF-2 was significantly enhanced in Ucp2−/− macrophages following 60 min of LPS treatment (Figure 5C). The weak increase in p38 phosphorylation after 30 min of LPS treatment resulted in an even stronger ATF-2 activation after 60 min of LPS treatment.
Our results show that LPS-induced activation of ERK and p38 signalling pathways are earlier in Ucp2−/− macrophages, which leads to a stronger activation of downstream targets of these kinases.
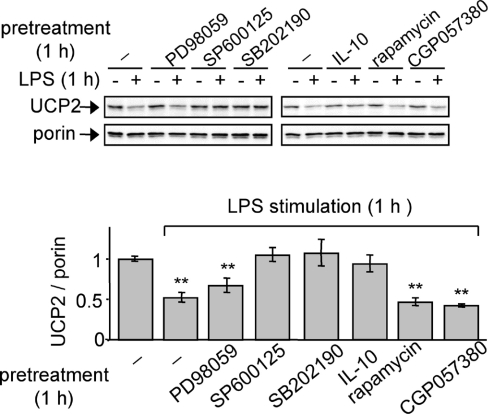
UCP2 down-regulation is mediated by p38 and JNK signalling pathways
Since UCP2 is rapidly down-regulated in response to LPS (Figure 3), the signalling pathways that may be implicated in this phenomenon were investigated. Macrophages were treated for 1 h with MAPK inhibitors prior to 1 h of LPS stimulation. The use of a JNK inhibitor (SP600125) and a p38 inhibitor (SB202190) prevented the LPS-mediated down-regulation of UCP2 protein (Figure 6). In contrast, there was no significant effect of an ERK inhibitor (PD98059) on UCP2 protein levels. Pre-incubation of macrophages with IL-10, an anti-inflammatory cytokine which inhibits MAPK activation [34], confirmed the dependency of UCP2 protein expression on MAPK pathways.
Figure 6. UCP2 down-regulation is mediated by the p38 and JNK signalling pathways.
Immunoblot analysis of UCP2 protein after 1 h of LPS (1 μg/ml) stimulation of macrophages pre-incubated with vehicle, IL-10 (100 ng/ml) or inhibitors of ERK (PD98059; 50 μM), JNK (SP600125; 25 μM), p38 (SB202190; 10 μM), mTOR (rapamycin; 1 μM) or Mnk-1 (CGP057380; 10 μM), (n=3).
We have previously shown that UCP2 mRNA levels did not change after LPS treatment ([20] and results not shown). We have tested the involvement of two MAPK-induced protein kinases involved in translational control in response to LPS [35,36]. Neither inhibition of the mTOR (mammalian target of rapamycin) by rapamycin nor inhibition of Mnk-1 (MAPK-interacting kinase) by its specific inhibitor CGP057380 affected UCP2 down-regulation (Figure 6).
These results suggest that p38 and JNK pathways are the signalling pathways involved in UCP2 down-regulation observed following LPS treatment. Meanwhile the absence of UCP2 is associated with increased activation of MAPK pathways, suggesting the existence of a self-amplification phenomenon.
Increased ROS production by Ucp2−/− macrophages mediates a greater resistance to NO-induced apoptosis
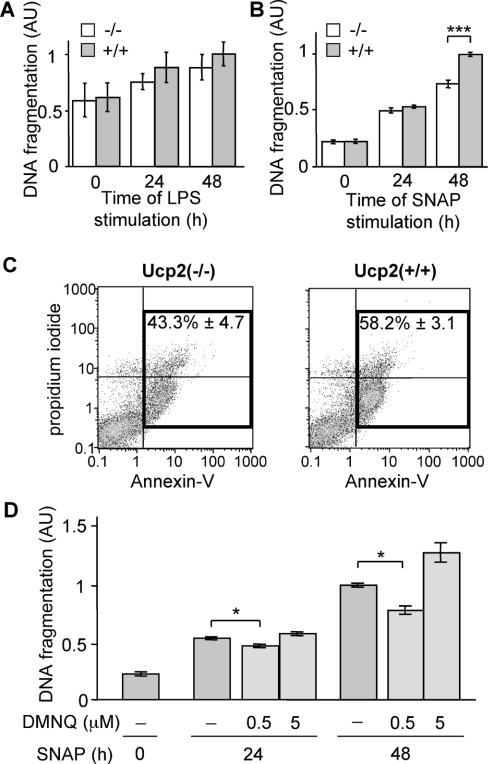
A late event that follows LPS-dependent activation of macrophages is NO-mediated apoptosis [25], which resolves the inflammatory reaction. Since NO production by Ucp2−/− macrophages is 2-fold higher than that of Ucp2+/+ macrophages (Figure 1A), we studied LPS-induced apoptosis. Macrophages were stimulated for 24 and 48 h with LPS and DNA fragmentation was assayed by measuring the presence of histone-associated DNA fragments. Surprisingly, despite the higher NO production observed in Ucp2−/− cells (Figure 1A), LPS-induced apoptosis of Ucp2−/− macrophages was similar to apoptosis of Ucp2+/+ macrophages (Figure 7A). These results suggest a better resistance of Ucp2−/− macrophages to NO-induced apoptosis.
Figure 7. Ucp2−/− macrophages are more resistant to NO-induced apoptosis.
(A) DNA fragmentation in macrophages stimulated with LPS (1 μg/ml) was analysed by ELISA as described in the Experimental section (n=3). (B and C) Macrophages were treated with SNAP (50 μM), an NO donor, for the indicated times. (B) DNA fragmentation was analysed by ELISA (n=5). (C) Annexin-V and propidium iodide staining was analysed by flow cytometry 48 h after SNAP treatment (n=5). (D) Apoptosis was induced by treating Ucp2+/+ macrophages with SNAP (50 μM) in the presence or absence of different concentrations of DMNQ, a ROS donor. DNA fragmentation was analysed by ELISA (n≥3). *P<0.05, ***P<0.005.
To test this hypothesis, both types of macrophages were treated with SNAP, an NO donor. Apoptosis was assessed by quantification of DNA fragmentation (Figure 7B), as described previously [25]. There was no difference in the apoptosis of Ucp2−/− and Ucp2+/+ macrophages following 24 h of SNAP treatment. Interestingly, after 48 h of treatment, there were significantly more Ucp2+/+ macrophages undergoing apoptosis than Ucp2−/− macrophages (Figure 7B), confirming our hypothesis that Ucp2−/− macrophages have a better resistance to NO-induced apoptosis. The detection of the loss of plasma membrane asymmetry by annexin-V/propidium iodide staining was also in agreement with this hypothesis (Figure 7C).
It has been reported previously that increased superoxide production by macrophages is in part responsible for their enhanced resistance to NO-induced cell death [37]. Moreover appropriate concentrations of NO and superoxide was able to reduce NO-induced apoptosis [38]. Consequently we investigated whether this phenomenon could explain the better resistance of Ucp2−/− macrophages to NO. Ucp2+/+ macrophages were treated as described above with SNAP in the presence of DMNQ, a ROS donor (Figure 7D). After SNAP treatment in the presence of 0.5 μM DMNQ, DNA fragmentation was significantly reduced compared with macrophages treated with SNAP alone. After 48 h, a higher concentration of DMNQ led to increased apoptosis, reflecting the cytotoxic/pro-apoptotic effects of ROS at high concentrations [39] (Figure 7D). We suggest that Ucp2−/− macrophages may be better protected against NO-mediated cell death than Ucp2+/+ macrophages because of their higher basal ROS production.
DISCUSSION
Beyond antimicrobial defense during the oxidative burst, ROS are key players in immune cell signalling [40]. In macrophages, the origin of LPS-induced ROS production is not limited to NADPH oxidase [13–15] and the involvement of mitochondria has been proposed [12,16,17]. In the present study, we aimed (i) to demonstrate the involvement of mitochondria in LPS-induced ROS signalling; (ii) to propose the mitochondrial protein UCP2 as a key regulator of this process; and (iii) to provide mechanistic evidence to explain the immune phenotypes of Ucp2−/− and Ucp2+/+ macrophages.
Our primary macrophage culture model was adequate since we reproduced previously published phenotypes [21,28]. Indeed, we confirmed the negative influence of UCP2 on NO and cytokine production in response to LPS. Moreover, UCP2 has been described as a negative regulator of cellular ROS production in macrophages [20,21] as well as in spleen mitochondria [28]. In the present paper, we show for the first time that mitochondria of Ucp2−/− macrophages produce more ROS than mitochondria of Ucp2+/+ macrophages.
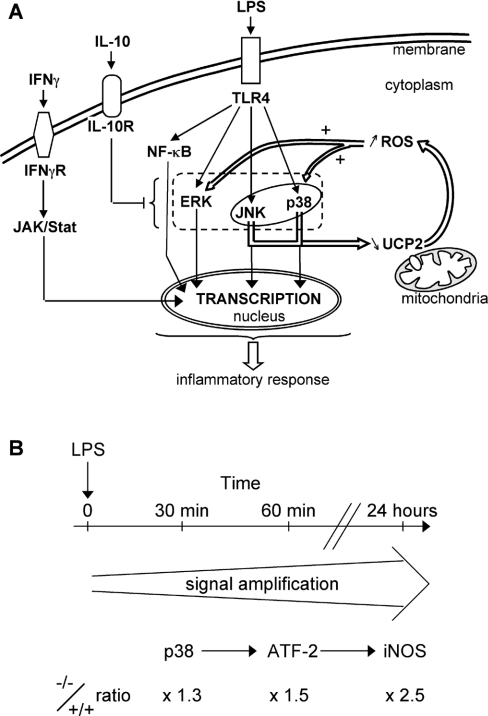
We have previously reported that UCP2 mRNA starts to decrease in peritoneal macrophages about 4 h after LPS treatment [20] and Kizaki et al. [21] described a down-regulation of UCP2 in RAW264 cells, a macrophage cell line, stimulated with LPS for 6 h. In fact, no intermediate times were tested. In the present paper, we show that UCP2 protein down-regulation is an early event required for macrophage activation and that the protein level is down-regulated before the mRNA level (30 min and 4 h respectively). Our results indicate that macrophage activation requires an early need to get rid of the UCP2 protein. We propose a signal amplification loop, in which UCP2 down-regulation is required to enable LPS-induced mitochondrial ROS signalling (Figure 8A).
Figure 8. Model for the role of UCP2 in LPS-induced mitochondrial ROS signalling.
(A) Recognition of LPS by TLR-4 activates JNK, p38 and ERK signalling pathways. Meanwhile, a ROS signalling pathway is set up. UCP2 is quickly down-regulated in response to LPS via the JNK and p38 pathways to increase mitochondrial ROS production. The mitochondrial part of this signal is regulated by UCP2, a physiological brake that has to be switched off in order to enhance mitochondrial ROS production. This mitochondrial ROS generation, as a feedback signal, stimulates the ERK and p38 pathways. In this way, mitochondria contribute to the macrophage inflammatory response. (B) Diagram showing the amplified signalling cascade responsible for the enhanced inflammatory state of Ucp2−/− macrophages.
Once the UCP2 protein level has reached its minimum level, stimulation of macrophages with LPS for 1 h eliminated the differences in fumarase/aconitase activities between Ucp2+/+ and Ucp2−/− macrophages observed under basal conditions. This interesting result indicates that 2-fold UCP2 down-regulation is sufficient to enable mitochondrial ROS production since UCP2 protein expression is not completely removed in LPS-treated macrophages. Therefore similar MAPK phosphorylation after 1 h of LPS stimulation in Ucp2−/− and Ucp2+/+ macrophages is a direct consequence of similar ROS production.
The enhanced ROS signalling promoted p38 and ERK activity in Ucp2−/− macrophages. These two MAPKs are known to play a key role in LPS-induced signal transduction pathways in macrophages [3,5,41]. Accordingly, ATF-2 phosphorylation, a transcription factor involved in iNOS induction [33] and a downstream target of p38, was clearly increased in LPS-treated Ucp2−/− macrophages. As a consequence, the inflammatory response of Ucp2−/− macrophages is up-regulated (Figures 1 and 8B). They produce twice as much NO, a major mediator in the antimicrobial response [42]. Their 2-fold higher transmigration ability is also a very important result since extravasation is an early key event in inflammation [43]. Finally, they are more resistant to NO-mediated cell death than Ucp2+/+ macrophages. Thus, taken together, it becomes clear that Ucp2−/− macrophages are more powerful immune cells than Ucp2+/+ macrophages. However, despite a higher ROS production under basal conditions, Ucp2−/− macrophages have neither elevated MAPK activity, NO and cytokine production nor iNOS expression. Therefore the difference in the redox state of Ucp2−/− and Ucp2+/+ macrophages is not sufficient to induce macrophage activation in the absence of an appropriate stimulus. This may explain why Ucp2−/− mice do not develop inflammatory events in basal conditions. UCP2 is only a brake that has to be switched off in order to allow mitochondrial ROS to amplify MAPK signalling and allow the setting-up of the inflammatory response (Figure 8A).
Unlike Bai et al. [28] who noted increased NF-κB activity in the spleen of Ucp2−/− mice, we observed differences neither in NF-κB activity nor in IκB degradation under basal conditions or after LPS stimulation in our in vitro macrophage culture system. The observation of Bai et al. is probably a consequence of interactions between the diversity of immune cells present in spleen.
IFNγ pretreatment of macrophages eliminated the differences in NO production and transmigration between Ucp2−/− and Ucp2+/+ macrophages (results not shown). IFNγ is responsible for macrophage priming through the JAK/STAT (Janus kinase/signal transducer and activator of transcription) signalling pathway [44,45]. In this way it increases the responsiveness of macrophages to LPS [21] without modification of UCP2 protein levels, eliminating the benefits of Ucp2−/− over Ucp2+/+ macrophages. Therefore IFNγ-activation of macrophages is independent of UCP2 (Figure 8A).
NO and ROS are two well-known pro-apoptotic agents. We found that Ucp2−/− macrophages are more resistant to NO-induced apoptosis than Ucp2+/+ macrophages and we suggest that it may be a consequence of their intrinsically higher ROS production. Indeed previous studies have demonstrated that an adequate amount of ROS production protects against NO-mediated apoptosis [37,38]. Thus UCP2 down-regulation leads to participation of a modified intracellular redox state in the inflammatory response as well as in the protection against the pro-apoptotic effects of NO.
Our present results clarify previous studies by giving a mechanistic explanation of their findings. We have shown that Ucp2−/− mice resist T. gondii infection due to higher elimination of T. gondii by Ucp2−/− macrophages [20]. In order to escape the immune response, T. gondii inhibit MAPK phosphorylation by promoting phosphatase activity in macrophages [46]. Since phosphatases are ROS-sensitive [12,14], enhanced ROS signalling in Ucp2−/− macrophages can counteract the action of T. gondii. In addition, a major weapon against T. gondii is NO. Therefore the difference in mouse survival is not simply a matter of microbicidal ROS production but rather is due to cell activation, leading to higher NO production, as shown in the present study. In another model, i.e. brain ischaemia, inhibition of TLR (Toll-like receptor) signalling contributes to ischaemic tolerance [47]. Cerebral ischaemia triggers acute inflammation which exacerbates primary brain damage [47,48]. Therefore unlike in Ucp2−/− mice, the LPS-induced ROS and MAPK signalling pathways are probably decreased in mice overexpressing UCP2. This can explain the reduced inflammatory response of mice overexpressing UCP2 [24] and their greater resistance to ischaemic brain damage [49]. Finally, the relevance of our in vitro data is corroborated by the increased NO and TNF-α production in Ucp2−/− mice in response to LPS [28].
In summary, the present study provides evidence of a crucial role of UCP2 as a regulator of mitochondrial ROS production and its signalling in early events leading to macrophage activation. Our results should be extended to any immune cell activation since TLR signalling and ROS signalling [40,50] are both important in the function of lymphocytes and phagocytes, which are UCP2-expressing cells [22].
Acknowledgments
This work was supported in part by the CNRS (Centre National de la Recherche Scientifique), INSERM (Institut National de la Santé et de la Recherche Médicale), the ARC (Association pour la Recherche contre le Cancer), the Institut de Recherche Servier and by ECFP6 funding (contract No. LSHM-CT-2003-503041). C. H. and Y. E. are supported by a grant from the Ministère de la Recherche and T. N. receives ECFP6 funding. We thank Dr F. Bouillaud, Dr J. L. Danan, Dr D. Emilie, Dr F. Geissmann, Dr C. Pecqueur and Dr B. Rocha for helpful discussions and Dr D. Marsh for careful reading of the manuscript. We are grateful to Dr H. Gram (Novartis Pharma AG, CH-4002 Basel, Switzerland) for the gift of the Mnk1 inhibitor CGP057380. We thank D. Chamereau for help in the breeding of mice.
References
- 1.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 4.Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., et al. Limits of a deletion spanning Tlr4 in C57BL/10ScCr mice. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 5.Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell. Signalling. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein S. L., Gold M. R., DeFranco A. L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4148–4152. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein S. L., Sanghera J. S., Lemke K., DeFranco A. L., Pelech S. L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J. Biol. Chem. 1992;267:14955–14962. [PubMed] [Google Scholar]
- 8.Hambleton J., Weinstein S. L., Lem L., DeFranco A. L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han J., Lee J. D., Bibbs L., Ulevitch R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 10.Janssen-Heininger Y. M. W., Poynter M. E., Baeuerle P. A. Recent advances towards understanding redox mechanisms in the activation of nuclear factor κB. Free Radical Biol. Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 11.Forman H. J., Torres M. Redox signaling in macrophages. Mol. Aspects Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 12.Cakir Y., Ballinger S. W. Reactive species-mediated regulation of cell signaling and the cell cycle: the role of MAPK. Antioxid. Redox Signaling. 2005;7:726–740. doi: 10.1089/ars.2005.7.726. [DOI] [PubMed] [Google Scholar]
- 13.Park H. S., Jung H. J., Park E. Y., Kim J., Lee W. J., Bae Y. S. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-κB. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 14.Forman H. J., Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am. J. Resp. Crit. Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 15.Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 16.Kamata H., Hirata H. Redox regulation of cellular signalling. Cell. Signalling. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 17.Woo C. H., Lim J. H., Kim J. H. Lipopolysaccharide induces matrix metalloproteinase-9 expression via a mitochondrial reactive oxygen species-p38 kinase-activator protein-1 pathway in Raw 264.7 cells. J. Immunol. 2004;173:6973–6980. doi: 10.4049/jimmunol.173.11.6973. [DOI] [PubMed] [Google Scholar]
- 18.Fleury C., Neverova M., Collins S., Raimbault S., Champigny O., Levi-Meyrueis C., Bouillaud F., Seldin M. F., Surwit R. S., Ricquier D., Warden C. H. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 19.Negre-salvayre A., Hirtz C., Carrera G., Cazenave R., Troly M., Salvayre R., Penicaud L., Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- 20.Arsenijevic D., Onuma H., Pecqueur C., Raimbault S., Manning B. S., Miroux B., Couplan E., Alves-Guerra A. G., Goubern M., Surwit R., et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 21.Kizaki T., Suzuki K., Hitomi Y., Tanigushi N., Saitoh D., Watanabe K., Onoe K., Day N. K., Good R. A., Ohno R.A. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9392–9397. doi: 10.1073/pnas.142206299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rousset S., Emre Y., Join-Lambert O., Hurtaud C., Ricquier D., Cassard-Doulcier A. M. The uncoupling protein 2 modulates the cytokine balance in innate immunity. Cytokine. 2006;35:135–142. doi: 10.1016/j.cyto.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Blanc J., Alves-Guerra M. C., Esposito B., Rousset S., Gourdy P., Ricquier D., Tedgui A., Miroux B., Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- 24.Horvath T. L., Diano S., Miyamoto S., Barry S., Gatti S., Alberati D., Livak F., Lombardi A., Morreno M., Goglia F., et al. Uncoupling proteins-2 and 3 influence obesity and inflammation in transgenic mice. Int. J. Obes. Relat. Metab. Disord. 2003;27:433–442. doi: 10.1038/sj.ijo.0802257. [DOI] [PubMed] [Google Scholar]
- 25.Xaus J., Comalada M., Valledor A. F., Lloberas J., Lopez-Soriano F., Argiles J. M., Bogdan C., Celada A. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood. 2000;95:3823–3831. [PubMed] [Google Scholar]
- 26.Pecqueur C., Alves-Guerra M. C., Gelly C., Levi-Meyrueis C., Couplan E., Collins S., Ricquier D., Bouillaud F., Miroux B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J. Biol. Chem. 2001;276:8705–8712. doi: 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- 27.Criscuolo F., Gonzalez-Barroso M. M., Le Maho Y., Ricquier D., Bouillaud F. Avian uncoupling protein expressed in yeast mitochondria prevents endogenous free radical damage. Proc. R. Soc. London, Ser. B. 2005;272:803–810. doi: 10.1098/rspb.2004.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai Y., Onuma H., Bai X., Medvedev A. V., Misukonis M., Weinberg J. B., Cao W., Robidoux J., Floering L. M., Daniel K. W., Collins S. Persistent nuclear factor-κB activation in Ucp2−/− mice leads to enhanced nitric oxide and inflammatory cytokine production. J. Biol. Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausladen A., Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J. Biol. Chem. 1994;269:29405–29408. [PubMed] [Google Scholar]
- 30.Gardner P., Fridovich I. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J. Biol. Chem. 1992;267:8757–8763. [PubMed] [Google Scholar]
- 31.Li Q. Y., Pedersen C., Day B. J., Patel M. Dependence of excitotoxic neurodegeneration on mitochondrial aconitase inactivation. J. Neurochem. 2001;78:746–755. doi: 10.1046/j.1471-4159.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- 32.Gardner P. Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 33.Bhat N. R., Feinstein D. L., Shen Q., Bhat A. N. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor κB, cAMP response element-binding protein, CCAAT/enhancer-binding protein-β, and activating transcription factor-2. J. Biol. Chem. 2002;277:29584–29592. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- 34.Haddad J. J., Saade N. E., Safieh-Garabedian B. Interleukin-10 and the regulation of mitogen-activated protein kinases: are these signalling modules targets for the anti-inflammatory action of this cytokine? Cell. Signalling. 2003;15:255–267. doi: 10.1016/s0898-6568(02)00075-x. [DOI] [PubMed] [Google Scholar]
- 35.Ueda T., Watanabe-Fukunaga R., Fukuyama H., Nagata S., Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth. Mol. Cell. Biol. 2004;24:6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinstein S. L., Finn A. J., Dave S. H., Meng F., Lowell C. A., Sanghera J. S., DeFranco A. L. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-β. J. Leukocyte Biol. 2000;67:405–414. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- 37.Brüne B., Götz C., Messmer U. K., Sandau K., Hirvonen M. R., Lapetina E. G. Superoxide formation and macrophage resistance to nitric oxide-mediated apoptosis. J. Biol. Chem. 1997;272:7253–7258. doi: 10.1074/jbc.272.11.7253. [DOI] [PubMed] [Google Scholar]
- 38.Sandau K., Pfeilschifter J., Brüne B. The balance between nitric oxide and superoxide determines apoptotic and necrotic death of rat mesangial cells. J. Immunol. 1997;158:4938–4946. [PubMed] [Google Scholar]
- 39.Raha S., Robinson B. H. Mitochondria, oxygen free radicals, and apoptosis. Am. J. Med. Genet. 2001;106:62–70. doi: 10.1002/ajmg.1398. [DOI] [PubMed] [Google Scholar]
- 40.Williams M. S., Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radical Biol. Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 41.Kim S. H., Kim J., Sharma R. P. Inhibition of p38 and ERK MAP kinases blocks endotoxin-induced nitric oxide production and differentially modulates cytokine expression. Pharmacol. Res. 2004;4:433–439. doi: 10.1016/j.phrs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Macmicking J., Xie Q. W., Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 43.Engelhardt B., Wolburg H. Mini-review: transendothelial migration of leukocytes: through the front door or around the side of the house? Eur. J. Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 44.Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu X., Herrero C., Li W. P., Antoniv T. T., Falck-Pedersen E., Koch A. E., Woods J. M., Haines G. K., Ivashkiv L. B. Sensitization of IFN-γ Jak-STAT signaling during macrophage activation. Nat. Immunol. 2002;3:859–865. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- 46.Denkers E. Y., Butcher B. A., Del Rio L., Kim L. Manipulation of mitogen- activated protein kinase/nuclear factor-κB-signaling cascades during intracellular Toxoplasma gondii infection. Immunol. Rev. 2004;201:191–205. doi: 10.1111/j.0105-2896.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 47.Kariko K., Weissman D., Welsh F. A. Inhibition of toll-like receptor and cytokine signaling: a unifying theme in ischemic tolerance. J. Cereb. Blood Flow Metab. 2004;24:1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- 48.Dirnagl U., Iadecola C., Moskowitz M. A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 49.Mattiasson G., Shamloo M., Gido M., Mathi K., Tomasevic G., Yi S., Warden C. H., Castilho R. F., Melcher T., Gonzalez-Zulueta M., et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 2003;9:1062–1067. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- 50.Verweij C. L., Gringhuis S. I. Oxidants and tyrosine phosphorylation: role of acute and chronic oxidative stress in T-and B-lymphocyte signaling. Antioxid. Redox Signaling. 2002;4:543–551. doi: 10.1089/15230860260196344. [DOI] [PubMed] [Google Scholar]