Abstract
Lu (Lutheran) blood group and BCAM (basal cell adhesion molecule) antigens both reside on two gp (glycoprotein) isoforms, Lu and Lu(v13), that differ by the size of their cytoplasmic tail. They are receptors of laminin-10/11 and are expressed in RBCs (red blood cells), epithelial cells of multiple tissues and vascular endothelial cells. To gain more insights into the biological function of Lu/BCAM gps, we looked for potential partners of their cytoplasmic tail. We isolated Ubc9 (ubiquitin-conjugating enzyme 9) protein by screening a human kidney library using the yeast two-hybrid system. Lu/Ubc9 interaction was validated by GST (glutathione S-transferase) pull-down and co-immunoprecipitation experiments. Endogenous Ubc9 formed a complex with endogenous or recombinant Lu gp in A498 and MDCK (Madin–Darby canine kidney) epithelial cells respectively. Replacement of Lys585 by alanine in the Lu gp abolished in vitro and ex vivo interactions of Lu gp with Ubc9 protein. Lu K585A mutant transfected in MDCK cells exhibited a normal basolateral membrane expression but was overexpressed at the surface of polarized MDCK cells as compared with wild-type Lu. Pulse–chase experiments showed extended half-life of Lu K585A gp at the plasma membrane, suggesting an impaired endocytosis of this mutant leading to protein accumulation at the membrane. Furthermore, we showed that the ability of MDCK-Lu K585A cells to spread on immobilized laminin was dramatically decreased. Our results support a physiological role for the direct interaction between Lu gp and Ubc9 protein and reveal a role for this enzyme in regulating the stability of Lu gp at the cell membrane.
Keywords: basal cell adhesion molecule (BCAM), glycoprotein, Lutheran, red blood cell, ubiquitin-conjugating enzyme 9 (Ubc9), yeast two-hybrid system
Abbreviations: BCAM, basal cell adhesion molecule; gp, glycoprotein; GST, glutathione S-transferase; DTT, dithiothreitol; IP, immunoprecipitation; Lu, Lutheran; mAb, monoclonal antibody; MDCK, Madin–Darby canine kidney, RBC, red blood cell; SABC, specific antibody binding capacity; SUMO, small ubiquitin-related modifier; Ubc9, ubiquitin-conjugating enzyme 9; mUbc9, mouse Ubc9; wt, wild-type; ZO-1, zonula occludens 1 protein
INTRODUCTION
Lu (Lutheran) and Lu(v13) are two gp (glycoprotein) isoforms that belong to the immunoglobulin superfamily and which differ only by the length of their cytoplasmic tail (59 and 19 amino acids respectively) (for reviews, see [1,2]). These two gps carry both Lu blood group and BCAM (basal cell adhesion molecule) antigens [3,4] and represent adhesion molecules for laminin-10/11 (α5 chain) in normal and in sickle RBCs (red blood cells) [5–7]. In addition to RBCs, Lu/BCAM antigens are predominantly expressed in the endothelium of blood vessel walls, on the surface of a subset of muscles and in the basal membrane of epithelial cells. In contrast with RBCs, Lu/BCAM antigens are highly expressed in epithelial cells [70000 copies/cell in Caco-2 (colon) or A498 (renal) cells]. Lu gps also exhibit laminin α5 binding properties in endothelial [8] as well as epithelial cells. Indeed, Lu gp is a specific receptor for laminin α5 chain in murine basement membranes [9], and its expression is directly correlated to the expression of laminin α5. In mouse embryos lacking laminin α5, the basal concentration of Lu gp is reduced, whereas the amount of Lu is increased in transgenic mice overexpressing laminin α5 [10]. However, although the interaction between Lu gps and laminin α5 has been clearly demonstrated, the biological function of Lu gps remains unknown.
The 40 C-terminal amino acids specific for the Lu gp cytoplasmic tail carry a dileucine motif involved in the basolateral targeting of this isoform in polarized MDCK (Madin–Darby canine kidney) epithelial cells [11] and contain phosphorylation sites consistent with a receptor signalling function. We recently demonstrated that protein kinase A-mediated phosphorylation of Lu gp positively regulates its adhesion function to laminin α5 in erythroid cells [12]. Both Lu and Lu(v13) isoforms carry in their common C-terminal cytoplasmic tail the RK573–574 motif necessary for direct interaction with erythroid spectrin [13]. As Lu and Lu(v13) represent quantitatively minor components of the RBC membrane (from 1500 to 4000 copies per cell), it is unlikely that these gps play a role in the mechanical properties and the stability of the RBC membrane. We have speculated that the interaction with spectrin may be critical for signalling and/or laminin receptor function. In order to identify new cytoplasmic partners of Lu gps that may regulate their expression and/or function, we have screened a human kidney cDNA library using a yeast two-hybrid system and the cytoplasmic domain of the Lu gp isoform as bait. Among the proteins found to potentially interact with the Lu gp, we focused our analysis on Ubc9 (ubiquitin-conjugating enzyme 9), a protein mainly known to conjugate SUMO-1 (small ubiquitin-related modifier 1; a ubiquitin-like modifier protein) to proteins. Ubc9 protein represents the only E2-type SUMO-conjugating enzyme described in vertebrates (for reviews, see [14,15]). We provide evidence for a direct ionic interaction between the Lys585 of Lu gp and Ubc9 that could play a role in the internalization rate and the turnover as well as in the adhesion properties of the Lu gp.
MATERIALS AND METHODS
Materials
Mouse monoclonal anti-RGS–His6 antibody was purchased from Qiagen (Courtaboeuf, France) and sheep anti-human Ubc9 (recognizes C-terminal peptide CEYEKRVRAQAKKFAPS) was purchased from AG Scientific (San Diego, CA, U.S.A.). mAb (monoclonal antibody) anti-Lub (clone LM342) was from Dr R.H. Fraser (Regional Donor Centre, Glasgow, U.K.), and mAb anti-Lu, clone F241, was from our institute in collaboration with Dr D. Blanchard [EFS (Etablissement Français du Sang), Nantes, France] as well as the rabbit polyclonal anti-Lu antibody, 602 (INTS, Les Ulis, Courtaboeuf, France). Mouse mAb anti-LEX A was from Clontech (Palo Alto, CA, U.S.A) and goat anti-GST (glutathione-S-transferase) from Amersham Biosciences. Mouse monoclonal anti-Glycophorin A, 3F4, was produced by EFS Loire Atlantique (Nantes, France). Dako was purchased from DakoCytomation (Carpinteria, CA, U.S.A.). Monoclonal rat anti-ZO-1 (zonula occludens 1 protein) and polyclonal rabbit anti-α-catenin antibodies were from Chemicon (Euromedex, France). Rat mAb anti-E-cadherin antibody was purchased from Interchim (Montluçon, France). RNase A, purified human laminin-10/11 mixture and 30% (w/v) BSA were purchased from Sigma (St. Louis, MO, U.S.A.). Propidium iodide was from Molecular Probes–Invitrogen (Leek, The Netherlands). Oligonucleotides used in PCR and mutagenesis were purchased from Eurogentec (Seraing, Belgium). Mutagenesis kit (QuikChange® XL site-directed mutagenesis) was from Stratagene (La Jolla, CA, U.S.A.). Yeast and cell culture media were purchased from Q-Biogene (Montreal, ON, Canada) and Invitrogen respectively. The pcDNA3 vector was obtained from Invitrogen. The in vitro sumoylation assay was performed using the sumoylation control kit distributed by Corgen (Guilford, CT, U.S.A.).
Construction of baits
The Lu gp cytoplasmic domain (residues 569–628, see Figure 3 for amino acid sequence) was amplified using forward primer 5′-GGTGGGAATTCTTTACTGCGTGAGACGCAAAGG-3′ and reverse primer 5′-ACCCGCTCGAGTCAGCACTCGTCTCCGAAG-3′. This cDNA EcoRI–XhoI fragment was fused in frame with the DNA-binding domain of LEXA in pLEX12 (a modified pBMT116 plasmid, carrying the tetracycline resistance gene). pLEX-Lu(v13) construct encoding Lu(v13) cytoplasmic domain (residues 569–588) was constructed by PCR introducing a stop codon in the pLEX-Lu construct. Oligonucleotides used were (mutated nucleotides are underlined): sense primer 5′-GAAGGGGGCTCCGTAGCCAGGGGAGCCA-3′ and antisense primer 5-′TGGCTCCCCTGGCTACGGAGCCCCCTTC-3′. Mutants of Lu were obtained by in vitro mutagenesis. Primers used were as follows: for Lu RR572–573AA, sense primer 5′-GGAATTCCTACTGCGTGGCAGCAAAAGGGGGCCC-3′ and antisense primer 5′-GGGCCCCCTTTTGCTGCCACGCAGTAGGAATTCC-3′; for Lu K574A, sense primer 5′-TACTGCGTGAGACGCGCTGGGGGCCCCTGCTGC-3′ and antisense primer 5′-GCAGCAGGGGCCCCCAGCGCGTCTCACGCAGTA-3′; for Lu RR582–583AA, sense primer 5′-CCCTGCTGCCGCCAGGCTGCTGAGAAGGGGGCTCC-3′ and antisense primer 5′-GGAGCCCCCTTCTCAGCAGCCTGGCGGCAGCAGGG-3′; for Lu E584A, sense primer 5′-CCCTGCTGCCGCCAGAGGAGAGCGAAGGGGGCTCCGCCG-3′ and antisense primer 5′-CGGCGGAGCCCCCTTCGCTCTCCTCTGGCGGCAGCAGGG-3′; for Lu K585A, sense primer 5′-CTGCCGCCAGCGTCGTGAGGCAGGGGCTCCGCCG-3′ and antisense primer 5′-CGGCGGAGCCCCTGCCTCACGACGCTGGCGGCAG-3′; and for Lu K585R, sense primer 5′-GCCAGCGTCGTGAGAGGGGGGCTCCACCGCC-3′ antisense primer 5′-GGCGGCGGAGCCCCCCTCTCACGACGCTGGC-3′. The inserts were sequenced using ABI-PRISM 310 genetic analyser (Applied Biosystems). Expression of fusion proteins in yeast was verified by Western blot using mouse mAb anti-LEX A (100 ng/ml).
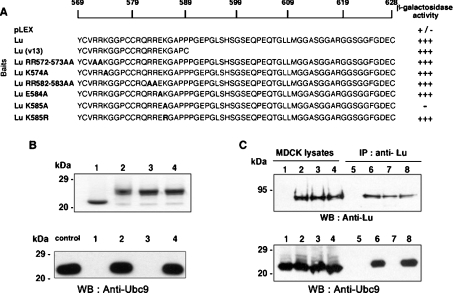
Figure 3. Mapping of the Ubc9-binding site in the cytoplasmic tail of Lu gp.
(A) Yeast two-hybrid experiments. Yeast was co-transformed with pACT2-Ubc9 and pLEX (DNA-binding domain alone), pLEX-Lu, -Lu(v13), -Lu RR572–573AA, -Lu K574A, -Lu RR582–583AA, -Lu E584A, -Lu K585A, or -Lu K585R. β-Galactosidase activity was determined on filters for each construct. Mutated amino acids are in bold type. (B) GST pull-down assays. Upper panel: Coomassie Blue staining was used to show that equivalent amounts of GST (1), GST–Lu (2), GST–Lu K585A (3) and GST–Lu K585R (4) were used. Lower panel: His6-tagged Ubc9 protein was incubated with GST (1), GST–Lu (2), GST–Lu K585A (3) or GST–Lu K585R (4) in pull-down assays. Proteins were eluted and analysed by Western blot (WB) using a sheep anti-Ubc9 antibody. An aliquot of the purified His6-tagged Ubc9 protein was loaded on to the gel as control (‘control’ lane). (C) IP of Lu gp in MDCK cells. Wt (1 and 5) and transfected MDCK clones expressing Lu gp (2 and 6) or mutants Lu K585A (3 and 7) or Lu K585R (4 and 8) were lysed and extracts were subjected to IP with monoclonal LM342 anti-Lub antibody. Presence of Lu gp (upper panel) and Ubc9 (lower panel) were tested by Western blot (WB) in lysates (1–4) and in IP eluates (5–8) using a rabbit polyclonal anti-Lu (602) and a sheep Ubc9 antibodies respectively.
Yeast two-hybrid screening and interaction experiments
Two-hybrid screening was performed as described in [16], in L40 strain expressing pLEX-Lu cytoplasmic tail transformed with 100 μg of oligo(dT) human kidney cDNA library (Clontech, Ozyme). The library was fused with the activation domain of GAL4 in pACT2. A standard lithium chloride transformation procedure [17] was used and transformation efficiency was estimated on selective medium lacking tryptophan and leucine (DO–WL). His+ clones were selected after 3–5 days of growth at 30 °C on selective medium lacking tryptophan, leucine and histidine (DO–WLH). β-Galactosidase activity of His+ clones was estimated either on filters, using X-Gal (5-bromo-4-chloroindol-3-yl β-D-galactopyranoside), or in liquid assays, using ONPG (o-nitrophenyl β-D-galactopyranoside) on pellets corresponding to 1 ml of culture at D600 (attenuance) of 0.5. The results are expressed as Miller units; 1 Miller unit=D420×(1000/time)×(1/D600) [31].
pLEX-Lu interacting clones were identified by sequencing of PCR-amplified products obtained from yeast plasmids (forward primer CGATGATGAAGATACCCCACC and reverse primer GAACTTGCGGGGTTTTTCAG). Sequences were submitted to a BLAST search NCBI (National Center for Biotechnology Information).
The Lu(v13) and the Lu mutant baits used in the specificity analysis were pLEX-Lu(v13), pLEX-Lu gp, RR572–573AA, -K574A, -RR582–583AA, -E584A, -K585A and -K585R, constructed as described above. Colonies from co-transformed yeast were picked, applied in duplicate on the media DO–WL and DO–WLH and left to grow for 3 days at 30 °C. β-Galactosidase activity of His+ clones was estimated either on filters or in liquid assays.
GST pull-down assays and Western blots
The Lu gp cytoplasmic domain was expressed in Escherichia coli BL21 (Stratagene) as GST fusion protein from pGEX-5X-3 plasmid (Amersham Biosciences) essentially as described by Smith and Johnson [18]. After cell lysis by sonication in PBS buffer containing 1% Triton X-100 and protease inhibitor cocktail (Roche), the fusion protein was purified by elution (20 mM glutathione and 50 mM pH 8 Tris) from glutathione beads (Amersham Bioscience). Mutants pGEX-5X-3-Lu K574A, K585A and K585R were constructed by mutagenesis using the Lu plasmid as a template. Oligonucleotides used for mutagenesis were as described above for pLEX constructs.
The Ubc9 cDNA (sequence accession number: NM_020987) was amplified using forward primer 5′-TCGCGGATCCTCGGGGATCGCCCTCAG-3′ and reverse primer 5′-ACGCGTCGACCGATGCCACAAGGTCGC-3′. The cDNA BamHI–SalI fragment was fused in frame with a His6-tagged motif in pQE80 plasmid (Qiagen). The His6-tagged Ubc9 protein was expressed in E. coli BL21. After cell lysis in 1 ml of 50 mM NaH2PO4, 300 mM NaCl, 10 mM pH 8 imidazole and 1 mg/ml of lysozyme for 30 min on ice, cells were sonicated on ice and centrifuged at 10000 g for 30 min. The His6-tagged Ubc9 protein was purified from the lysate using the Ni-NTA spin column kit (Qiagen).
For in vitro interaction, 1 μg (0.05 nmol) of His6-tagged Ubc9 protein was incubated with 2 μg (0.05 nmol) of GST, GST–Lu gp and the Lu gp mutants Lu K574A, K585A and K585R for 1 h at 37 °C with gentle shaking in 250 mM pH 8 imidazole, 20 mM pH 7.5 Hepes, 75 mM KCL, 1 mM EDTA, 2 mM MgCl2, 2 mM DTT (dithiothreitol), 0.5% Nonidet P40 and protease inhibitor cocktail. After extensive washings, two successive elutions of proteins bound to glutathione–Sepharose beads were performed (20 mM glutathione and 50 mM pH 8 Tris).
SDS/PAGE was performed using 15% polyacrylamide gels by the method of Laemmli [19]. Blots were performed on nitrocellulose membranes [20] and probed with anti-RGS–His6 (1:100000) or anti-Ubc9 (1:3000) mAb using the ECL® (enhanced chemiluminescence) system according to the manufacturer's instructions (Amersham Biosciences).
Cell culture and transfection
Human kidney carcinoma epithelial cells A498 (American Type Culture Collection: HB44) were maintained in MEM (minimal essential medium) Glutamax I supplemented with 10% (v/v) fetal bovine serum, 100 units/ml antibiotic antimycotic (Invitrogen), 1 mM sodium pyruvate and 0.1 mM Non-essential Amino Acids, in a humidified atmosphere at 37 °C with 5% CO2. MDCK cells (American Type Culture Collection: CCL-34) were maintained in DMEM (Dulbecco's modified Eagle's medium) Glutamax I supplemented with 10% fetal bovine serum, 100 units/ml antibiotic antimycotic (Invitrogen) and 0.1 mM Non-essential Amino Acids, in a humidified atmosphere at 37 °C with 5% CO2.
Stable MDCK cells expressing wt (wild-type) and mutated Lu gps generated by in vitro mutagenesis as described above for the generation of Y2H baits were obtained as previously described [11]. Briefly, transfected cells were first selected in the presence of 0.6 g/l Geneticin and then Lub-positive cells were detected by flow cytometry using the anti-Lub mAb LM342 and amplified by a round of selection using magnetic beads coated with anti-mouse IgG (Dynabeads-M-450; Dynal AS, Oslo, Norway) as recommended by the manufacturer. This procedure generated pools with at least 90% of Lu-positive cells. Cellular clones were also generated by serial limit dilution and clones showing similar Lu antigen membrane expression [SABC (specific antibody binding capacity) units] were selected (SABC: 538200 for Lu, 526270 for K585A and 467190 for K585R mutants).
IP (immunoprecipitation) assay
Cells (107 and 3×107 A498 cells and stably transfected MDCK cells expressing Lu gp wt, Lu K585A and K585R) were lysed for 20 min at room temperature in 50 mM pH 8 Tris/HCl, 150 mM NaCl, 1 mM DTT and 1% N-octyl-β-D-glucopyranoside (Bachem, Heidelberg, Germany) with protease inhibitor cocktail. Cell extracts were incubated overnight at +4 °C with the anti-Lub mAb or an irrelevant antibody (anti-ZO-1) bound to Protein A–Sepharose (Amersham Bioscience) or incubated only with Protein A–Sepharose as negative control. After extensive washings in lysis buffer, the co-immunoprecipitated proteins were eluted from the Protein A–Sepharose in Laemmli buffer at 100 °C for 5 min and analysed by Western blot using a rabbit polyclonal anti-Lu antibody 602 (1:5000) and an anti-Ubc9 mAb (1:3000).
Confocal fluorescence microscopy
MDCK cells stably transfected with Lu gp wt and the Lu gp mutants K585A and Lu K585R were grown on to 12 mm diameter, 0.4 μM pore Costar Transwell polycarbonate filters (5×105 cells/filter). The transepithelial resistance was measured daily using a Millipore electrical resistance apparatus (Millipore, Bedford, MA, U.S.A.). At days 3, 7 and 11 of culture, polarized cells were fixed 20 min with 4% (w/v) paraformaldehyde, treated with 50 mM NH4Cl in PBS, permeabilized 10 min with 0.5% Triton X-100 (PBS) and incubated with F241 anti-Lu mAb (1:10) in Dako for 1 h at room temperature. Filters were washed with PBS/0.5% BSA and incubated with an Alexa Fluor® 488-conjugated anti-mouse antibody (1:200) (Molecular Probes, Invitrogen) for 1 h at room temperature, washed with PBS–0.5% BSA and treated with RNase before incubation with propidium iodide. Samples were examined by confocal microscopy using a NIKON EC-1 system equipped with 63/1.4 objective.
Membrane steady-state expression of Lu gps
Polarized MDCK monolayers expressing Lu or Lu K585A gp mutant were cultured for 7 days on to 12 mm diameter Costar Transwell polycarbonate filters as described above. Cells were incubated at the basolateral side with 0.5 mg/ml Sulfo-NHS (N-hydroxysuccinimido)-LC-biotin (Pierce) diluted in biotinylation buffer (10 mM Hepes, 150 mM NaCl, 0.2 mM CaCl2 and 0.2 mM MgCl2, pH 7.5) at 4 °C for 30 min. The apical side was incubated with cold PBS. Filters were washed twice with cold PBS, incubated with 10 mM glycine for 10 min at 4 °C and washed twice with cold PBS. Filters were excised and cells were lysed in 800 μl of lysing buffer (150 mM NaCl and 20 mM Tris, pH 8.5; 1% Triton X-100) containing protease inhibitor cocktail (Roche) at 4 °C for 45 min. Lysates were centrifuged at 10000 g for 20 min and supernatants were incubated with Protein A–Sepharose (Amersham Bioscience) and goat serum at 4 °C for 3 h. After this preclearing step, Lu gps were immunoprecipitated by incubating the supernatants with Protein A–Sepharose and anti-Lu mouse mAb F241 overnight at 4 °C. The beads were washed three times with lysis buffer and Lu gps were eluted with 10% (w/v) SDS for 5 min at 100 °C. Half of the eluted proteins were diluted with Laemmli 3× buffer (total Lu gps) and the other half was diluted in 500 μl of lysis buffer and incubated for 3 h with immunopure immobilized streptavidin beads (Pierce) to isolate membrane Lu gps. Beads were washed three times with lysis buffer and Lu gps were eluted in 20 μl of Laemmli buffer for 5 min at 100 °C. Eluates from both steps (total and membrane Lu gps) were run on 8% polyacrylamide gel under reducing conditions and analysed by immunoblotting using a rabbit polyclonal anti-Lu antibody 602 (1:5000).
Membrane targeting assays of Lu gps
Delivery of newly synthesized Lu and Lu K585A gps to the membrane and their turnover were determined by performing a pulse-labelled biotin-targeting assay. Polarized MDCK monolayers expressing Lu or Lu K585A gps were cultured as described above. Newly synthesized proteins were labelled by adding 150 μCi of [35S]methionine/[35S]cysteine (Invitrogen) in the cell culture medium for 20 min incubation at 37 °C. Cells were washed with complete medium and incubated in non-radioactive medium for 60, 90, 120, 150 and 180 min at 37 °C. After each incubation time, cells were washed twice with cold PBS and incubated at the basolateral side with 0.5 mg/ml Sulfo-NHS-LC-biotin (Pierce). IP of total Lu gps and isolation of the membrane fraction were performed as described for the steady-state experiments. All proteins were analysed by SDS/PAGE on 8% polyacrylamide gel under reducing conditions followed by Western blot probed with rabbit polyclonal anti-Lu antibody 602 (1:5000). Samples of biotinylated Lu proteins were analysed by autoradiography to quantify the newly delivered membrane proteins as a function of time.
Morphological cell adhesion assay
The plate wells were coated with 2 μg/ml of laminin-10/11 or 1% BSA overnight at 4 °C. Wells were then washed twice with PBS and were subsequently coated with 1% BSA at 37 °C for 1 h before two additional washes. MDCK-WT, -Lu or -Lu K585A cells (105 cells) were washed twice with serum-free medium and added to the wells. Cells were incubated in serum-free medium for 3 h at 37 °C. After this incubation time, spread and round cells were quantified in four representative areas by microscopy (×100; Leitz) using a computerized image analysis system (Biocom VisioL@b 2000). The counted cells were then averaged and presented as percentage of spread cells.
RESULTS
Identification of Ubc9 as Lu cytoplasmic tail partner in yeast two-hybrid screening
Yeast two-hybrid screening was performed using a human kidney cDNA library with the cytoplasmic tail of Lu gp isoform as bait (amino acids 569–628). Among 106 transformant clones, ten clones representative of five different proteins were retained for further studies among which was the Ubc9.
Three independent clones were isolated, one clone encoding a full-length Ubc9 and two clones encoding a truncated form of Ubc9 deleted for the first eight amino acids. To control the specificity of Lu–Ubc9 interaction, co-transformation experiments were performed in yeast using pACT2-Ubc9 full-length isolated clone in combination with either empty pLEX or pLEX-Lu. Auxotrophy for histidine and β-galactosidase activity were then assessed. Yeast transformed with pACT2-Ubc9 and pLEX-Lu, but not pLEX, was able to grow in the absence of histidine (results not shown). β-Galactosidase activity was quantified with a liquid assay. Yeast transformed with pACT2-Ubc9 and pLEX-Lu had an activity of 3.15 Miller units, while the activity of yeast transformed with pACT2-Ubc9 and pLEX was less than 0.73 Miller units. These results indicated that Ubc9 protein interacts with Lu gp in the yeast two-hybrid system.
Lu gp interacts directly in vitro with Ubc9 independently of SUMO
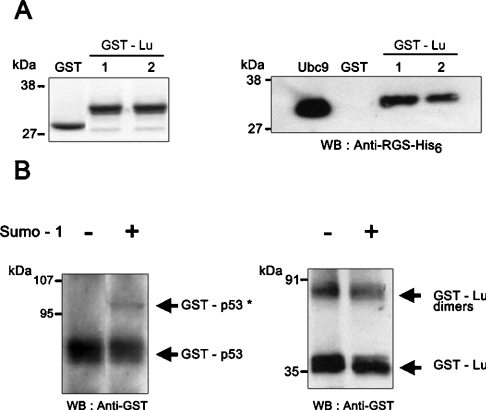
To confirm that Ubc9 protein and the cytoplasmic domain of Lu gp interact directly, we performed in vitro GST pull-down assays using GST and GST–Lu fusion protein incubated with recombinant His6-tagged Ubc9 protein. Coomassie Blue staining allowed us to assess that equivalent amounts of GST and GST–Lu were used for each analysis (Figure 1A, left panel). Western blots of glutathione-eluted samples probed with anti-His6 antibody demonstrated that GST–Lu gp interacted with His6-tagged Ubc9 protein in contrast with GST alone (Figure 1A, right panel). Western blots were also probed with anti-GST antibody to ensure that no degradation of GST proteins occurred during the assay (results not shown). These in vitro experiments confirmed the direct interaction between Ubc9 protein and the cytoplasmic domain of Lu gp revealed by the two-hybrid screening.
Figure 1. In vitro interaction between Lu and Ubc9 studied by GST pull-down assays.
(A) Left panel: Coomassie Blue staining showing that equivalent amounts of GST and GST–Lu were used for each analysis and that no degradation of GST proteins occurred during the assay. Right panel: His6-tagged Ubc9 protein was incubated with GST or GST–Lu in pull-down assays and the eluted proteins were analysed by Western blot (WB) using anti-RGS–His6 antibody. Two successive elutions were performed for GST–Lu interaction (lanes 1 and 2). An aliquot of the purified His6-tagged Ubc9 protein was loaded on to the gel as control (Ubc9 lane). (B) In vitro sumoylation assay with GST–Lu. GST–Lu was incubated with human Aos1/Uba2 (E1), UBC9 (E2) and with or without human SUMO-1. GST–p53 was used as positive control. GST proteins were detected by Western blot using a sheep anti-GST antibody. Sumoylated form of GST–p53* was detected at 96 kDa. No sumoylated form of GST–Lu was detected at the expected molecular mass (53 kDa). Dimers of GST–Lu were detected at 70 kDa.
Ubc9 is known to conjugate SUMO-1 to lysine side chains in target molecules [14]. As the cytoplasmic domain of Lu, which carries two lysine residues (amino acids 574 and 585), may be a target for SUMO-1 in addition to Ubc9, we performed an in vitro sumoylation assay on GST–Lu using GST–p53 as a positive control. As expected, sumoylated form of GST–p53 was detected at 96 kDa (Figure 1B, left panel). In contrast with GST–p53, we did not observe any sumoylation of GST–Lu, expected at 53 kDa (Figure 1B, right panel). These results indicated that SUMO could not be conjugated with the cytoplasmic tail of Lu gp under our experimental conditions.
Lu gps interact with Ubc9 in A498 epithelial cells
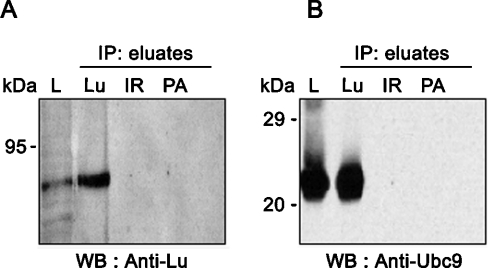
The association between Lu gp and Ubc9 was further investigated in A498 human epithelial cells by IP assays using monoclonal anti-Lub antibody LM342, which recognizes both Lu gp isoforms. As shown in Figure 2 (lane L), A498 cells express Lu gp isoforms and Ubc9 endogenously. After IP with the anti-Lu antibody, the presence of Lu gp and Ubc9 was revealed by Western blot using the anti-Lu rabbit polyclonal antibody 602 (Figure 2A) and the sheep anti-Ubc9 antibody (Figure 2B) respectively, indicating that Ubc9 was co-immunoprecipitated with Lu gp. Lu gp and Ubc9 proteins were not detected when an irrelevant antibody or Protein A–Sepharose alone was used for IP (Figure 2B). These results indicated that the endogenous Lu gp interacts with Ubc9 in A498 epithelial cells.
Figure 2. Ex vivo interaction of Lu and Ubc9 by co-IP.
A498 cells (107) were lysed and extracts were subjected to IP with monoclonal LM342 anti-Lub (Lu), irrelevant antibody (IR) and Protein A–Sepharose (PA). Presence of Lu gps (A) and Ubc9 (B) were studied by Western blot (WB) in lysates (L) and in IP eluates using a rabbit anti-Lu (602) and a sheep anti-human Ubc9 antibodies respectively.
Mapping of the Ubc9-binding site in the cytoplasmic tail of Lu gp
To determine the minimal domain of the Lu cytoplasmic tail involved in this interaction and to identify the amino acids that interact with Ubc9, we used the cytoplasmic tail of Lu(v13) isoform that is deleted of the 40 C-terminal amino acids of Lu, in yeast two-hybrid experiments (Figure 3A). The pLEX-Lu(v13) plasmid (encoding amino acids 569–588) was transformed simultaneously with pACT2-Ubc9. Co-transformants were tested for histidine auxotrophy and β-galactosidase activity on filters. We found that pLEX-Lu(v13)/pACT2-Ubc9 co-transformants grew on deficient DO–WLH medium similarly to pLEX-Lu/pACT2-Ubc9 (results not shown). Moreover, β-galactosidase activity estimated on filters was identical for Lu and Lu(v13) (Figure 3A). Together, these results indicated that the Ubc9-binding site on Lu gps involves amino acids located in the common cytoplasmic sequence of Lu and Lu(v13).
Electrostatic interactions are known to be important for Ubc9 targets in mammalian cells [21]. The common cytoplasmic domain of Lu and Lu(v13) includes two stretches of positively charged amino acids, RRK (amino acids 572–574) and RREK (amino acids 582–585). The RRK motif is entirely conserved between human, bovine, mouse and rat, whereas the RREK motif is partially conserved in these species. In order to map the binding site of Ubc9 in Lu gp cytoplasmic tail, the arginine residues, the lysine residues and the glutamic acid residue of these motifs were first mutated into alanine separately. Interaction with Ubc9 was tested using the yeast two-hybrid system. As shown in Figure 3(A), the interaction of Lu RR572–573AA, Lu K574A, Lu RR582–583AA and Lu E584A mutants with Ubc9 was conserved, whereas mutation of Lys585 into alanine completely abolished this interaction. In order to investigate whether the interaction with Ubc9 involves the lysine intrinsically or the positive charge of this amino acid, we mutated Lys585 into arginine, another positively charged amino acid. Yeast growth on DO–WLH (results not shown) and β-galactosidase activity indicated that the Lu K585R mutant interacted with Ubc9 as efficiently as the Lu wt construct (Figure 3A).
To confirm the involvement of Lys585 in Ubc9 binding, GST pull-down in vitro experiments were performed using purified His6-tagged Ubc9 protein and GST–Lu mutants K585A and K585R and GST–Lu K574A as control. Coomassie Blue staining allowed us to assess that equivalent amounts of GST, GST–Lu, GST–Lu K585A and GST–Lu K585R were used for each analysis and that no degradation of GST proteins occurred during the assay (Figure 3B, upper panel). As revealed with the anti-Ubc9 antibody, only K585A mutation totally abolished GST–Lu interaction with Ubc9 confirming the loss of interaction observed in yeast (Figure 3B, lower panel, lane 3), whereas mutation K585R had no effect on Ubc9 binding to GST–Lu (Figure 3B, lower panel, lane 4). Together, results obtained from GST pull-down and yeast two-hybrid experiments pointed out Lys585 as the critical residue involved in Lu gps binding to Ubc9.
To analyse the effect of these substitutions in the cytoplasmic tail of Lu gp on Ubc9 binding in a cellular context, mutations K585A and K585R were introduced in the full-length Lu cDNA cloned in the pcDNA3 vector. MDCK cells were stably transfected by wt or mutant constructs, and IP assays were performed. As control, aliquots of each cellular extract were analysed by Western blot using anti-Lu (Figure 3C, upper panel, left) and anti-Ubc9 antibodies (Figure 3C, lower panel, left) to verify that equivalent amounts of proteins were present in the starting material. As shown in Figure 3(C) (lower panel, right), Ubc9 was co-immunoprecipitated only with Lu and Lu K585R mutant, but not with Lu K585A mutant confirming ex vivo that this last mutation leads to a loss of interaction with Ubc9. Equal amounts of Lu gps were revealed in each immunoprecipitated sample, except for non-transfected MDCK cells (Figure 3C, upper panel, right). It is noteworthy that the interaction site of Ubc9 is conserved between dog and human as endogenous Ubc9 from MDCK cells bound to recombinant human Lu gp.
These results indicated that Lys585 of the Lu cytoplasmic tail was involved in the interaction with Ubc9 in renal epithelial cells and that the replacement by another positively charged amino acid like arginine maintained Lu/Ubc9 interaction.
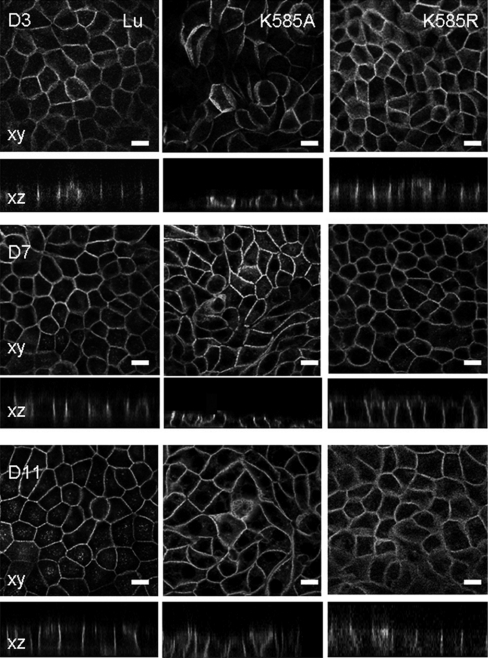
MDCK cells normally express Lu K585A mutant at the lateral surface but exhibit abnormal morphology during polarization
When grown on filters, MDCK-Lu and MDCK-Lu K585R pool cells exhibited normal epithelioid shape, from day 3 up to day 11, establishing monolayers of closely apposed cuboid cells with a fluorescent staining of Lu gp restricted to the lateral domain (Figure 4). In contrast, MDCK-Lu K585A cells displayed a persistent different cellular phenotype along the culture on filters. The shape and size of the cells remained irregular, with many non-polygonal elongated cells. In orthogonal projections, cells appeared more flattened and their height was reduced (mean 8 μm versus 20 μm for the MDCK-Lu and MDCK-Lu K585R cells). Cells were normally polarized, as indicated by ZO-1, α-catenin and E-cadherin staining (results not shown) and expressed Lu K585A protein at the lateral surface indicating that K585A mutation did not disturb the trafficking of Lu gp to the membrane.
Figure 4. Immunofluorescence studies of the Lu mutants in MDCK cells.
Stably transfected MDCK-Lu gp, -Lu K585A and -Lu K585R cells were grown on filters from day 3 (D3) up to day 11 (D11). Confocal imaging shows fluorescence staining of Lu gp in xy (en face view) and xz (side views) sections. Scale bars, 10 μM.
To control that these results were not due to different expression levels of recombinant Lu proteins, flow cytometry studies were performed to analyse the surface expression of Lu gps and select equivalent clones (SABC: 538200 for MDCK-Lu, 526270 for MDCK-Lu K585A and 467190 for MDCK-Lu K585R). Furthermore, immunofluorescence studies of clones expressing different levels of Lu gps showed similar results, indicating that the phenotype of polarized MDCK-Lu K585A cells was associated with the K585A mutation of Lu gp.
Lu K585A is overexpressed at the membrane of polarized MDCK cells
As wt and mutated forms of Lu gp were normally polarized in MDCK cells, we investigated the potential role of Ubc9 in regulating the expression and the stability of Lu proteins at the membrane of polarized MDCK cells.
In a first approach, pools of MDCK-Lu and MDCK-Lu K585A cells were analysed for the steady-state expression of Lu gps at the membrane. Sulfo-NHS-LC-biotin was used to label total membrane proteins at the basolateral side of polarized cells. Total Lu gps were immunoprecipitated using anti-Lu monoclonal antibody F241 and the immunoprecipitated proteins were divided into two halves. The first one was directly analysed by Western blot to estimate the total amount of Lu gps. The second half was used to estimate the amount of Lu gps present in the cell membrane, after an additional round of capture of biotinylated Lu gps on streptavidin-coupled agarose beads. All the Western blots were probed with the rabbit polyclonal anti-Lu antibody 602. As shown in Figure 5, equivalent amounts of Lu gp were immunoprecipitated from both cell types (IP total Lu) but the membrane fraction of Lu K585A mutant was clearly higher as compared with Lu wt, indicating that Lu K585A is overexpressed at the plasma membrane.
Figure 5. Expression level of Lu and Lu K585A mutant at the membrane of polarized MDCK cells grown on Transwell filters.
Western blot using rabbit polyclonal anti-Lu antibody (602) showing the total amounts of Lu and Lu K585A gps after IP using mouse anti-Lu antibody (F241) compared with the biotinylated Lu and Lu K585A gps expressed at the membrane.
These results were not related to the expression level of recombinant Lu gps since similar comparative data were obtained using two MDCK clones expressing the same level of Lu gps (SABC: 538200 for MDCK-Lu and 526270 for MDCK-Lu K585A), with an overexpression of the Lu K585A mutant at the cell membrane after cell polarization (results not shown).
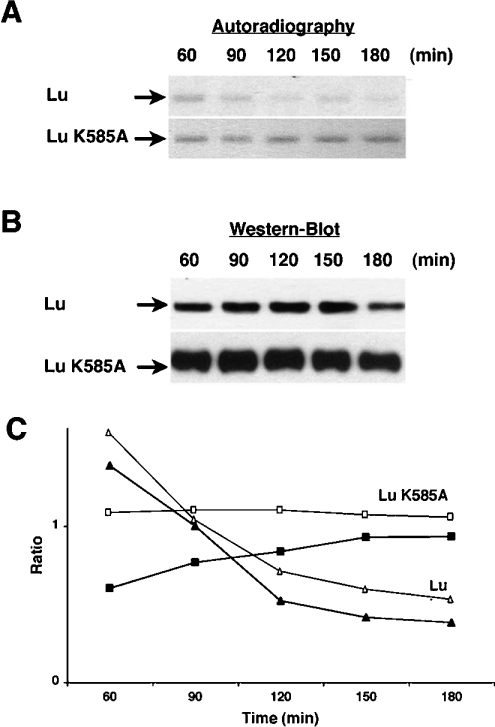
To investigate the overexpression of Lu K585A mutant at the membrane of polarized MDCK cells, the membrane delivery and turnover of Lu proteins were analysed using a pulse–chase experiment combined with biotinylation of surface proteins. Pools of polarized MDCK-Lu and MDCK-Lu K585A cells were radiolabelled for 20 min with [35S]methionine/[35S]cysteine and then incubated at 37 °C for 60, 90, 120, 150 and 180 min in non-radioactive complete medium. Sulfo-NHS-LC-biotin was used to label total membrane proteins at the basolateral side. Total Lu gps were immunoprecipitated using anti-Lu monoclonal antibody F241 and the biotinylated fraction, corresponding to the Lu gp expressed at the cell membrane, was isolated by streptavidin–agarose beads as described for the steady-state analysis. Biotinylated Lu proteins, representing total Lu gp at the membrane, include a radioactive newly synthesized Lu population of variable extent depending on the time of chase. The amounts of radioactive Lu gps at the membrane were determined at 60, 90, 120, 150 and 180 min by SDS/PAGE of biotinylated Lu gps followed by autoradiography (Figure 6A). The chase analysis was started at 60 min as our previous studies indicated that the newly delivered Lu gp at the membrane, after 20 min of radiolabelling, reached a peak between 60 and 90 min of chase [11]. Newly synthesized Lu and Lu K585A gps were correctly addressed to the basolateral membrane as they were both present at the cell surface after 60 min of chase (see autoradiography in Figure 6A). This result indicated that the Lu–Ubc9 interaction was not involved in the membrane targeting of Lu gp, in agreement with the results obtained by confocal analysis (see above). In contrast, there was a significant difference in the turnover of the wt and the mutated Lu gps as Lu K585A mutant showed increased stability and extended half-life at the membrane over time. Indeed, the newly delivered amounts of this mutant at 60 min remained unchanged at the membrane up to 180 min while Lu wt proteins decreased during this time.
Figure 6. The expression level and the turnover of Lu and Lu K585A mutant at the membrane of polarized MDCK cells.
MDCK cells expressing Lu and Lu K585A were grown on Transwell filters. (A) Newly delivered Lu and Lu K585A gps at the cell surface as determined by autoradiography of biotinylated Lu gps. (B) Western blot showing total amounts of Lu and Lu K585A gps expressed at the membrane at each time of chase, using rabbit polyclonal anti-Lu antibody (602). (C) The curves show the ratio of ‘radiolabelled biotinylated Lu/total biotinylated Lu’ at the membrane reflecting the turnover of newly delivered Lu and Lu K585A gps at the cell surface as a function of time. The curves represent mean values obtained with cellular pools (Lu: △, n=2; Lu K585A: □, n=2) and clones (Lu: ▲, n=4; Lu K585A: ■, n=4).
To ensure that the decrease in radioactive wt Lu gp at the membrane was not due to degradation of Lu proteins during the experiment, samples of biotinylated Lu gps from each chase time were analysed by SDS/PAGE followed by Western blot probed with the rabbit anti-Lu antibody 602. As shown in Figure 6(B), equivalent amounts of Lu gp were extracted from the membrane at each chase time. Similarly, equivalent quantities of the Lu K585A mutant were present at the membrane through the chase time. Figure 6(B) also shows a clear overexpression of Lu K585A mutant at the membrane when compared with wt Lu. This observation strengthened our results obtained under steady-state conditions showing that Lu K585A accumulates at the membrane during polarization of MDCK cells.
To quantify the turnover of both proteins, the intensity of all bands was determined using NIH Image 1.63 and the ratio ‘radio-labelled biotinylated Lu/total biotinylated Lu’ was calculated for each chase time. As shown in Figure 6(C), radiolabelled Lu gp decreased at the membrane over time with almost half of the proteins being internalized after 120 min as compared with 60 min. Conversely, Lu K585A mutant showed an unusually stable membrane expression. Indeed, newly delivered Lu K585A proteins did not undergo significant internalization up to 300 min of chase (results not shown), indicating that the lack of interaction with Ubc9 through Lys585 could disturb the turnover of Lu gp at the membrane of polarized MDCK cells.
Clones expressing similar amounts of Lu and Lu K585A gps, as determined by flow cytometry (SABC: 538200 for MDCK-Lu and 526270 for MDCK-Lu K585A) and Western blot (Figure 3C, upper panel, lanes 2 and 3), gave similar results (results not shown), indicating that the results obtained with the pools were not dependent on the expression level of Lu gps.
Cell spreading on laminin is impaired in MDCK-Lu K585A cells
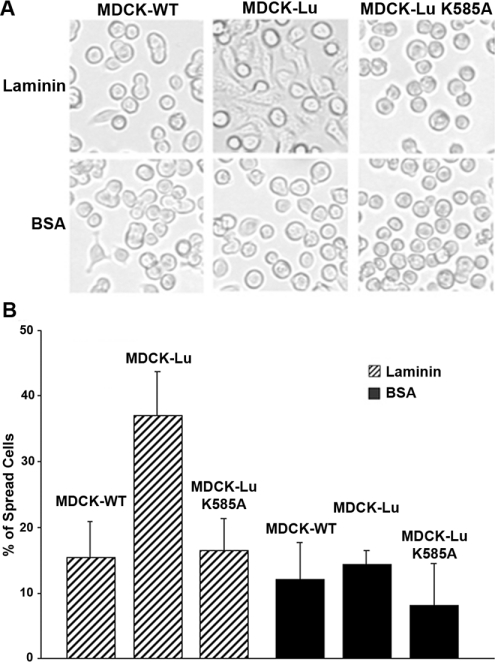
To test if the K585A mutation has an effect on the function of cell adhesion on laminin, we examined the ability of MDCK-Lu and MDCK-Lu K585A cells to spread on laminin α5 using a morphological adhesion assay. Microwell culture dishes were coated with 2 μg of purified laminin-10/11 (containing α5 chain) or BSA as control. As shown in Figure 7, a large number of MDCK-Lu cells spread on the laminin-coated wells after 3 h of incubation. In contrast, MDCK-WT and MDCK-Lu K585A cells did not spread on laminin and showed a round morphology. Neither the transfected nor the parental cells spread on the wells coated with BSA. This result indicated that the cells expressing the Lu K585A mutant, which did not interact with Ubc9, exhibit impaired morphological adhesion properties to laminin α5 as compared with MDCK-Lu cells.
Figure 7. Morphological cell adhesion assay of MDCK-WT, -Lu and -Lu K585A cells to laminin-10/11.
MDCK-WT, -Lu or -Lu K585A cells (105) were incubated at 37 °C for 3 h in wells coated with 2 μg/ml of laminin-10/11 or 1% BSA. (A) Photos showing cell morphology of each cell line. (B) The histogram presents the percentage of spread cells after 3 h of adhesion on laminin-10/11 or BSA. Results represent the means for four experiments.
DISCUSSION
In the present study, we have identified the enzyme Ubc9 as a direct cytoplasmic partner of Lu gps, in vitro and ex vivo. The co-IP of Ubc9 and Lu gp from A498 cells, expressing both proteins endogenously, assesses the physiological meaning of the two-hybrid and GST pull-down interactions.
In addition to epithelial cells, the Lu gp is expressed in the endothelium of blood vessel walls and in RBCs as well as in the erythroid lineage at the end of erythroid maturation [22]. In a first approach to investigate the Lu–Ubc9 interaction in an erythroid context, we have performed a co-IP assay in transfected K562 cells expressing recombinant human Lu. As observed for epithelial cells, Ubc9 was co-immunoprecipitated with Lu gp (E. Collec, C. Rahuel and C. Levankin, unpublished work). These results indicated that the interaction between Ubc9 and Lu gp was not restricted to the epithelial lineage. It could be assumed that this interaction might occur in all cell types where these two proteins are expressed.
Lys585 of Lu gp is directly involved in the binding of Ubc9 since mutation K585A abolished the interaction. Conversely, K585R mutation had no deleterious effect on the Lu–Ubc9 interaction, indicating that Ubc9 binds to Lu gp by an electrostatic interaction. Lys585 is included in a charged amino acid cluster RREK. We found that Lys585 was the critical amino acid for Lu gp interaction with Ubc9, since yeast two-hybrid experiments showed that the Lu RR582–583AA and E584A mutants interacted with Ubc9 similarly to the wt Lu gp.
Most of the known Ubc9 interacting partners are nuclear proteins or proteins translocated in the nucleus (for a review, see [14]). In only rare cases, Ubc9 has been reported to interact with proteins independently of its sumoylation enzymatic activity and to modulate the activity of transcriptional machinery [23,24]. Our results indicated that Ubc9 interacts with Lu independently of SUMO. First, the sumoylation of molecules by Ubc9 involves a lysine of the targeted protein that cannot be replaced by another charged amino acid like arginine [25–27]. As demonstrated in the present study, the replacement of the Lys585 by an arginine did not impair Lu/Ubc9 interaction and did not alter the morphology of the MDCK epithelial cells. Furthermore, Lys585 is not included in a SUMO consensus motif (ΨKXE, where Ψ is a hydrophobic amino acid and X any amino acid). Accordingly, in vitro sumoylation experiments performed with GST–Lu and GST–p53 used as control, did not reveal a sumoylation of Lu gp.
To investigate the role of Ubc9–Lu interaction in a cellular context, we performed fluorescence imaging using confocal microscopy. These experiments revealed that the Lu K585A mutant was addressed to the plasma membrane and displayed a correct basolateral expression in MDCK polarized cells. In previous studies, we demonstrated that the basolateral expression of Lu was associated with the presence of a dileucine motif within the specific 40 amino acids of Lu gp cytoplasmic tail (position 608–609) [11]. Accordingly, we show that Ubc9–Lu interaction is not necessary for correct targeting of the Lu gp to the lateral plasma membrane of epithelial polarized MDCK cells. Thus Ubc9 is most probably not involved in the trafficking of Lu gp from the Golgi apparatus to the plasma membrane.
Assays performed to co-localize Lu and Ubc9 by confocal microscopy were unsuccessful most likely because it is an enzymatic and therefore very rapid interaction. Lu gp is found mainly in plasma membranes, whereas Ubc9 has been shown to exhibit mainly nucleocytoplasmic localization [14]. mUbc9 (mouse Ubc9) was found abundantly in microsomal membranes, was less abundant in nuclei, mitochondria and plasma membranes and was almost absent from the cytosol [28]. In our experiments, Ubc9 was expressed in the cytosol and the nucleus of MDCK cells (results not shown). This suggests that the interaction between Lu and Ubc9 could occur in the cytoplasm during the traffic after internalization. Our hypothesis is that a small proportion of Ubc9 could interact rapidly and reversibly with Lu gp to regulate its trafficking during or after internalization.
To gain more insights into the role of the Lu–Ubc9 interaction, we analysed the turnover of wt and mutant Lu gps in the plasma membrane. We found that Lu K585A mutant had an extended half-life at the membrane as compared with the wt, resulting in accumulation of this mutated protein at the cell surface. mUbc9 has been described to interact directly with glucose transporters GLUT1 and GLUT4 and to modulate their membrane expression levels in opposite directions through a post-transcriptional mechanism [28]. Overexpression of mUbc9 in skeletal-muscle cells led to a severe reduction of endogenous GLUT1 in the cells while expression of a dominant-negative form of mUbc9 lacking the catalytic site resulted in an overexpression of GLUT1. This could be compared with our reported results as absence of interaction with Ubc9 is related to an accumulation of Lu K585A mutant at the cell surface, suggesting that Ubc9 could play a key role in the turnover of Lu gp at the membrane through the endocytic pathway.
Lu K585A-transfected MDCK cells displayed an abnormal morphology. Cells were certainly polarized, as indicated by ZO-1, α-catenin and E-cadherin fluorescence staining, but they failed to organize as a regular epithelioid shape with high apposed cuboid cells, remaining smaller as compared with MDCK-Lu cells even at day 11 of culture. Non-polarized MDCK-Lu and MDCK-Lu K585A cells exhibited similar amounts of Lu proteins at the membrane as determined by flow cytometry and Western blot (see Figure 3C, upper panel, lanes 2 and 3). The expression level of Lu K585A mutant at the membrane was dramatically increased during polarization as determined by protein biotinylation and IP (see Figures 5 and 6). We postulate that the turnover of Lu gp at the membrane is differently regulated in polarized and non-polarized cells and that the atypical morphology of MDCK-Lu K585A cells during polarization could be a consequence of the abnormal high abundance of an adhesion molecule like Lu gp at the cell surface that could disorganize normal cell–cell contact.
Lu/BCAM proteins, as the unique erythroid receptors for laminin α5, play an important role in sickle cell disease where they could contribute to the abnormal adhesiveness of sickle RBCs to the endothelium and the subendothelial matrix. In a recent study, we showed that cell adhesion on laminin is modulated by phosphorylation of Lu gp [12]. Lu/BCAM proteins have also been recognized as laminin α5 receptors in kidney epithelial cells, in smooth muscle cells and endothelial cell lines [8,10,29]. It was previously established that laminin-10 induced spreading of human corneal epithelial cells by interacting with Lu gps [30]. Here, we show that Ubc9 interacts with Lu gp and could influence cell adhesion to laminin. Indeed, morphological adhesion assays to laminin α5 indicate that MDCK-Lu K585A cells, in contrast with MDCK-Lu cells, did not spread on laminin. This result suggests that the loss of Lu–Ubc9 interaction has a consequence on cell spreading. Further investigations should document if signalling events induced by Ubc9 binding regulate laminin adhesion properties of Lu gps.
After demonstrating that Lu and Lu(v13) were differentially delivered to the cell membrane of polarized epithelial cells [11], the present study shows for the first time that the turnover of Lu gp at the cell membrane is modulated by the interaction with Ubc9. Our studies demonstrate that Ubc9 could be a new component of the endocytic machinery of Lu gps and suggest a potential role for Ubc9 in regulating the turnover of plasma membrane proteins. These findings help to understand the mechanisms that regulate the expression of an adhesion molecule like Lu/BCAM at the cell membrane and could be of interest to further understand and characterize the adhesion function of these molecules in sickle cell disease and during development.
Acknowledgments
We are grateful to Yolande Kroviarski and Colette Galand for helpful advice on GST pull-down and yeast two-hybrid experiments and to Anne Filipe for helpful advice on cell adhesion assay. This investigation was supported in part by the INTS, INSERM. This paper must therefore be hereby marked ‘advertisement’ in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
References
- 1.Cartron J. P., Colin Y. Structural and functional diversity of blood group antigens. Transfus. Clin. Biol. 2001;8:163–199. doi: 10.1016/s1246-7820(01)00142-2. [DOI] [PubMed] [Google Scholar]
- 2.Daniels G. Terminology for red cell antigens – 1999 update. Immunohematology. 1999;15:95–99. [PubMed] [Google Scholar]
- 3.El Nemer W., Rahuel C., Colin Y., Gane P., Cartron J. P., Le Van Kim C. Organization of the human LU gene and molecular basis of the Lu(a)/Lu(b) blood group polymorphism. Blood. 1997;89:4608–4616. [PubMed] [Google Scholar]
- 4.Rahuel C., Le Van Kim C., Mattei M. G., Cartron J. P., Colin Y. A unique gene encodes spliceoforms of the B-cell adhesion molecule cell surface glycoprotein of epithelial cancer and of the Lutheran blood group glycoprotein. Blood. 1996;88:1865–1872. [PubMed] [Google Scholar]
- 5.El Nemer W., Gane P., Colin Y., Bony V., Rahuel C., Galacteros F., Cartron J. P., Le Van Kim C. The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J. Biol. Chem. 1998;273:16686–16693. doi: 10.1074/jbc.273.27.16686. [DOI] [PubMed] [Google Scholar]
- 6.Udani M., Jefferson S., Daymont C., Zen Q., Telen M. J. B-CAM/Lu protein: a novel laminin receptor overexpress by sickle erythrocytes. Blood. 1996;88:6a. [Google Scholar]
- 7.Zen Q., Batchvarova M., Twyman C. A., Eyler C. E., Qiu H., De Castro L. M., Telen M. J. B-CAM/LU expression and the role of B-CAM/LU activation in binding of low- and high-density red cells to laminin in sickle cell disease. Am. J. Hematol. 2004;75:63–72. doi: 10.1002/ajh.10442. [DOI] [PubMed] [Google Scholar]
- 8.Vainionpaa N., Kikkawa Y., Lounatmaa K., Miner J. H., Rousselle P., Virtanen I. Laminin-10 and Lutheran blood group glycoproteins in adhesion of human endothelial cells. Am. J. Physiol. Cell Physiol. 2006;290:C764–C775. doi: 10.1152/ajpcell.00285.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kikkawa Y., Moulson C. L., Virtanen I., Miner J. H. Identification of the binding site for the Lutheran blood group glycoprotein on laminin α5 through expression of chimeric laminin chains in vivo. J. Biol. Chem. 2002;277:44864–44869. doi: 10.1074/jbc.M208731200. [DOI] [PubMed] [Google Scholar]
- 10.Moulson C. L., Li C., Miner J. H. Localization of Lutheran, a novel laminin receptor, in normal, knockout, and transgenic mice suggests an interaction with laminin α5 in vivo. Dev. Dyn. 2001;222:101–114. doi: 10.1002/dvdy.1169. [DOI] [PubMed] [Google Scholar]
- 11.El Nemer W., Colin Y., Bauvy C., Codogno P., Fraser R. H., Cartron J. P., Le Van Kim C. L. Isoforms of the Lutheran/basal cell adhesion molecule glycoprotein are differentially delivered in polarized epithelial cells. Mapping of the basolateral sorting signal to a cytoplasmic di-leucine motif. J. Biol. Chem. 1999;274:31903–31908. doi: 10.1074/jbc.274.45.31903. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier E., Rahuel C., Wautier M. P., El Nemer W., Gane P., Wautier J. L., Cartron J. P., Colin Y., Le Van Kim C. Protein kinase A-dependent phosphorylation of Lutheran/basal cell adhesion molecule glycoprotein regulates cell adhesion to laminin α5. J. Biol. Chem. 2005;280:30055–30062. doi: 10.1074/jbc.M503293200. [DOI] [PubMed] [Google Scholar]
- 13.Kroviarski Y., El Nemer W., Gane P., Rahuel C., Gauthier E., Lecomte M. C., Cartron J. P., Colin Y., Le Van Kim C. Direct interaction between the Lu/B-CAM adhesion glycoproteins and erythroid spectrin. Br. J. Haematol. 2004;126:255–264. doi: 10.1111/j.1365-2141.2004.05010.x. [DOI] [PubMed] [Google Scholar]
- 14.Melchior F. SUMO – nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- 15.Wilson V. G., Rangasamy D. Intracellular targeting of proteins by sumoylation. Exp. Cell Res. 2001;271:57–65. doi: 10.1006/excr.2001.5366. [DOI] [PubMed] [Google Scholar]
- 16.Nicolas G., Fournier C. M., Galand C., Malbert-Colas L., Bournier O., Kroviarski Y., Bourgeois M., Camonis J. H., Dhermy D., Grandchamp B., Lecomte M. C. Tyrosine phosphorylation regulates αII spectrin cleavage by calpain. Mol. Cell. Biol. 2002;22:3527–3536. doi: 10.1128/MCB.22.10.3527-3536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gietz D., St Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications Proc. Natl. Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong H., Hateboer G., Perrakis A., Bernards R., Sixma T. K. Crystal structure of murine/human Ubc9 provides insight into the variability of the ubiquitin-conjugating system. J. Biol. Chem. 1997;272:21381–21387. doi: 10.1074/jbc.272.34.21381. [DOI] [PubMed] [Google Scholar]
- 22.Bony V., Gane P., Bailly P., Cartron J. P. Time-course expression of polypeptides carrying blood group antigens during human erythroid differentiation. Br. J. Haematol. 1999;107:263–274. doi: 10.1046/j.1365-2141.1999.01721.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaul S., Blackford J. A., Jr, Cho S., Simons S. S., Jr Ubc9 is a novel modulator of the induction properties of glucocorticoid receptors. J. Biol. Chem. 2002;277:12541–12549. doi: 10.1074/jbc.M112330200. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi S., Shibata H., Kurihara I., Yokota K., Suda N., Saito I., Saruta T. Ubc9 interacts with chicken ovalbumin upstream promoter-transcription factor I and represses receptor-dependent transcription. J. Mol. Endocrinol. 2004;32:69–86. doi: 10.1677/jme.0.0320069. [DOI] [PubMed] [Google Scholar]
- 25.Bies J., Markus J., Wolff L. Covalent attachment of the SUMO-1 protein to the negative regulatory domain of the c-Myb transcription factor modifies its stability and transactivation capacity. J. Biol. Chem. 2002;277:8999–9009. doi: 10.1074/jbc.M110453200. [DOI] [PubMed] [Google Scholar]
- 26.Lin X., Liang M., Liang Y. Y., Brunicardi F. C., Feng X. H. SUMO-1/Ubc9 promotes nuclear accumulation and metabolic stability of tumor suppressor Smad4. J. Biol. Chem. 2003;278:31043–31048. doi: 10.1074/jbc.C300112200. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez M. S., Desterro J. M., Lain S., Midgley C. A., Lane D. P., Hay R. T. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 1999;18:6455–6461. doi: 10.1093/emboj/18.22.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giorgino F., de Robertis O., Laviola L., Montrone C., Perrini S., McCowen K. C., Smith R. J. The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1125–1130. doi: 10.1073/pnas.97.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolcato-Bellemin A. L., Lefebvre O., Arnold C., Sorokin L., Miner J. H., Kedinger M., Simon-Assmann P. Laminin α5 chain is required for intestinal smooth muscle development. Dev. Biol. 2003;260:376–390. doi: 10.1016/s0012-1606(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 30.Hasenson S., Maatta M., Rousselle P., Kikkawa Y., Miner J. H., Tervo T., Virtanen I. The immortalized human corneal epithelial cells adhere to laminin-10 by using Lutheran glycoproteins and integrin α3β1. Exp. Eye Res. 2005;81:415–421. doi: 10.1016/j.exer.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Miller J. H. Plainview, NY: Cold Spring Harbor Laboratory; 1972. Experiments in Molecular Genetics; pp. 352–355. [Google Scholar]