Abstract
Zac1, a novel seven-zinc-finger transcription factor, preferentially binds GC-rich DNA elements and has intrinsic transactivation activity. To date, the NLS (nuclear localization signal) of Zac1 has not been empirically determined. We generated a series of EGFP (enhanced green fluorescence protein)-tagged deletion mutants of Zac1 and examined their subcellular localization, from which we defined two NLSs within the DNA-binding (or zinc-finger) domain. Fusion proteins consisting of the two EGFP-tagged zinc-finger clusters (zinc finger motifs 1–3 and 4–7) were located exclusively in the nucleus, demonstrating that each of the zinc-finger clusters is sufficient for nuclear localization. Physical interactions between these two zinc-finger clusters and importin α1 were demonstrated using an in vitro glutathione S-transferase pull-down assay. Finally, our results indicate that the association of Zac1 with importin α1 is also involved in regulating the transactivation activity of Zac1 on the p21WAF1/CIP1 gene and protein expression.
Keywords: gene regulation, importin α1, nuclear localization, p21, zinc finger, zinc-finger protein which regulates apoptosis and cell-cycle arrest 1 (Zac1)
Abbreviations: ChIP, chromatin immunoprecipitation; C2H2, Cys2His2; DAPA, DNA-affinity precipitation assay; DAPI, 4′,6-diamidino-2-phenylindole; EGFP, enhanced green fluorescent protein; GR, glucocorticoid receptor; GST, glutathione S-transferase; HA, haemagglutinin; IBB domain, importin-β-binding domain; NLS, nuclear localization signal; NLSZF, NLS zinc-finger cluster; NPC, nuclear pore complex; NR, nuclear receptor; PACAP1-R, type I pituitary-adenylate-cyclase-activating polypeptide receptor; PLAG, pleomorphic adenoma gene; PLAGL, PLAG-like; RLU, relative light units; Sp1, specificity protein 1; Zac1, zinc-finger protein which regulates apoptosis and cell-cycle arrest 1
INTRODUCTION
Zac1 (zinc-finger protein which regulates apoptosis and cell cycle arrest 1) and p53 have been identified as components of a functional screening system that is mediated through their common ability to induce the expression of the type I PACAP1-R (type I pituitary-adenylate-cyclase-activating polypeptide receptor) gene [1,2]. It has been proposed that Zac1 binds to GC-rich DNA elements through its seven-zinc-finger motif and that this interaction is required for the expression of PACAP1-R and cytokeratin [1,3]. Activation of PLAG1 (pleomorphic adenoma gene 1) is the most frequent gain-of-function mutation found in pleomorphic adenomas of the salivary glands [4]. PLAG1 belongs to the highly conserved PLAG superfamily of seven N-terminal copies of a C2H2 (Cys2His2)-type zinc-finger motif, which is also present in two other members, PLAGL1 (PLAG-like 1; also called Zac1) and PLAGL2 [5].
The initial cytoplasmic event in the NLS (nuclear localization signal)-dependent transportation process of nuclear proteins is the binding of the NLS-containing cargo protein to the importin α/β heterodimer [6–8]. Importin α has an NLS-binding site for the cargo protein; this complex then interacts, via the IBB domain (importin-β-binding domain) of importin α, with importin β, which in turn interacts with the NPC (nuclear pore complex). The nuclear transfer of the trimeric cargo–importin α/β complex through the NPC is energy-dependent and appears to require GTP hydrolysis by Ran. RanGTP then causes importin α to dissociate from importin β in the nucleus. The binding relationship of importin α/β to its NLS cargo is necessarily bipolar, because they need to form a highly selective and tight complex in the cytoplasm, and, after getting into the nucleus, the complex needs to be switched to a lower-affinity state in the nucleus to release the cargo protein. Structural analysis of importin α has revealed three functional domains: an IBB domain at the N-terminus, a hydrophobic central domain known as the ‘armadillo’ repeat domain, and a short acidic domain at the C-terminus. The importin α adaptor family consists of various isoforms, which have distinct binding to their corresponding cargo proteins and exhibit diverse expression patterns in a tissue-specific manner [9–11].
Zac1 is a nuclear zinc-finger protein with both transactivation and DNA-binding activity. More importantly, the dimerization and DNA-binding ability of Zac1 are both required for its transactivation activity [1–3]. However, the NLS(s) and transport proteins responsible for the nuclear localization of Zac1 still remain elusive. In the present study, we have demonstrated that the seven C2H2 zinc fingers of Zac1 are not only involved in its DNA-binding ability, dimerization and transactivation activity, but also direct the nuclear localization of Zac1 through interacting with importin α1 via two newly identified NLSs within the zinc-finger motif of Zac1.
MATERIALS AND METHODS
Plasmids and recombinant proteins
Various Zac1-coding regions were synthesized by PCR and subcloned into the EcoRI and SalI sites of the pEGFP-C2 vector (Clontech). PCR-amplified fragments encoding various importin α1 fragments were subcloned into the EcoRI/XhoI sites of pSG5.HA, which has promoters for expression in vitro and in mammalian cells and also provides an N-terminal HA (haemagglutinin) tag for the expressed protein [12]. pDsRed.flag.importin α1 was generated by cloning EcoRI/BamHI-digested PCR fragments into the pDsRed.flag vector at the EcoRI/BamHI sites derived from the pDsRed.Flag.SUMO1 plasmid (a gift from Dr Bon-Chu Chung, Academia Sinica, Taipei, Taiwan, Republic of China) [13]. p21-LUC reporter gene and pSG5.HA.Zac1 expression vector have been described previously [14,15].
Bacterial expression vectors for various GST (glutathione S-transferase)–Zac1 fusions were constructed by inserting the appropriate PCR fragments into the EcoRI/XhoI sites of pGEX-4T1 (GE Healthcare).
Cell culture, transient transfection assays and immunofluorescence microscopy
HeLa cells were grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% charcoal/dextran-treated foetal bovine serum. Transient transfections and luciferase assays were performed in 24-well culture dishes as described previously [16]. The expression of pEGFP fusion proteins in 24-well culture dishes were observed by fluorescence microscopy (model DMURE2, Leica) and analysed with the Image-Pro® Plus (Media Cybernetics) 15 h after transfection. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) (Roche). For luciferase assays, total transfected DNAs for reporter analysis were adjusted to 1 μg by adding an appropriate amount of empty vector DNA. Luciferase activity of the transfected cell extracts is presented as the mean±S.D. RLU (relative light units) for three transfected independent experiments. The expression of many control vectors for monitoring transfection efficiency have been shown to be affected greatly by Zac1, therefore internal controls were not used. Instead, the reproducibility of observed effects on the luciferase activity was determined in multiple independent transfection experiments.
Protein–protein interaction assays
For GST pull-down assays, 35S-labelled proteins were produced by the TNT T7-coupled reticulocyte lysate system (Promega), and GST fusion proteins were produced in Escherichia coli BL21 cells. Radioactively labelled importin α1 proteins were translated in vitro, followed by incubating with various immobilized GST–Zac1 fusion proteins, eluted and analysed by SDS/PAGE as described previously [17].
Immunoblot analysis
Amounts of 5 μg of pSG5.HA.Zac1 and/or pSG5.HA.importin α1 were transfected into HeLa cells. At 48 h after transfection, cell lysates were prepared in lysis buffer (100 mM Tris/HCl, pH 8.0, 150 mM NaCl, 0.1% SDS and 1% Triton X-100) at 4 °C. The lysates were separated by SDS/PAGE, transferred on to a PVDF membrane (Millipore) and detected by antibodies against p21, p53, α-tubulin (C19, DO-1 and H-51 respectively; Santa Cruz Biotechnology) or HA (3F10; Roche).
ChIP (chromatin immunoprecipitation) assay
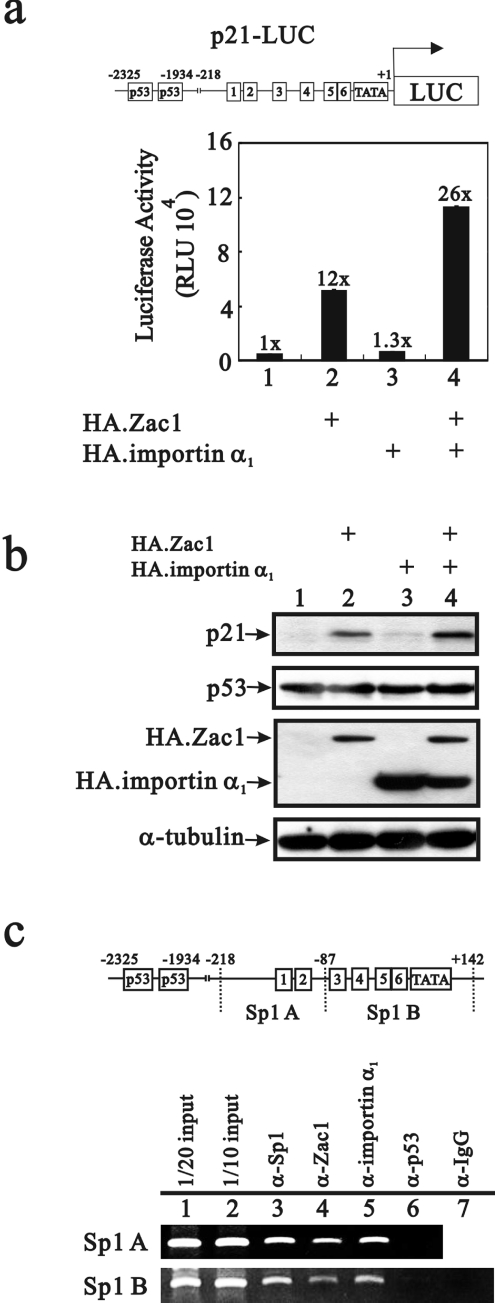
ChIP assays were performed as described previously [18] in HeLa cells with some modifications. Cells were cross-linked with 1% formaldehyde at 37 °C for 15 min. The pellets were lysed with lysis buffer (1% SDS, 10 mM EDTA and 50 mM Tris/HCl, pH 8.0) at 4 °C for 30 min. The lysates were sonicated (Sonicator 3000; Misonix) to shear the size of DNA to ∼200 to 1000 bp. Sonicated extracts (200 mg) were diluted 1:10 with dilution buffer (20 mM Tris/HCl, pH 8.0, 2 mM EDTA, 1% Triton X-100, 200 mM NaCl and proteinase inhibitor kit) followed by incubation with 20 μl of salmon sperm DNA-saturated 50% Protein A/G–Sepharose at 4 °C for 30 min for pre-cleaning. ChIP was performed with 1 μg of anti-Sp1 (specificity protein 1) antibody (Upstate), anti-Zac1 antibody, anti-(importin α1) antibody, anti-p53 antibody or anti-(normal mouse IgG) (G-18, C-20 and DO-1 respectively; Santa Cruz Biotechnology) with rotating at 4 °C overnight. The following day, chromatin–antibody complexes were eluted from the solution by incubating with 40 μl of salmon sperm DNA-saturated 50% Protein A/G–Sepharose at 4 °C for 2 h. The beads were harvested and washed as already described. Cross-linking was reversed by heating at 65 °C overnight, followed by treating with 100 μg/ml proteinase K at 50 °C for 1 h. The DNA was extracted with the gel/PCR DNA fragments extraction kit (Geneaid) and dissolved in 60 μl water. All PCRs were performed on a PerkinElmer thermocycler (GeneAmp PCR system 2400) using Promega PCR Master Mix. The primer pairs Sp1 A (−218/−87), 5′-TCCGGGACCGGCTGGCCT-3′ (forward) and 5′-GCTCGGCCCACCGCGCCG-3′ (reverse), and Sp1 B (−86/+142), 5′-GCGGGTCCCGCCTCCTTG-3′ (forward) and 5′-TCTGGGCCGCCGGCCCGG-3′ (reverse) were used to amplify the p21 promoter region.
Modified DAPA (DNA-affinity precipitation assay)
One oligonucleotide containing biotin-labelled sense strand primer (5′-gtactaacaGGGGCCCCatttaatcat-3′) for the Zac1-binding site (G4C4 type) was used in the assays. HeLa nuclear extracts were prepared as described previously [19] and were incubated with DAPA binding buffer [10 mM Tris/HCl, pH 7.5, 50 mM KCl and 1 mM DTT (dithiothreitol)]. Poly(dI-dC)·(dI-dC) competitor was incubated with the nuclear extracts, followed by incubating with Zac1 double-stranded oligonucleotide. After the incubation, anti-HA antibody (F-7; Santa Cruz Biotechnology), anti-Sp1 antibody, anti-Zac1 antibody and anti-(importin α1) antibody were added to the reaction mixture and incubated. The specific protein–DNA–agarose complex was washed and analysed by SDS/PAGE. Detection was performed adding streptavidin–horseradish peroxidase (Panomics) and immunoblot analysis.
RESULTS
The N-terminal region of Zac1 allows nuclear accumulation of an EGFP (enhanced green fluorescent protein) fusion protein
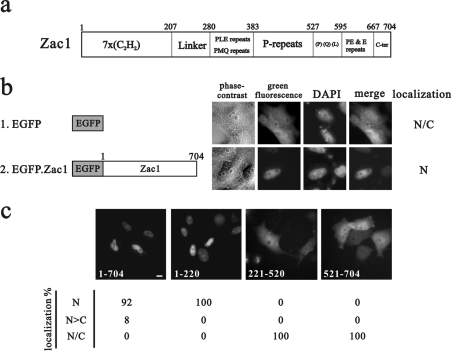
Previous studies have demonstrated that Zac1 is a nuclear zinc-finger protein with transactivation and DNA-binding activity [1–3]. We analysed the sequence of Zac1 and found that it contains no typical NLS (Figure 1a and results not shown). First, we examined the subcellular localization of EGFP-tagged full-length Zac1 in HeLa cells and found that the fusion protein localized exclusively in the nucleus (Figure 1b). To determine the region of Zac1 responsible for its nuclear localization we then constructed EGFP-tagged fusion proteins with various functional motifs of Zac1. The N-terminal region (amino acids 1–220) of Zac1 targeted EGFP to the nucleus (Figure 1c), whereas constructs containing any other regions of Zac1 (amino acids 221–520 or 521–704) had subcellular distributions similar to that of EGFP alone. To further validate these observations, the subcellular localization of the EGFP-fusion constructs was monitored across a variety of cell lines, including C2C12, CV-1, HEK-293 (human embryonic kidney 293) and C33A cells. In all of the cells analysed, the cellular localization patterns of the EGFP-fusion proteins were identical with that obtained in HeLa cells (results not shown), suggesting that the seven-zinc-finger motif plays a role in determining the nuclear localization of Zac1.
Figure 1. The NLS of Zac1 is identified in its N-terminal region.
(a) The functional domains of mouse Zac1. (b and c) Schematic representations of various EGFP-tagged constructs used to transfect HeLa cells. Representative HeLa cells were transiently transfected with these constructs. Nuclei are identified by blue fluorescence after DAPI staining. The intracellular distribution of various EGFP-fusion proteins is shown underneath (c). Approx. 100 transfected cells were examined by fluorescence microscopy and classified by the presence of green fluorescence: exclusively in the nucleus (N), mainly in the nucleus (N>C) or evenly distributed throughout the nucleus and cytoplasm (N/C). Scale bar, 10 μm.
Two distinct zinc-finger clusters in the Zac1 N-terminal region are sufficient for nuclear localization
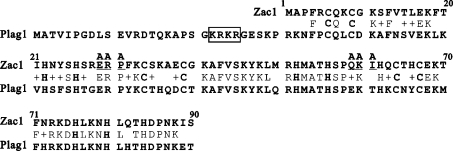
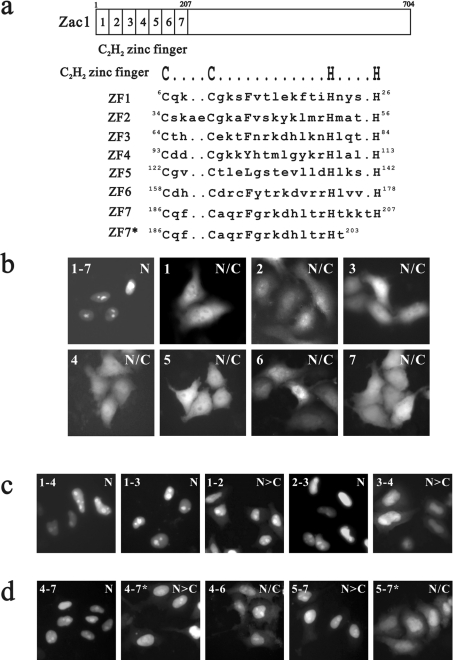
A sequence alignment of Zac1 and PLAG1 shows the putative NLS signal of PLAG1 (Figure 2, marked with an open box). A study of PLAG1 demonstrated one putative NLS (K22RKR25) for interacting with importin α1 and it attributed a functional role in nuclear import to the zinc-finger motif at amino acids 84–244, despite the fact that the study failed to demonstrate its association with importin α1 [20]. To characterize the role of the seven copies of the C2H2-type zinc-finger motif of Zac1 in detail in the process of nuclear import (Figure 3a), we constructed a series of EGFP-tagged fusion proteins with different zinc-finger combinations, including one, two, three or four zinc-finger motifs (Figures 3b–3d). We found that the seven zinc fingers in the N-terminal domain of Zac1 function in concert to determine its nuclear transportation. Two clusters, zinc-finger motifs 1–3 and 4–7, were functionally identified to facilitate nuclear localization (Figures 3c and 3d).
Figure 2. Sequence alignment of Zac1 (amino acids 1–90; zinc finger motifs 1–3) and PLAG1 (amino acids 1–120).
Sequence alignment is illustrated for identical amino acids by capital letters and for similar amino acids by plus signs (+) in between the two sequences. The highly conserved cysteine (C) and histidine (H) residues within the C2H2-zinc finger motif of Zac1 and PLAG1 are shown in bold capital letters. The KRKR sequence in the open box is the putative NLS of PLAG1. Two linkers (underlined) were swapped with alanine residues (indicated as A) by site-directed mutagenesis.
Figure 3. Two zinc-finger clusters function as nuclear import signals for Zac1.
(a) The diagram shows seven C2H2-type zinc-finger motifs, within which the conserved cysteine (C) and histidine (H) residues are shown in upper case letters. ZF7* is a truncated ZF7 and its amino acid sequence is illustrated. (b–d) Representative fluorescence images are shown for the HeLa cells transiently transfected with various combinations of zinc-finger motifs compiling the Zac1–EGFP fusion proteins. Approx. 100 transfected cells were examined by fluorescence microscopy, and each of the EGFP-fusion proteins is classified by N, N>C and N/C as in Figure 1.
Any single zinc-finger motif failed to import the EGFP-fusion protein into the nucleus (Figure 3b), whereas any two (zinc-finger motifs 1–2, 2–3 or 3–4) or three (zinc-finger motifs 4–6 or 5–7) adjacent zinc fingers in combination partially, but not completely, facilitated nuclear import (Figures 3c and 3d). A defected zinc-finger motif 7 partially impaired the nuclear import capacities of zinc-finger motifs 4–7* and 5–7* (Figure 3d, marked with an asterisk), suggesting that zinc-finger motif 7, to a certain extent, also contributes to the nuclear import of Zac1. The two zinc-finger clusters, zinc-finger motifs 1–3 and 4–7, are designated NLSZF1 and NLSZF2 respectively in the context of their involvement in nuclear import. Because multiple zinc-finger motifs are required for nuclear translocation, we analysed the effects of the linkers between zinc-finger motifs 1 and 2 or 2 and 3 on the nuclear localization of Zac1 in HeLa cells by introducing site-directed mutations at amino acids E29RP31 or Q59KI61, or both (Figure 2; underlined residues were mutated into alanine residues). We found that the linkers between each zinc-finger motif of NLSZF1 partially impaired the ability of the zinc fingers in nuclear import (results not shown).
Zac1 interacts directly with importin α1 and this interaction is associated with its two nuclear localization clusters, NLSZF1 and NLSZF2
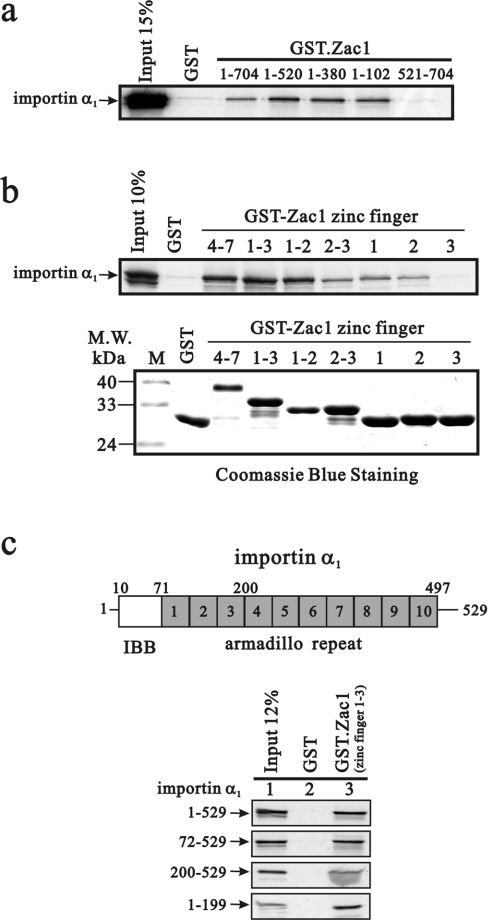
Previous studies have shown that some zinc-finger proteins interact directly with nuclear-import-related proteins and contribute to protein nuclear localization [20,21]. Therefore, to identify its nuclear import pathway, we tested whether Zac1 interacts physically with importin α1. We generated a series of GST-fusion proteins with various Zac1 fragments. These GST-fusion constructs were used to pull down importin α1 that was translated in vitro. Amino acids 1–102 of Zac1 formed the shortest fragment that interacted directly with importin α1 in vitro (Figure 4a). Therefore we analysed the interaction between importin α1 and a set of different zinc-finger combinations. The major importin-α1-binding sites within Zac1 were the zinc-finger motifs 1–2, 1–3 and 4–7 (Figure 4b). However, weaker interactions were observed with zinc-finger motifs 2–3, zinc-finger motif 1 and zinc-finger motif 2, but no apparent interaction was found with zinc-finger motif 3.
Figure 4. Zac1 physically interacts with importin α1 through its zinc-finger motif in vitro.
(a and b) Full-length importin α1 was translated in vitro and incubated with bead-bound GST or various GST–Zac1 fusion proteins as indicated. Bound proteins were eluted, separated by SDS/PAGE and visualized by autoradiography. For comparison, the leftmost lane of each panel shows 10 or 15% of the input protein used in the binding assay reactions. M.W., molecular mass (kDa) (c) Location of the IBB domain and ten armadillo repeats. Various importin α1 fragments were translated in vitro and incubated with bead-bound GST or GST–Zac1ZF1−3 fusion protein. Results are representative of two independent experiments.
Importin α acts as an adaptor molecule between the NLS-containing cargo protein and importin β. A recent study showed that importin α transports CaMKIV to the nucleus without the involvement of importin β [22]. Therefore we focused on the relationship between importin α and Zac1, without taking into account importin β, in the so-called importin pathway. We identified further a potential Zac1-interacting site in importin α1 using GST pull-down assays. Our analysis revealed that Zac1 bound mainly to importin α1 through its armadillo repeats and acidic domains (Figure 4c).
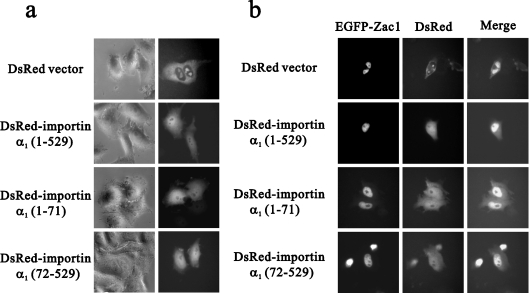
The red fluorescent protein DsRed protein primarily distributes in the cytoplasm, but various importin α1 fragments (full-length, N-terminal and N-truncated) fused to pDsRed vectors show equal distribution in the cytoplasm and nucleus (Figure 5a). We showed that importin α1 and Zac1 co-localized in the nucleus of HeLa cells (Figure 5b) and importin α1 fragments (amino acids 1–71 and 72–529) showed similar subcellular distribution patterns to that of full-length importin α1 (amino acids 1–529) in both the cytoplasm and nucleus, regardless of whether Zac1 was present or not (Figure 5). On the basis of the co-localization of N-terminally truncated importin α1, lacking the IBB domain, it is suggested that importin α1 transports Zac1 to the nucleus without the involvement of importin β (Figure 5b).
Figure 5. Importin α1 co-localizes with Zac1 in the nucleus.
HeLa cells were transfected with (a) 0.5 μg of DNA encoding DsRed conjugated to the indicated fragments of importin α1 fusion proteins with various regions of importin α1 as indicated, or (b) 0.5 μg of DNA encoding EGFP-tagged full-length Zac1 and DsRed conjugated to the indicated fragments of importin α1. Approx. 100 transfected cells were examined by fluorescence microscopy.
Importin α1 co-operates with Zac1 in the induction of the p21WAF1/CIP1 gene and protein in HeLa cells
We have shown previously that Zac1 induces p21WAF1/CIP1 (p21) promoter activity and protein expression through a p53-dependent pathway in HeLa cells [23]. Transiently transfected importin α1 had little or no effect on the p21 promoter or its protein induction (Figure 6a, compare bars 1 and 3, and 6b, lanes 1 and 3), whereas co-transfection of importin α1 acted synergistically with Zac1 in the induction of the p21 gene and protein (Figures 6a and 6b, lanes 2 and 4). Therefore we suspect from the nuclear co-localization of importin α1 and Zac1 that Zac1/importin α1 might function in a p53-dependent co-activator complex through a protein–protein mechanism. The co-expression of importin α1 with Zac1 might enhance the Zac1 co-activator function by increasing the amount of Zac1 nuclear transportation (results not shown). Importantly, our ChIP analysis revealed that the working complex on the Sp1-responsive elements of the p21 promoter in HeLa cells contained Sp1, Zac1 and importin α1, but not p53 (Figure 6c).
Figure 6. Importin α1 acts synergistically with Zac1 on the p21 promoter and protein induction in HeLa cells.
(a and b) HeLa cells were transiently transfected with the p21-LUC reporter gene (0.4 μg) and pSG5HA.Zac1 (0.3 μg) and/or pSG5HA.importin α1 (0.3 μg) reporter constructs. Luciferase activity in the transfected cell extracts is indicated in RLU. Numbers above the columns indicate the fold induction in RLU relative to that of the control cells in which only p21-LUC was transfected. (b) HeLa cell extracts were subjected to Western blot analysis probing with anti-p21, anti-p53, anti-HA and anti-α-tubulin antibodies. (c) HeLa cell extracts were used for ChIP assays followed by PCR analysis with two sets of primer for both: Sp1 A (−218/−87) and Sp1 B (−86/+142). Results (b and c) are representative of two independent experiments.
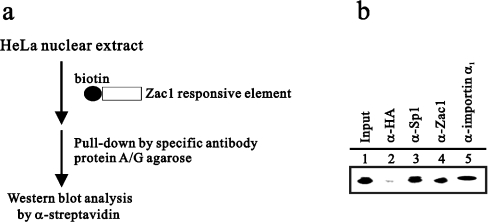
Our newly defined NLSs in Zac1 contains seven canonical C2H2-type zinc fingers which have been reported in the recognition of and specific binding to GC-rich DNA elements [3]. The importin-α1-binding sites of Zac1 were also identified within the zinc-finger region. Hence we performed a modified DAPA to demonstrate the composition of the Zac1–DNA complex in HeLa cells (Figure 7a). HeLa nuclear extracts were incubated with a GC-rich (G4C4) DNA element conjugated with biotin and immunoprecipitated with the indicated antibodies, including HA (as control), Sp1, Zac1 and importin α1. In Figure 7(b), it is demonstrated that not only was Zac1 able to bind to this GC-rich DNA element, but also Sp1 and importin α1 were found in the Zac1–DNA complex.
Figure 7. Importin α1 associates with the Zac1–DNA-responsive element complex.
(a) A modified DAPA carried out as described in the Materials and methods section is shown schematically. (b) HeLa nuclear extracts were subjected to analysis by the modified DAPA using the antibodies indicated and analysed by Western blot analysis. Results are representative of two independent experiments.
DISCUSSION
Zac1, a novel seven-zinc-finger transcription factor, specifically binds to GC-rich DNA elements and, functionally, it promotes cell-cycle arrest and apoptosis, and acts as a transcriptional cofactor for a number of nuclear receptors, p53 and Apaf-1 (apoptotic protease-activating factor 1) [14,23,24]. Previous studies have revealed that the structure of the zinc-finger motif and the basic residues within the zinc-finger motif also act as critical nuclear localization determinants in erythroid Kruppel-like factor [21,25]. These studies also demonstrated that complete removal of the zinc-finger motifs or the basic residues within them is necessary to disrupt its nuclear import ability. Both NLSZF1 and NLSZF2 of Zac1 contain at least ten basic residues. Because multiple zinc-finger motifs (NLSZF1 or NLSZF2) are required for nuclear translocation, we examined NLSZF1 to investigate whether the linkers between each zinc-finger motif are possible nuclear localization determinants. Our data indicate that these mutated linkers between each zinc-finger motif (motif 1–2 or 2–3) only partially impaired the ability of the zinc fingers in nuclear import, suggesting that the linkers are not the crucial residues that affect the subcellular localization of Zac1 (results not shown).
In the present study, we first demonstrated that two clusters, zinc-finger motifs 1–3 and 4–7, were functionally identified to facilitate nuclear localization (Figures 3c and 3d). Subsequently, our GST pull-down analysis demonstrated that at least two importin-α1-binding sites, zinc fingers 1–3 and 4–7, were identified within the N-terminal region of Zac1. Zinc fingers 1–2 also interact most robustly with importin α1, but this fragment was not good enough for fully translocalizing the EGFP-fusion protein into the nucleus, suggesting that the ability of Zac1 to bind importin α1 might not be the only determinant for nuclear translocalization. A similar observation of EGFP–zinc finger 2–3 fusion also supports this idea that 74% of the fusions show, predominately, translocalization in the nucleus (Figure 3c), whereas its association with importin α1 was not as good as those of zinc fingers 1–3 and 4–7 (Figure 4b). Therefore, in conclusion, the zinc-finger motif of Zac1 plays an important role in the nuclear localization of Zac1, but, based on our results, there are some other factors that fine-tune its tendency to be imported into the nucleus by, presumably, interacting with some other nuclear transporting proteins besides importin α1.
In the sequence analysis, there are many NR (nuclear receptor)-binding motifs in importin-related proteins, suggesting the possibility that importin α1 interacts with NRs and co-activators [26–29]. A previous study has demonstrated that importin 7, importin 8 and importin α associate with the GR (glucocorticoid receptor) even in the absence of hormone, suggesting that hormone-controlled localization of GR might by exerted immediately downstream of the import-receptor binding [30]. Moreover, we found that androgen receptor transcriptional activation was affected by importin α1 and importin α1–Zac1 complex in HeLa cells (results not shown). Other studies have demonstrated that Ran-binding proteins, such as RanBPM and Mog1, are bifunctional proteins that play roles both in nucleocytoplasmic transport and in transcription factor recruitment [31,32]. In the present study, our ChIP and DAPA data support the idea that importin α1 plays important roles in p21 gene transcription regulation mediated through assisting transcription factors and subsequently promoting the anchorage of these transcription factors on the p21 promoter.
Our work suggests that the nuclear import of Zac1 might be mediated through association with importin α1, but the N-terminal IBB domain of importin α1 which is involved in importin β association and auto-inhibition [33] is not responsible for Zac1 binding. The interacting complex thus formed in the nucleus might also participate in regulating gene expression. In summary, the existence of the importin α1–Zac1 complex in the nucleus provides a new direction in studying the functions of importin α1, i.e. its involvement in Zac1 nuclear importation and its participation in p21 gene regulation via Zac1 and, more importantly, in understanding the molecular mechanisms by which the potential transcriptional activity of importin α1 is regulated.
Acknowledgments
We thank Dr B. C. Chung (Academia Sinica, Taipei, Taiwan, Republic of China) for DsRed expression vector. This work was supported by grants from the National Health Research Institute, Taipei, Taiwan, Republic of China (NHRI-EX92-9224NC, NHRI-EX93-9224NC and NHRI-EX94- 9224NC to S.-M.H.).
References
- 1.Varrault A., Ciani E., Apiou F., Bilanges B., Hoffmann A., Pantaloni C., Bockaert J., Spengler D., Journot L. hZAC encodes a zinc finger protein with antiproliferative properties and maps to a chromosomal region frequently lost in cancer. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8835–8840. doi: 10.1073/pnas.95.15.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spengler D., Villalba M., Hoffmann A., Pantaloni C., Houssami S., Bockaert J., Journot L. Regulation of apoptosis and cell cycle arrest by Zac1, a novel zinc finger protein expressed in the pituitary gland and the brain. EMBO J. 1997;16:2814–2825. doi: 10.1093/emboj/16.10.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann A., Ciani E., Boeckardt J., Holsboer F., Journot L., Spengler D. Transcriptional activities of the zinc finger protein Zac are differentially controlled by DNA binding. Mol. Cell. Biol. 2003;23:988–1003. doi: 10.1128/MCB.23.3.988-1003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kas K., Voz M. L., Roijer E., Astrom A. K., Meyen E., Stenman G., Van de Ven W. J. Promoter swapping between the genes for a novel zinc finger protein and β-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat. Genet. 1997;15:170–174. doi: 10.1038/ng0297-170. [DOI] [PubMed] [Google Scholar]
- 5.Kas K., Voz M. L., Hensen K., Meyen E., Van de Ven W. J. Transcriptional activation capacity of the novel PLAG family of zinc finger proteins. J. Biol. Chem. 1998;273:23026–23032. doi: 10.1074/jbc.273.36.23026. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A. Importin α: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Cingolani G., Petosa C., Weis K., Muller C. W. Structure of importin-β bound to the IBB domain of importin-α. Nature. 1999;399:221–229. doi: 10.1038/20367. [DOI] [PubMed] [Google Scholar]
- 8.Pemberton L. F., Paschal B. M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 9.Nachury M. V., Ryder U. W., Lamond A. I., Weis K. Cloning and characterization of hSRP1γ, a tissue-specific nuclear transport factor. Proc. Natl. Acad. Sci. U.S.A. 1998;95:582–587. doi: 10.1073/pnas.95.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuji L., Takumi T., Imamoto N., Yoneda Y. Identification of novel homologues of mouse importin α, the αs subunit of the nuclear pore-targeting complex, and their tissue-specific expression. FEBS Lett. 1997;416:30–34. doi: 10.1016/s0014-5793(97)01092-2. [DOI] [PubMed] [Google Scholar]
- 11.Kamei Y., Yuba S., Nakayama T., Yoneda Y. Three distinct classes of the α-subunit of the nuclear pore-targeting complex (importin-α) are differentially expressed in adult mouse tissues. J. Histochem. Cytochem. 1999;47:363–372. doi: 10.1177/002215549904700310. [DOI] [PubMed] [Google Scholar]
- 12.Chen D., Ma H., Hong H., Koh S. S., Huang S. M., Schurter B. T., Aswad D. W., Stallcup M. R. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 13.Chen W. Y., Lee W. C., Hsu N. C., Huang F., Chung B. C. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1) J. Biol. Chem. 2004;279:38730–38735. doi: 10.1074/jbc.M405006200. [DOI] [PubMed] [Google Scholar]
- 14.Huang S. M., Stallcup M. R. Mouse Zac1, a transcriptional coactivator and repressor for nuclear receptors. Mol. Cell. Biol. 2000;20:1855–1867. doi: 10.1128/mcb.20.5.1855-1867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu R. C., Schonthal A. H. Activation of p53–p21waf1 pathway in response to disruption of cell-matrix interactions. J. Biol. Chem. 1997;272:29091–29098. doi: 10.1074/jbc.272.46.29091. [DOI] [PubMed] [Google Scholar]
- 16.Liu P. Y., Hsieh T. Y., Chou W. Y., Huang S. M. Modulation of glucocorticoid receptor-interacting protein 1 (GRIP1) transactivation and co-activation activities through its C-terminal repression and self-association domains. FEBS J. 2006;273:2172–2183. doi: 10.1111/j.1742-4658.2006.05231.x. [DOI] [PubMed] [Google Scholar]
- 17.Ma H., Hong H., Huang S. M., Irvine R. A., Webb P., Kushner P. J., Coetzee G. A., Stallcup M. R. Multiple signal input and output domains of the 160-kilodalton nuclear receptor coactivator proteins. Mol. Cell. Biol. 1999;19:6164–6173. doi: 10.1128/mcb.19.9.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma H., Shang Y., Lee D. Y., Stallcup M. R. Study of nuclear receptor-induced transcription complex assembly and histone modification by chromatin immunoprecipitation assays. Methods Enzymol. 2003;364:284–296. doi: 10.1016/s0076-6879(03)64016-4. [DOI] [PubMed] [Google Scholar]
- 19.Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braem C. V., Kas K., Meyen E., Debiec-Rychter M., Van De Ven W. J., Voz M. L. Identification of a karyopherin α2 recognition site in PLAG1, which functions as a nuclear localization signal. J. Biol. Chem. 2002;277:19673–19678. doi: 10.1074/jbc.M112112200. [DOI] [PubMed] [Google Scholar]
- 21.Quadrini K. J., Bieker J. J. Kruppel-like zinc fingers bind to nuclear import proteins and are required for efficient nuclear localization of erythroid Kruppel-like factor. J. Biol. Chem. 2002;277:32243–32252. doi: 10.1074/jbc.M205677200. [DOI] [PubMed] [Google Scholar]
- 22.Kotera I., Sekimoto T., Miyamoto Y., Saiwaki T., Nagoshi E., Sakagami H., Kondo H., Yoneda Y. Importin α transports CaMKIV to the nucleus without utilizing importin β. EMBO J. 2005;24:942–951. doi: 10.1038/sj.emboj.7600587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S. M., Schonthal A. H., Stallcup M. R. Enhancement of p53-dependent gene activation by the transcriptional coactivator Zac1. Oncogene. 2001;20:2134–2143. doi: 10.1038/sj.onc.1204298. [DOI] [PubMed] [Google Scholar]
- 24.Rozenfeld-Granot G., Krishnamurthy J., Kannan K., Toren A., Amariglio N., Givol D., Rechavi G. A positive feedback mechanism in the transcriptional activation of Apaf-1 by p53 and the coactivator Zac-1. Oncogene. 2002;21:1469–1476. doi: 10.1038/sj.onc.1205218. [DOI] [PubMed] [Google Scholar]
- 25.Pandya K., Townes T. M. Basic residues within the Kruppel zinc finger DNA binding domains are the critical nuclear localization determinants of EKLF/KLF-1. J. Biol. Chem. 2002;277:16304–16312. doi: 10.1074/jbc.M200866200. [DOI] [PubMed] [Google Scholar]
- 26.Shank L. C., Paschal B. M. Nuclear transport of steroid hormone receptors. Crit. Rev. Eukaryotic Gene Expression. 2005;15:49–73. doi: 10.1615/critreveukaryotgeneexpr.v15.i1.40. [DOI] [PubMed] [Google Scholar]
- 27.Romanelli M. G., Tato L., Lorenzi P., Morandi C. Nuclear localization domains in human thyroid transcription factor 2. Biochim. Biophys. Acta. 2003;1643:55–64. doi: 10.1016/j.bbamcr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Kawana K., Ikuta T., Kobayashi Y., Gotoh O., Takeda K., Kawajiri K. Molecular mechanism of nuclear translocation of an orphan nuclear receptor, SXR. Mol. Pharmacol. 2003;63:524–531. doi: 10.1124/mol.63.3.524. [DOI] [PubMed] [Google Scholar]
- 29.Amazit L., Alj Y., Tyagi R. K., Chauchereau A., Loosfelt H., Pichon C., Pantel J., Foulon-Guinchard E., Leclerc P., Milgrom E., Guiochon-Mantel A. Subcellular localization and mechanisms of nucleocytoplasmic trafficking of steroid receptor coactivator-1. J. Biol. Chem. 2003;278:32195–32203. doi: 10.1074/jbc.M300730200. [DOI] [PubMed] [Google Scholar]
- 30.Freedman N. D., Yamamoto K. R. Importin 7 and importin α/importin β are nuclear import receptors for the glucocorticoid receptor. Mol. Biol. Cell. 2004;15:2276–2286. doi: 10.1091/mbc.E03-11-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J. M., Deschenes R. J., Fassler J. S. Role for the Ran binding protein, Mog1p, in Saccharomyces cerevisiae SLN1-SKN7 signal transduction. Eukaryotic Cell. 2004;3:1544–1556. doi: 10.1128/EC.3.6.1544-1556.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao M. A., Cheng H., Quayle A. N., Nishitani H., Nelson C. C., Rennie P. S. RanBPM, a nuclear protein that interacts with and regulates transcriptional activity of androgen receptor and glucocorticoid receptor. J. Biol. Chem. 2002;277:48020–48027. doi: 10.1074/jbc.M209741200. [DOI] [PubMed] [Google Scholar]
- 33.Harreman M. T., Hodel M. R., Fanara P., Hodel A. E., Corbett A. H. The auto-inhibitory function of importin α is essential in vivo. J. Biol. Chem. 2003;278:5854–5863. doi: 10.1074/jbc.M210951200. [DOI] [PubMed] [Google Scholar]