Abstract
Neutrophils kill micro-organisms using microbicidal products that they release into the phagosome or into the extracellular space. The secretory machinery utilized by neutrophils is poorly characterized. We show that the small GTPase Rab27a is an essential component of the secretory machinery of azurophilic granules in granulocytes. Rab27a-deficient mice have impaired secretion of MPO (myeloperoxidase) into the plasma in response to lipopolysaccharide. Cell fractionation analysis revealed that Rab27a and the Rab27a effector protein JFC1/Slp1 (synaptotagmin-like protein 1) are distributed principally in the low-density fraction containing a minor population of MPO-containing granules. By immunofluorescence microscopy, we detected Rab27a and JFC1/Slp1 in a minor subpopulation of MPO-containing granules. Interference with the JFC1/Slp1–Rab27a secretory machinery impaired secretion of MPO in permeabilized neutrophils. The expression of Rab27a was dramatically increased when promyelocytic HL-60 cells were differentiated into granulocytes but not when they were differentiated into monocytes. Down-regulation of Rab27a in HL-60 cells by RNA interference did not affect JFC1/Slp1 expression but significantly decreased the secretion of MPO. Neither Rab27a nor JFC1/Slp1 was integrated into the phagolysosome membrane during phagocytosis. Neutrophils from Rab27a-deficient mice efficiently phagocytose zymosan opsonized particles and deliver MPO to the phagosome. We conclude that Rab27a and JFC1/Slp1 permit MPO release into the surrounding milieu and constitute key components of the secretory machinery of azurophilic granules in granulocytes. Our results suggest that the granules implicated in cargo release towards the surrounding milieu are molecularly and mechanistically different from those involved in their release towards the phagolysosome.
Keywords: azurophilic granule, exocytosis, myeloperoxidase, Rab27a, small GTPase, synaptotagmin-like protein (Slp)
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; EGFP, enhanced green fluorescent protein; GS, Griscelli syndrome; GST, glutathione S-transferases; HRP, horseradish peroxidase; LOD, log likelihood ratio; LPS, lipopolysaccharide; MMP-9, matrix metalloproteinase-9; MPO, myeloperoxidase; RBD, Rab-binding domain; siRNA, small interference RNA; SLO, streptolysin O; Slp1, synaptotagmin-like protein 1; VAMP-2, vesicle-associated membrane protein-2
INTRODUCTION
Human neutrophils play a central role in innate immunity by combating bacterial and fungal infections. Neutrophils kill micro-organisms by releasing microbicidal products into the phagosome or into the extracellular space. In resting neutrophils these microbicidal molecules are segregated and stored in secretory organelles, thus protecting the host from uncontrolled activation. Mature neutrophils contain four types of exocytosable storage organelles: azurophilic (primary) granules, specific (secondary) granules, gelatinase (tertiary) granules and secretory vesicles [1]. These secretory organelles contain a battery of molecules that contribute to the accurate implementation of many neutrophil functions [2]. Azurophilic granules contain MPO (myeloperoxidase) and defensins [3], in addition to other antimicrobial peptides. Specific granules contain lactoferrin [4] and MMP-9 (matrix metalloproteinase-9) [5], both modulators of the inflammatory response, which are also the main cargo of tertiary granules [6]. Neutrophil granules also contain a set of membrane proteins that are translocated to the plasma membrane during activation thus replenishing the plasma membrane with diverse receptor molecules that are essential for neutrophil function [7]. It is generally accepted that granule exocytosis during neutrophil activation is hierarchical [1]; thus the secretory vesicles are mobilized first, while tertiary granules, specific granules and azurophilic granules are sequentially mobilized in response to increasingly stronger stimuli. The hierarchy that characterizes the secretory process correlates with the different roles of their secretory cargo in adhesion, migration, chemotaxis, phagocytosis and production of ROS (reactive oxygen species). It has been proposed that this hierarchy relies on quantitative rather than qualitative differences in the secretory machinery of the various granule populations [1]. However, the exocytotic machinery of the neutrophil granules remains incompletely understood. Importantly, low-molecular-mass GTP-binding proteins have been implicated in the regulation of vesicular traffic in the secretory pathways of several types of cells including neutrophils [8], supporting the hypothesis that Rab GTPases, which help to regulate the specificity of vesicular transport including exocytosis [9], play a central role in this mechanism.
The Rab proteins are Ras-like small GTPases that act as membrane organizers. Together with their specific effectors, they are thought to regulate both the efficiency and specificity of vesicular transport. In particular, Rab27a is believed to play a central role in regulated secretion in a wide range of non-neuronal secretory cells [10]. Interestingly, of the more than 60 Rab GTPases that have been identified in humans, Rab27a is the only one directly associated with a human disease. Patients with a genetic defect in the Rab27a gene suffer a rare severe immunodeficiency disorder associated with partial pigmentary dilution [type 2 GS (Griscelli syndrome)] [11,12]. GS is characterized by impaired melanosome transport, which causes the pigmentary disorder, and by immunological abnormalities that are responsible for the poor prognosis of the disease [11]. The immunodeficiency observed in GS has been associated with impaired function of the T-lymphocytes [11], which fail to secrete the content of their lytic granules [13], and with impaired natural killer cell function [14]. Importantly, although the roles of Rab27a and its effectors have not been characterized in neutrophil function, two previous case reports have suggested that patients with GS may have defects in the function of their granulocytes. One of these studies showed abnormal bactericidal activity in the neutrophils of some of the patients evaluated [14], while the other report indicated that neutrophils from GS patients were abnormal in their phagocytic ability [15]. These studies support the hypothesis that neutrophil function is impaired in Rab27a-deficient patients and suggest that this may contribute to the pathophysiology of GS. Interestingly, Rab27a has been associated with the exocytic mechanism of a group of secretory organelles denominated lysosome-related organelles. This group includes melanosomes, lytic granules, MHC class II compartments, platelet-dense granules and basophil granules [16]. Neutrophil azurophilic granules are also lysosome-related organelles [16]. The exocytic mechanism of neutrophil granules and in particular of azurophilic granules is poorly understood, and the possible role of Rab27a in this process has not been shown.
We recently identified the Rab27a effector JFC1/Slp1 (synaptotagmin-like protein 1) from a B-lymphoblast-derived cDNA library and with the neutrophil NADPH oxidase cytosolic factor p67phox as bait [17]. Like Rab27a, JFC1/Slp1 is widely expressed in tissues with a secretory function, and a high level of expression is observed in leucocytes. The C2A domain of JFC1/Slp1 exhibits the phosphoinositide-binding site K(K/R)KTXXK(K/R) found in several members of the synaptotagmin family and binds to phosphatidylinositol (3,4,5)trisphosphate [18]. The N-terminus of JFC1/Slp1 contains a domain that is homologous with the Rab-binding module [RBD (Rab-binding domain)] present in Rabphilin3a, an effector of the small GTPase Rab3a. JFC1/Slp1 specifically binds to Rab27a [19,20] but not to other Rab proteins [21]. Our previous studies indicate that JFC1/Slp1 and Rab27a play an essential role in exocytosis [19]. In the present study, we demonstrate that Rab27a is a key component of the secretory machinery of azurophilic granules in granulocytes and we suggest that this is performed in conjunction with the Rab-effector JFC1/Slp1.
EXPERIMENTAL
Cell culture and transfection
The human promyelocytic leukaemia cell line HL-60 [A.T.C.C. (American Type Culture Collection)] was cultured in DMEM (Dulbecco's modified Eagle's medium; Gibco-BRL) supplemented with 20% fetal bovine serum (Hyclone), 0.292 mg/ml glutamine, 50 units/ml penicillin and 50 μg/ml streptomycin at 37 °C in 5% CO2/air. HL-60 cells were transfected by nucleoporation using a nucleoporator apparatus (Amaxa Biosystems). For these experiments, 5×106 cells were resuspended in 100 μl of Solution V (Amaxa Biosystems) in the presence of 20 μM Rab27a-specific siRNA (small interference RNA; Dharmacon) or non-silencing siRNA. The Rab27a-specific siRNAs used were: 5′-ggagagguuucguagcuua, Rab27a(1) and 5′-ccaguguacuuuaccaaua, Rab27a(2). Nucleofection was performed using the electrical setting T-O1. The cells were then replated in complete medium, incubated at 37 °C in 5% CO2/air and used for analysis 72 h post-transfection.
Differentiation, transfection and fractionation of HL-60 promyelocytic cells
For differentiation, HL-60 cells were treated with DMSO (1.3%, v/v), PMA (3.2 nM) or left untreated for 48 h at 37 °C in 5% CO2/air. For stimulated secretion, HL-60 cells (5×106) were transfected by nucleofection with Rab27a-specific siRNA or with control siRNA (C2, Akt-specific and C3, non-silencing siRNA). The cells were differentiated to granulocytes 24 h after transfection and, 48 h later, the cells were stimulated with PMA (1 μg/ml) for 30 min at 37 °C or left untreated (control). The cells were spun down, the supernatant was recovered and the pellets were lysed (M-PER; Pierce). MPO concentration was determined by ELISA (Assay Design). The protein concentration in the samples was analysed by the Bradford [49] method using a protein assay kit (Bio-Rad) and BSA as the standard. For each experiment, the cells were harvested and analysed for the expression of Rab27a. In some experiments, transfected HL-60 cells were resuspended in hypo-osmotic buffer [20 mM Tris/HCl, pH 7.4, containing 10 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, Complete™ antiprotease cocktail (Roche Diagnostics) and 1 mM PMSF]. The cells were placed on ice for 20 min and passed through a 27 gauge syringe needle 40 times. Lysed cells were centrifuged at 1000 g for 5 min and supernatants were spun down at 5000 g for another 5 min at 4 °C. The new supernatants were then centrifuged at 100000 g for 1 h at 4 °C. These supernatants were recovered and saved for further analysis. Pellets were washed with PBS and spun down again at 100000 g for 1 h. The pellets were resuspended in M-PER lysis buffer (Pierce). The samples were analysed for the presence of Rab27a and JFC1/Slp1 by Western blot.
Neutrophil isolation and fractionation
Neutrophils were isolated from a normal donor's blood by Ficoll density centrifugation as previously described [22]. The cellular fractionation was carried out using a two-layer Percoll density gradient after nitrogen cavitation exactly as described in [23]. The particulate fractions are referred to as α (more dense), β and γ (less dense). For sucrose-density-gradient fractionation, neutrophil lysates were spun down at 400 g for 30 min, the supernatants were placed on top of a continuous sucrose gradient (20–70%) and spun down at 150000 g for 1 h at 4 °C. Aliquots were collected from the top to the bottom and analysed for the expression of granule markers. The spectrophotometric determination of MPO activity in each fraction was measured using CytoStore (Calgary, Canada). In some experiments, human neutrophils were transfected by nucleofection with the vector expressing the plasma-membrane-binding domain of JFC1/Slp1 (C2A domain) [18] as a chimaera with EGFP (enhanced green fluorescent protein) (EGFP–C2A), or with the EGFP empty vector as a control. The cells were fixed 2 h after transfections and analysed by confocal microscopy.
Immunofluorescence
Human neutrophils or differentiated HL-60 cells were seeded at 70% confluence in an eight-well chambered coverglass [pre-treated with poly(L-lysine) at 0.01% in PBS], fixed with 3.7% (w/v) paraformaldehyde, permeabilized with 0.01% saponin and blocked with a solution of 1% BSA in PBS. In order to stain the nucleus, some samples were incubated with DAPI (4′,6-diamidino-2-phenylindole) for 5 min at 21 °C. Samples were labelled with the indicated primary antibodies, overnight at 4 °C, and the appropriate combinations of the secondary antibodies (488 nm) and/or (594 nm) Alexa Fluor®-conjugated donkey anti-rabbit, anti-mouse and/or anti-goat (Molecular Probes). Cells were stored in Fluoromount-G (Southern Biotechnology) and analysed using a Bio-Rad (Zeiss) 2100 Rainbow Radiance LSCM (laser-scanning confocal microscope) attached to a Nikon TE2000-U microscope at 21 °C with a ×60 oil-Plan-Apo-1.4na objective. For visualization, fluorescence associated with Alexa Fluor® 594-labelled secondary antibody was excited using the 543 nm laser line, and collected using a standard Texas Red filter. Fluorescence associated with Alexa Fluor® 488-labelled secondary antibodies was visualized using the 488 nm laser line, and collected using a standard FITC filter set. Images were collected using the Bio-Rad Laser Sharp 2000 (version 3.2) software and processed using Image J and Adobe Photoshop CS.
Co-immunoprecipitation assays
For co-immunoprecipitation of Rab27a with JFC1/Slp1, 1×107 human neutrophils were isolated as described above and treated with 2.5 mM di-isopropyl fluorophosphate (Sigma) for 10 min at 4 °C. Then, neutrophils were resuspended in a lysis buffer consisting of 50 mM Tris/HCl (pH 7.4), containing 150 mM NaCl and 2% N-octyl-β-D-glucopyranoside or 1% Triton X-100 and supplemented with protease inhibitors. The cells were kept in ice for 30 min and spun down at 13000 g for 15 min at 4 °C to eliminate the cell debris. The level of expression of JFC1/Slp1 and Rab27a in the samples was analysed by Western blot. The immunoprecipitation reactions were carried out overnight at 4 °C by the addition of the anti-JFC1/Slp1 affinity-purified antibody or an irrelevant antibody as control [rabbit anti-HA (haemagglutinin) epitope; Santa Cruz Biotechnology] to the precleared lysates and by further addition of anti-rabbit IgG beads (TrueBlot™; eBiosciences). The presence of Rab27a and JFC1/Slp1 in the immunoprecipitates was determined by Western-blot analysis using specific monoclonal and polyclonal antibodies respectively. In some experiments, JFC1/Slp1 was co-immunoprecipitated with Rab27a from HL-60 cell lysates using a rabbit anti-Rab27a-specific antibody and Protein A–agarose. In these experiments, the presence of Rab27a and JFC1/Slp1 in the immunoprecipitates was determined by Western blot using specific polyclonal antibodies and Protein A-conjugated HRP (horseradish peroxidase) to detect the bound antibody.
Stimulated secretion in SLO (streptolysin O)-permeabilized human neutrophils
Secretory assays using SLO-permeabilized cells were performed as previously described [24]. Briefly, neutrophils were washed twice with PBS and resuspended in permeabilization buffer [24]. Human neutrophils (2.5×106) were resuspended in permeabilization buffer (100 μl) and transferred to a tube containing 2 μl of 2500 units/ml SLO in the presence of 2 μM GST (glutathione S-transferases) or GST–C2A(JFC1/Slp1) and incubated at 37 °C for 5 min. The cells were stimulated with phorbol ester (PMA; 0.5 μg/ml). The reactions were stopped by transferring the samples to ice and immediately centrifuged at 16000 g for 5 min at 4 °C. The released MPO was analysed by ELISA (Assay Design) following the manufacturer's instructions. The purification of GST–C2A(JFC1/Slp1) was described previously [25].
Antibodies
The mouse monoclonal and rabbit polyclonal antibodies raised against Rab27a were described elsewhere [19]. The polyclonal antibody raised against JFC1/Slp1 was described previously [19]. Other antibodies used were: goat polyclonal anti-human MMP-9, goat polyclonal anti-human MPO, and mouse anti-VAMP-2 (vesicle-associated membrane protein-2) (Santa Cruz Biotechnology); rabbit polyclonal anti-VAMP-8 (Abcam, Cambridge, MA, U.S.A.) and rabbit polyclonal anti-actin (Sigma). The polyclonal antibody directed against the NADPH oxidase subunit p22phox was raised in rabbits, using the peptide CNPPPRPPAEARKKPSE.
Rab27acct/cct mice
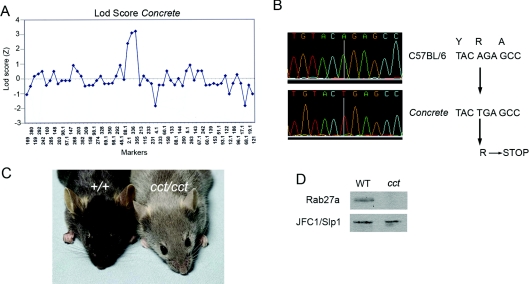
Mice homozygous for the Rab27a allele Concrete (cct) were identified based on their hypopigmented appearance in third generation (G3) animals carrying mutations induced at random by N-ethyl-N-nitrosourea [26] on a pure C57BL/6 background. The hypopigmentation phenotype was mapped by outcrossing the founder to C3H/HeN mice and intercrossing the F1 hybrid progeny. On 32 meioses (16 F2 mice, 15 of which showed the mutant phenotype), linkage to chromosome 9 was observed with a LOD (log likelihood ratio) score above 3, with confinement between markers D9Mit336 and D9Mit355 (Figure 1A). The Rab27a locus resides within this interval, and a nonsense transversion (AGA>TGA; R54>STOP) was observed within the Rab27a coding sequence (Figure 1B). Rab27acct/cct homozygotes were expanded and maintained as a homozygous stock for use in these studies. For in vivo studies of MPO secretion, mice were injected intraperitoneally with 100 μg of LPS (lipopolysaccharide). Smooth LPS, isolated and purified from Escherichia coli O111:B4 (Alexis), was used. Blood samples were collected before and at 4 and 8 h post injection.
Figure 1. The Concrete phenotype results from mutation of the Rab27a gene.
(A) Transgenomic LOD (LOD score) analysis shows a single peak of linkage on mouse chromosome 9. A total of 59 informative markers (horizontal axis) were included in the analysis and 32 meioses were genotyped at all markers. (B) Concrete (cct) corresponds to a single nucleotide substitution in the Rab27a gene. A→T transversion results in a stop codon replacing an arginine. The Consed display shows bi-directional sequencing of Rab27a cDNA. (C) Mice homozygous for the Rab27a allele Concrete present a hypopigmented appearance. (D) Leucocytes from Concrete mice lack the expression of Rab27a as detected by Western blot.
Isolation of murine leucocytes
Murine blood (∼1 ml per animal) was obtained by cardiac puncture. Red blood cells were lysed using 20 ml of lysing buffer (168 mM NH4Cl, 10 mM KHCO3 and 100 μM EDTA, pH 7.3) for 2 min at room temperature. Residual red blood cells were separated using a two-layer Percoll gradient (52 and 78%). Leucocytes were isolated from the 52–78% interface and washed twice with PBS before use. For neutrophil isolation, a three-layer Percoll gradient was used (52, 62 and 78%). Neutrophils were isolated from the 62–78% interface, washed and used in phagocytosis assays.
Phagocytosis assays
Human or murine neutrophils (5×104) were transferred to an eight-well chambered coverglass containing 200 μl of RPMI 1640/10% (v/v) fetal calf serum. The cells were incubated for 30 min at 37 °C in a humidified incubator under 5% CO2/air. Texas Red-conjugated opsonized zymosan (5×106 particles; Molecular Probes) was added and the cells were further incubated for 15 min. In some experiments, the extracellular particles were quenched using Trypan Blue. The cells were fixed and JFC1/Slp1, Rab27a, MPO and LAMP-2 (lysosome-associated membrane protein-2) were detected by immunofluorescence and confocal microscopy analysis.
SDS/PAGE and Western blotting
Proteins were separated by PAGE in the presence of 1% (w/v) SDS. Proteins were transferred on to nitrocellulose membranes for 120 min at 60 V and 4 °C. The membranes were blocked with PBS containing 5% (w/v) blotting grade blocker non-fat dry milk (Bio-Rad) and 0.05% (w/v) Tween 20 (pH 7.4). The proteins were detected by probing the membranes with the indicated primary antibodies at appropriate dilutions. The detection system used comprises HRP-conjugated secondary antibodies (Caltag Laboratories) and the LumiGlo chemiluminescence substrate (Upstate Biotechnology). Detection of the transferred proteins was performed on chemiluminescence films (Amersham Bioscience).
Statistical analysis
Continuous variables were expressed as means±S.E.M. The statistical significance of the difference of the means was analysed by ANOVA. P<0.05 was considered statistically significant.
RESULTS
Secretion of MPO is dramatically impaired in Rab27acct/cct mice
Several recent reports have highlighted the role of the small GTPase Rab27a and its effectors in exocytosis [10,19,27]. In particular, Rab27a plays a fundamental role in the exocytic mechanism of lysosome-related organelles including melanosomes [28], lytic granules [29], platelet-dense granules [30] and basophil granules [31]. Since neutrophil function might be altered in Rab27a deficiency [14,15], we hypothesized that Rab27a might play a central role in the exocytosis of neutrophil lysosome-related organelles: the azurophilic granule. To evaluate the role of Rab27a in the exocytosis of azurophilic granules in vivo, we utilized Rab27acct/cct mice. Concrete mice present a hypopigmented appearance (Figure 1C) and do not express Rab27a although they express detectable amounts of the Rab27a effector JFC1/Slp1 (Figure 1D). We analysed the release of MPO, an enzyme that is mainly expressed in neutrophils, in Rab27a-deficient mice after intraperitoneal injection of LPS. The results presented in Figure 2(A) show that the plasma concentration of MPO in Rab27a-deficient mice is significantly lower than that in the control population after LPS treatment. Significant differences were observed at 4 and 8 h after injection; however, no differences were detected for the basal level of MPO before LPS injection. Importantly, no significant differences were observed in the number of circulating neutrophils in the peripheral blood of control and Rab27acct/cct mice (Figure 2B). These results clearly indicate that Rab27a plays a central role in the process of regulated (inducible) MPO secretion, suggesting that defects in MPO exocytosis are part of the spectrum of cellular immunological disorders observed in Rab27a deficiency, together with T-lymphocyte and natural killer cell abnormalities.
Figure 2. The secretion of MPO is markedly impaired in Rab27a-deficient mice.
(A) Rab27a−/− mice (open circles) and wild-type mice (closed circles) were challenged with a single intraperitoneal injection of LPS (+) or vehicle (−). Blood samples were collected before (0 h) and 4 and 8 h after injection. The samples were spun down and plasma was collected and analysed for the presence of MPO using a mouse-specific ELISA (HyCult Biotechnology). The statistical significance of the difference of the means was analysed by ANOVA. *P<0.01; **P<0.001. (B) Differential leucocyte counts in Rab27a-deficient and control mice were performed using a HEMAVET-950 Hematology system (n=3). Black columns, wild-type mice; grey columns, Rab27a-deficient mice. WBC, white blood cells; NE, neutrophils; LY, lymphocytes; MO, monocytes; EO, eosinophils; BA, basophils.
Expression and subcellular distribution of Rab27a and JFC1/Slp1 in human neutrophils
Rab27a regulates the efficiency and specificity of exocytosis through interaction with specific effectors.
We evaluated the subcellular distribution of Rab27a and its effector JFC1/Slp1 in human neutrophils after cell fractionation, using a well-characterized method for the separation of neutrophils granules [23]. With this method, three different granule populations are identified and designated the α-fraction (most dense), the β-fraction (intermediate density), and the γ-fraction (least dense) with the cytosol on top. Rab27a was mainly detected in the low-density γ fraction and was slightly detected in the β and α fractions (Figure 3A). The Rab27a effector JFC1/Slp1 was also mainly distributed in the γ fraction with a minor proportion being detected in the β fraction and the cytosol (Figure 3A). Using a continuous sucrose gradient, we confirmed that Rab27a and JFC1/Slp1 are present in the low-density granule fraction (Figure 3B), a subpopulation of granules that is considered more readily releasable than high-density granules. The presence of JFC1/Slp1 in the cytosol is in agreement with our previous study showing that a major characteristic of this Rab effector is to cycle between membrane and cytosol in a phosphorylation-dependent manner [32]. Coinciding with the distribution of Rab27a and JFC1/Slp1, we detected a minor population of MPO-containing granules in the low-density granule fractions (Figures 3A and 3B). This is in agreement with a previous study showing that although MPO is predominantly present in the high-density (α) band, minor subpopulations of the MPO-positive granules comprising 10 and 5% of the MPO-containing granules are present in the intermediate density (β) and low-density (γ) bands respectively [33]. This also concurs with an earlier study, which suggested that azurophilic granules are heterogeneous in terms of density [34]. Importantly, VAMP-2, a protein involved in the exocytosis of neutrophil secretory organelles [35], also fractionated in the Rab27a/JFC1/Slp1-enriched fraction (Figure 3A). Conversely, VAMP-8, a SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) protein involved in exocytosis in pancreatic acinar cells [36], was detected not only in the γ but also in the α and β fractions, suggesting a different role for proteins of this family in the exocytosis of neutrophil granules. MMP-9 and p22phox, which co-distributed between the low-density and the intermediate density fractions, were included as controls of the fractionation procedure.
Figure 3. Rab27a and JFC1/Slp1 are constituents of a common secretory machine and co-localize with MPO in neutrophil granules.
(A) Human neutrophils were disrupted by nitrogen cavitation and the post-nuclear supernatant was separated using a two-layer Percoll gradient as described in the Experimental section. After centrifugation, three different bands were identified, designated from the bottom, the α-band, the β-band, and the γ-band with the clear cytosol (Cyt) on top. Cytosol (1.8×106 cell equivalents) or membranes (4.3×106 cell equivalents) were resolved by SDS/PAGE and the subcellular localization of the indicated proteins was analysed by Western blot. Upper panel, secretory proteins; lower panel, granule markers. (B) Neutrophils were disrupted and fractionated using a continuous sucrose gradient. Samples were analysed by Western blot for the presence of the indicated markers and for MPO activity by an enzymatic assay. (C) Endogenous JFC1/Slp1 and endogenous Rab27a were detected by immunofluorescent confocal microscopy in conjunction with the azurophilic granule marker MPO as indicated in the Figure. Endogenous JFC1/Slp1 and Rab27a were detected using specific rabbit polyclonal antibodies and for the detection of MPO we used an antibody raised in goat. For detection, the secondary antibodies Alexa fluor®-conjugated donkey anti-rabbit (488 or 594), and donkey anti-goat (594 or 488) were used. In some experiments, the nucleus was visualized by staining DNA with DAPI. Arrows indicate the co-localization of JFC1/Slp1 or Rab27a in punctate structures labelled for the indicated granule markers. Arrowheads indicate MPO-containing granules with no detectable JFC1/Slp1 or Rab27a. Scale bar, 10 μm. (D) Co-localization of endogenous JFC1/Slp1 and endogenous Rab27a was detected by immunofluorescent confocal microscopy in human neutrophils. In these experiments, neutrophils were labelled for immunofluorescent detection of Rab27a (red) or JFC1/Slp1 (green) using a mouse monoclonal and a rabbit polyclonal antibody respectively or negative controls (results not shown). For detection, the secondary antibodies Alexa fluor®-conjugated donkey anti-rabbit (488) and donkey anti-mouse (594) were used. Scale bar, 10 μm. (E) Rab27a is co-immunoprecipitated with JFC1/Slp1 from human neutrophil lysates. Human neutrophils were isolated, di-isopropyl fluorophosphate-treated and lysed as described in the Experimental section. An aliquot of the sample was saved to establish the level of expression of JFC1/Slp1 and Rab27a (input). The immunoprecipitation (IP) reactions were carried out by the addition of anti-JFC1/Slp1 polyclonal antibody to the precleared lysate, overnight at 4 °C, and by the subsequent addition of TrueBlot beads (eBioscience). An irrelevant antibody (Irr Ab, anti-HA epitope) was used as negative control. Immunoprecipitated Rab27a and JFC1/Slp1 were determined by Western-blot analysis (WB) using specific monoclonal and polyclonal antibodies respectively. The results are representative of three different experiments with similar results. (F), JFC1/Slp1 and Rab27a were co-immunoprecipitated from HL-60 granulocytes using an anti-Rab27a polyclonal antibody as described in the Experimental section.
To evaluate the subcellular distribution of endogenous Rab27a and JFC1/Slp1 in relationship to that of the azurophilic granule marker, MPO, we analysed their subcellular localization by immunofluorescence and confocal microscopic analysis. We detected the presence of Rab27a and JFC1/Slp1 in co-localization with MPO but, as expected from the cellular fractionation studies, only a subpopulation of MPO-containing granules was labelled with these secretory proteins (Figure 3C). These results support the inference that the great degree of heterogeneity observed in azurophilic granules is not limited to electron density, size, shape and density [34], but extends to their content of Rab proteins and their effectors. Based on these findings, on the previously described role of Rab27a in exocytosis [10,19] and on the observation that only a minor subpopulation of azurophilic granules is believed to undergo exocytosis [37], we hypothesized that the Rab27a-containing pool of azurophilic granules can undergo exocytosis and co-exists with a non-releasable pool of azurophilic granules that lack associated secretory machinery. To confirm that Rab27a and JFC1/Slp1 are constituents of a common secretory machine in neutrophils, we evaluated their subcellular distribution and interaction by confocal microscopy and co-immunoprecipitation assays respectively. In Figure 3(D), we show that endogenous Rab27a and JFC1/Slp1 co-localize at punctate structures that resemble secretory organelles in human neutrophils. We also found that endogenous Rab27a co-immunoprecipitates with endogenous JFC1/Slp1 in granulocytes (Figures 3E and 3F), indicating that the interaction of these secretory proteins might have physiological significance in these cells.
Secretion of MPO is impaired by interference with the Rab27a–JFC1/Slp1 secretory machinery
The Rab27a effector JFC1/Slp1 contains a RBD in its N-terminus and tandem C2 domains in its C-terminus. We previously showed that the Rab27a-binding domain of a truncated JFC1/Slp1, lacking the tandem C2 domains, localizes on vesicles that do not distribute in the proximity of the plasma membrane [18]. We also showed that the C2A domain of JFC1/Slp1 localizes exclusively to the plasma membrane when expressed as an EGFP chimaera [18]. These observations led to the hypothesis that JFC1/Slp1 plays the part of a connector, docking Rab27a-bearing vesicles to the plasma membrane [19]. In the present study, we show that the C2A domain of JFC1/Slp1 localizes to the plasma membrane in human neutrophils when expressed as an EGFP chimaera (Figure 4A). Then, to demonstrate that the Rab27a–JFC1/Slp1 machinery is involved in the exocytosis of neutrophil secretory organelles, we used a cellular approach based on the principle that the intracellular presence of the C2A domain of JFC1/Slp1 would compete with endogenous JFC1/Slp1 for a docking site on the plasma membrane and thereby impair secretion. We have used this approach successfully in the past to demonstrate the participation of JFC1/Slp1 and Rab27a in exocytosis in prostate carcinoma cells [19]. In the present study, we used SLO [38], a bacterial pore-forming toxin, to establish a semi-intact assay system for the analysis of granule secretion in human neutrophils. In Figure 4(B), we show that the secretion of the azurophilic granule marker MPO is dramatically impaired by interference with the Rab27a–JFC1/Slp1 apparatus in human neutrophils stimulated with phorbol ester. These results correlate with the results of immunofluorescence studies presented above showing that Rab27a and JFC1/Slp1 co-localize at MPO-containing granules. These results suggest for the first time that Rab27a and JFC1/Slp1 are part of the secretory machinery leading to exocytosis of a subset of neutrophil granules.
Figure 4. Interference with the Rab27a/JFC1/Slp1 pathway inhibits secretion of MPO in human neutrophils.
(A) Human neutrophils were transfected by nucleofection with the vector expressing the plasma-membrane-binding domain of JFC1/Slp1 (C2A domain) [18] as a chimaera with EGFP (EGFP–C2A), or with the EGFP empty vector as control. The cells were fixed and analysed by confocal microscopy. Scale bar, 3 μm. (B) Neutrophils were permeabilized with SLO in the presence of the C2A domain of JFC1/Slp1 (GST–C2A, grey columns) or an equimolar amount of GST protein as control (black columns). Subsequently, the cells were stimulated with PMA (0.5 μg/ml) for the indicated time. The conditioned medium and cellular pellets were collected and used for subsequent analysis. MPO was evaluated using a human ELISA kit (Assay Design). Results are the means±S.E.M. for four different experiments.
Rab27a plays a central role in the exocytosis of azurophilic granules in granulocytes
Neutrophils are non-dividing cells with poor survival after isolation. Consequently, exogenous gene expression or gene down-regulation in neutrophils is challenging. Researchers have partially overcome these difficulties by performing studies in well-developed cell-free systems [39], with permeabilized neutrophils, or with cell lines that resemble neutrophils in some ways. In particular, HL-60 promyelocytic cells can be differentiated to form granulocytes by treatment with DMSO or retinoic acid [40]. HL-60-derived granulocytes express active NADPH oxidase, undergo phagocytosis, and have the capacity to undergo exocytosis in response to stimuli, although their repertoire of secretory organelles is limited to azurophilic granules [41]. First, we evaluated the expression of Rab27a and its effector JFC1/Slp1 in HL-60 cells. As shown in Figure 5, Rab27a is poorly expressed in undifferentiated HL-60 cells. However, dramatic up-regulation of the expression of Rab27a occurs after differentiation to the granulocyte lineage. In contrast, we observed relatively low expression of Rab27a in HL-60 cells differentiated to the monocyte lineage. Moreover, the effect was specific for Rab27a since not all Rab proteins were up-regulated under identical experimental conditions (i.e. Rab11; Figure 5A). Altogether, these results indicate that Rab27a up-regulation parallels the functional maturation of myeloid cells and support the idea that Rab27a function is important in mature granulocytes. We also observed that expression of the Rab27a-specific effector JFC1/Slp1 is up-regulated during HL-60 differentiation to granulocytes (Figure 5B). However, this effect was of a lesser magnitude than that observed for Rab27a, perhaps because a relatively large amount of JFC1/Slp1 is already expressed in undifferentiated cells. We then analysed the cellular distribution of Rab27a and JFC1/Slp1 in HL-60 granulocytes by immunofluorescence and confocal microscopy. In Figure 5(C) we show that Rab27a and JFC1/Slp1 localize to punctate structures that resemble granules in HL-60 differentiated granulocytes. To confirm that these structures were secretory organelles, we simultaneously detected the marker of secretory organelles VAMP-2 and we showed that Rab27a and JFC1/Slp1 largely co-localize with this marker, supporting the utilization of HL-60 as a cellular model for the analysis of Rab27a function.
Figure 5. Rab27a is up-regulated during granulocyte differentiation.
(A, B) HL-60 cells were treated with DMSO (1.3%, v/v), PMA or left untreated, and 48 h after induction, the cells were harvested, lysed and analysed for the expression of Rab27a, Rab11 or JFC1/Slp1 by Western blot. Rab27a up-regulation was evident in HL-60 cells differentiated to the neutrophilic lineage (DMSO) but not in HL-60 cells differentiated to monocytes (PMA). (C) Analysis of the subcellular distribution of Rab27a and JFC1/Slp1 in HL-60 cells by immunofluorescence. The subcellular distribution of JFC1/Slp1 and Rab27a was evaluated in relationship to that of the secretory protein VAMP-2 by immunofluorescence. The arrows indicate punctate structures showing co-localization of the indicated proteins.
To elucidate the function of Rab27a in granulocytes, we first optimized the down-regulation of Rab27a expression using siRNA. HL-60 cells transfected with Rab27a-specific siRNA showed a dramatic decrease (∼85%) in the level of expression of Rab27a, while three different control siRNAs had no effect (Figure 6A). Interestingly, although some Rab effectors are known to be unstable in the absence of their Rab counterparts, as is the case for the Rab27a effector melanophilin [42], the expression of JFC1/Slp1 was not affected by Rab27a down-regulation (Figure 6B) or in Rab27a-deficient neutrophils (Figure 1D). This is probably explained in part by the natural abundance of JFC1/Slp1 in the cytosol, since this pool of protein is not in contact with membrane-bound Rab27a. Importantly, the relative distribution of JFC1/Slp1 in the cytosol increases after Rab27a down-regulation (Figure 6C), supporting a role for Rab27a as a docking molecule for JFC1/Slp1 on the secretory organelles. This further supports a role for JFC1/Slp1 as a Rab27a effector in granulocytes.
Figure 6. Rab27a deficiency impairs the secretion of MPO in granulocytes.
(A) Down-regulation of Rab27a in HL-60 by siRNA. HL-60 cells were transfected by nucleofection with Rab27a-specific siRNA [Rab27a(1) and Rab27a(2), please refer to the Experimental section for sequences], or with control siRNA (C1, JFC1/Slp1-specific; C2, Akt-specific; and C3, non-silencing siRNA). The cells were differentiated to granulocytes 24 h after transfection and, 48 h later, were harvested and analysed for the expression of Rab27a. (B) Experiments were performed as described above except that lysates were evaluated for the expression of JFC1/Slp1 as well as Rab27a. In these experiments, the siRNA Rab27a(2) was utilized. (C) The distribution of JFC1/Slp1 in the cytosol of granulocytes increases with down-regulation of Rab27a. HL-60 cells were transfected by nucleofection with Rab27a-specific siRNA and subsequently differentiated to granulocytes. Cells were disrupted by nitrogen cavitation and the fractions were separated by differential centrifugation. The abundance of the indicated proteins in the different fractions was evaluated by Western blot. PGK1 (phosphoglycerate kinase 1) was used as a marker for the cytosolic fraction (Cyt). Mem indicates particulate fraction. (D) HL-60 cells were transfected with Rab27a-specific siRNA or with non-silencing siRNA and differentiated as described above and in the Experimental section. The cells were harvested, washed with PBS and stimulated with PMA (1 μg/ml) for 30 min. The cells were spun down, and the concentration of MPO in the supernatants and the pellets was determined by ELISA. Results are the means±S.E.M. for three different experiments.
To evaluate the role of Rab27a in the secretory pathway of azurophilic granules, we analysed the exocytotic capacity of HL-60 cells after Rab27a knockdown. We found that MPO secretion was significantly decreased in Rab27a-down-regulated granulocytes when compared with HL-60 cells transfected with non-silencing control siRNA (Figure 6D). Of note, the secretion of MPO was markedly reduced but not abolished in Rab27a-down-regulated cells. This is most likely explained by the fact that residual Rab27a is present in siRNA-treated cells. Altogether, these results suggest a central role for Rab27a in the secretory function of granulocytes and in particular, in the release of the content of azurophilic granules.
Rab27a does not play a significant role in the mobilization of azurophilic granules to the phagosome
Phagocytosis of an invading micro-organism plays a central role in innate immunity. The capacity of neutrophils to efficiently protect the host organism from an invading micro-organism depends in part on their ability to mobilize organelles containing microbicidal proteins to the phagosome. Although a low proportion of MPO-containing granules is released into the extracellular milieu during activation, azurophilic granules are mostly considered to degranulate into the phagosome during bacteria engulfment. This facilitates the bactericidal activity of cationic granule proteins that are primarily responsible for the destruction of the bacteria [43]. Interestingly, phagocytosis defects have been observed in some but not all patients with GS [14,15], posing the question as to whether Rab27a is involved in the transport of granules towards the phagosome. We evaluated the behaviour of Rab27a-containing vesicles during phagocytosis in human neutrophils. Our results indicate that neither JFC1/Slp1 nor Rab27a integrates into the phagosome membrane during phagocytosis of opsonized zymosan (Figure 7A). Taking account of the fact that only a subpopulation of azurophilic granules contains JFC1/Slp1 and Rab27a (Figure 3), we suggest that the subpopulation of azurophilic granules responsible for MPO release into the surrounding milieu is qualitatively different from the subpopulation of granules involved in MPO release into the phagosome. Supporting this, we found that Rab27a-deficient neutrophils can efficiently phagocytose opsonized zymosan particles and deliver MPO to the phagosome (Figure 7B). However, in these experiments we analysed the behaviour of Rab27a in relation to mature phagosomes, and we are unable to discount a potential role for Rab27a during early stages of phagosome formation or through a ‘kiss-and-run’ [44] type of secretory process.
Figure 7. Rab27a does not play a significant role in the mobilization of azurophilic granules to the phagosome.
(A) Rab27a and JFC1/Slp1 do not integrate with the membrane of mature phagosomes. Human neutrophils were exposed to Texas Red-conjugated opsonized zymosan particles (Molecular Probes) for 15 min at 37 °C. The cells were fixed and the subcellular distribution of the indicated proteins was detected by immunofluorescence analysis and confocal microscopy as described in the Experimental section. Cells were analysed from top to bottom and a representative image of the Z-analysis is shown. LAMP-2, lysosome associated membrane protein-2; N, nuclear lobe; IF, immunofluorescence. (B) Neutrophils obtained from Rab27a-deficient mice were used in phagocytosis assays as described in the Experimental section. Rab27a-deficient neutrophils efficiently phagocytose opsonized zymosan particles and deliver MPO to the phagosome. Neutrophils were isolated from peripheral blood from Rab27a-deficient (Rab27a−/−) or wild-type (WT) mice and exposed to Texas Red-conjugated opsonized zymosan particles as above. The cells were fixed and the subcellular distribution of the indicated proteins was detected by immunofluorescence analysis and confocal microscopy.
DISCUSSION
Exocytosis and the fusion of neutrophil granules with the phagosome are crucial events in inflammation and host defence. Tight regulation of the exocytic process is particularly important in neutrophils, because unrestricted release of the toxic contents of neutrophil granules would be injurious to the host. However, many of the molecular mechanisms that control differential exocytosis of the various secretory organelles found in neutrophils remain obscure. In this study, we present strong evidence that the small GTPase Rab27a plays a central role in the regulation of azurophilic granule exocytosis in neutrophils.
Patients with type 2 GS (deficient in Rab27a) develop an immunodeficiency disorder characterized by malfunction of cytotoxic T-lymphocytes, which show deficient exocytosis of lytic granules, and by impaired natural killer cell function. Little attention has been paid to neutrophil dysfunction observed in GS except for two previous case reports, which suggested that patients with GS may have defects in the function of their granulocytes. However, the findings were subject to multiple interpretations. One of the studies reported abnormal granulocyte bactericidal activity in some of the patients evaluated [14], while the other study reported abnormalities in the phagocytic activity of neutrophils in a subpopulation of the patients under study [15]. Therefore, to elucidate the role of Rab27a in neutrophil function in further detail, we first analysed the response of Rab27acct/cct mice to LPS and found that the secretion of MPO is dramatically impaired in this animal model. These findings have physiological implications and suggest that Rab27a is an essential component of the secretory machinery of azurophilic granules. Then, we investigated the subcellular distribution of Rab27a by cell fractionation and immunofluorescence microscopy. We determined that Rab27a co-localizes with MPO, a marker of azurophilic granules. Importantly, only a minor fraction of the MPO-positive granules has been shown to undergo exocytosis in response to stimuli like fMet-Leu-Phe (N-formylmethionyl-leucylphenylalanine) and calcium ionophores [37], and azurophilic granules are known to fuse primarily with the phagosome during phagocytosis. This correlates with the results presented here showing that only a minor fraction of azurophilic granules contains Rab27a in their membranes. This supports the hypothesis that azurophilic granules are heterogeneous in their use of secretory machinery.
In the present study, we also show that interference with the Rab27a secretory machinery using the plasma-membrane-binding domain of JFC1/Slp1 impairs the secretion of MPO in human neutrophils (Figure 4). Therefore it becomes apparent that Rab27a and JFC1/Slp1 are important constituents of the secretory machinery of peroxidase-positive granules in neutrophils. To corroborate the finding that Rab27a actually plays an active role in the secretion of MPO, we examined HL-60 differentiated granulocytes, which contain azurophilic granules but not tertiary or specific granules. In these cells, down-regulated expression of Rab27a causes impairment of MPO release, supporting the data obtained in our experiments in vivo and in human neutrophils. Interestingly, neutrophil secretory organelles differ in their propensity to respond to stimuli. For example, secretory vesicles and tertiary, secondary and primary granules differ in the Ca2+ levels necessary to induce half-maximal marker release and in their tendency to respond to receptor-mediated stimulation [37]. A possible explanation for this phenomenon is that different Rab or Rab effectors may be involved in the secretory mechanism of organelles with different tendencies to undergo exocytosis. So far, 11 possible Rab27a effectors have been described in many cellular systems [27]. We show in the present study that JFC1/Slp1, a Rab27a effector originally isolated as a binding partner of a cytosolic factor of the neutrophil NADPH oxidase, is highly expressed in neutrophils and co-localizes with Rab27a. We also show that the C2A domain of JFC1/Slp1, a phosphoinositide-interacting module that has been shown to inhibit secretion in other cellular systems [19], has the ability to down-regulate the secretion of MPO from human neutrophils. We therefore suggest that JFC1/Slp1 might play an important role in the secretory machinery of human neutrophil granules, although we do not disregard the possibility that other Rab27a effectors co-participate in this complex secretory mechanism. Interestingly, proteomic analysis of human neutrophil granules demonstrated that MUNC13-4, another Rab27a effector shown to control the secretion of lysosomes from haemopoietic cells [45], is present in low-density secretory organelles [46]. At present, however, our findings represent the first evidence of the participation of Rab27a and JFC1/Slp1 in the exocytosis of neutrophil secretory organelles. The role of other Rab GTPases and effectors remains to be elucidated.
Although the secretion of MPO and other proteins with microbicidal activity to the extracellular milieu may be necessary to combat micro-organisms that have not been phagocytosed, their release is potentially deleterious to the host. For example, MPO activity has been associated with the pathogenesis of atherosclerosis, hypertension and diabetic vascular complications [47,48]. Therefore uncontrolled MPO release represents a potential hazard and its release must be tightly regulated. The central role of Rab27a in the regulated secretion of MPO would therefore be expected to have important physiological consequences, and suggest that a better understanding of the Rab27a-dependent secretory machinery might lead to the design of effective molecular strategies for the treatment of conditions in which exocytosis is altered. This could have implications in the control of primary immunodeficiencies with alterations in the secretory pathway of leucocytes like GS, and in the control of inflammatory vascular diseases in which the secretion of MPO has been implicated.
Acknowledgments
This study was supported by US Public Health Service grant AI-024227, and by the Sam and Rose Stein Endowment Fund. We thank Dr Miguel Seabra for the gift of reagents. We thank Dr W. Kiosses for assistance with confocal microscopy analysis.
References
- 1.Borregaard N., Cowland J. B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 2.Lehrer R. I. Primate defensins. Nat. Rev. Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 4.Legrand D., Elass E., Carpentier M., Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opdenakker G., Van den Steen P. E., Van D. J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S., Patel K. D. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J. Leukoc. Biol. 2005;78:279–288. doi: 10.1189/jlb.1004612. [DOI] [PubMed] [Google Scholar]
- 7.Sengelov H., Kjeldsen L., Kroeze W., Berger M., Borregaard N. Secretory vesicles are the intracellular reservoir of complement receptor 1 in human neutrophils. J. Immunol. 1994;153:804–810. [PubMed] [Google Scholar]
- 8.Philips M. R., Abramson S. B., Kolasinski S. L., Haines K. A., Weissmann G., Rosenfeld M. G. Low molecular weight GTP-binding proteins in human neutrophil granule membranes. J. Biol. Chem. 1991;266:1289–1298. [PubMed] [Google Scholar]
- 9.Pfeffer S. R. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- 10.Tolmachova T., Anders R., Stinchcombe J., Bossi G., Griffiths G. M., Huxley C., Seabra M. C. A general role for Rab27a in secretory cells. Mol. Biol. Cell. 2004;15:332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griscelli C., Durandy A., Guy-Grand D., Daguillard F., Herzog C., Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am. J. Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- 12.Menasche G., Pastural E., Feldmann J., Certain S., Ersoy F., Dupuis S., Wulffraat N., Bianchi D., Fischer A., Le Deist F., de Saint B. G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 13.Haddad E. K., Wu X., Hammer J. A., III, Henkart P. A. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J. Cell Biol. 2001;152:835–842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein C., Philippe N., Le Deist F., Fraitag S., Prost C., Durandy A., Fischer A., Griscelli C. Partial albinism with immunodeficiency (Griscelli syndrome) J. Pediatr. 1994;125:886–895. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- 15.Harfi H. A., Brismar J., Hainau B., Sabbah R. Partial albinism, immunodeficiency, and progressive white matter disease: a new primary immunodeficiency. Allergy Proc. 1992;13:321–328. doi: 10.2500/108854192778816933. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Angelica E. C., Mullins C., Caplan S., Bonifacino J. S. Lysosome-related organelles. FASEB J. 2000;14:1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- 17.McAdara-Berkowitz J. K., Catz S. D., Johnson J. L., Ruedi J. M., Thon V., Babior B. M. JFC1/Slp1, a novel tandem C2 domain-containing protein associated with the leukocyte NADPH oxidase. J. Biol. Chem. 2001;276:18855–18862. doi: 10.1074/jbc.M011167200. [DOI] [PubMed] [Google Scholar]
- 18.Catz S. D., Johnson J. L., Babior B. M. The C2A domain of JFC1/Slp1 binds to 3′-phosphorylated phosphoinositides and directs plasma membrane association in living cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11652–11657. doi: 10.1073/pnas.172382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson J. L., Ellis B. A., Noack D., Seabra M. C., Catz S. D. The Rab27a binding protein JFC1/Slp1 regulates androgen-dependent secretion of prostate specific antigen and prostate specific acid phosphatase. Biochem. J. 2005;391:699–710. doi: 10.1042/BJ20050380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strom M., Hume A. N., Tarafder A. K., Barkagianni E., Seabra M. C. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 2002;277:25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda T. S., Fukuda M., Ariga H., Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1–4 and Slac2 functions as a novel Rab27A binding domain. J. Biol. Chem. 2002;277:9212–9218. doi: 10.1074/jbc.M112414200. [DOI] [PubMed] [Google Scholar]
- 22.Markert M., Andrews P. C., Babior B. M. Measurement of O2− production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 1984;105:358–365. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- 23.Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J. Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai A., Yoshie S., Nashida T., Shimomura H., Fukuda M. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J. Cell Sci. 2004;117:1945–1953. doi: 10.1242/jcs.01048. [DOI] [PubMed] [Google Scholar]
- 25.Catz S. D., Johnson J. L., Babior B. M. Characterization of the nucleotide-binding capacity and the ATPase activity of the PIP3-binding protein JFC1/Slp1. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11230–11235. doi: 10.1073/pnas.191369598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du X., Tabeta K., Hoebe K., Liu H., Mann N., Mudd S., Crozat K., Sovath S., Gong X., Beutler B. Velvet, a dominant Egfr mutation that causes wavy hair and defective eyelid development in mice. Genetics. 2004;166:331–340. doi: 10.1534/genetics.166.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J. Biochem. (Tokyo) 2005;137:9–16. doi: 10.1093/jb/mvi002. [DOI] [PubMed] [Google Scholar]
- 28.Wu X., Wang F., Rao K., Sellers J. R., Hammer J. A., III Rab27a is an essential component of melanosome receptor for myosin Va. Mol. Biol. Cell. 2002;13:1735–1749. doi: 10.1091/mbc.01-12-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stinchcombe J. C., Barral D. C., Mules E. H., Booth S., Hume A. N., Machesky L. M., Seabra M. C., Griffiths G. M. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak E. K., Gautam R., Reddington M., Collinson L. M., Copeland N. G., Jenkins N. A., McGarry M. P., Swank R. T. The regulation of platelet-dense granules by Rab27a in the ashen mouse, a model of Hermansky–Pudlak and Griscelli syndromes, is granule-specific and dependent on genetic background. Blood. 2002;100:128–135. doi: 10.1182/blood.v100.1.128. [DOI] [PubMed] [Google Scholar]
- 31.Goishi K., Mizuno K., Nakanishi H., Sasaki T. Involvement of Rab27 in antigen-induced histamine release from rat basophilic leukemia 2H3 cells. Biochem. Biophys. Res. Commun. 2004;324:294–301. doi: 10.1016/j.bbrc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 32.Johnson J. L., Pacquelet S., Lane W. S., Eam B., Catz S. D. Akt regulates the subcellular localization of the Rab27a-binding protein JFC1/Slp1 by phosphorylation. Traffic. 2005;6:667–681. doi: 10.1111/j.1600-0854.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 33.Kjeldsen L., Sengelov H., Lollike K., Nielsen M. H., Borregaard N. Isolation and characterization of gelatinase granules from human neutrophils. Blood. 1994;83:1640–1649. [PubMed] [Google Scholar]
- 34.Egesten A., Breton-Gorius J., Guichard J., Gullberg U., Olsson I. The heterogeneity of azurophil granules in neutrophil promyelocytes: immunogold localization of myeloperoxidase, cathepsin G, elastase, proteinase 3, and bactericidal/permeability increasing protein. Blood. 1994;83:2985–2994. [PubMed] [Google Scholar]
- 35.Mollinedo F., Martin-Martin B., Calafat J., Nabokina S. M., Lazo P. A. Role of vesicle-associated membrane protein-2, through Q-soluble N-ethylmaleimide-sensitive factor attachment protein receptor/R-soluble N-ethylmaleimide-sensitive factor attachment protein receptor interaction, in the exocytosis of specific and tertiary granules of human neutrophils. J. Immunol. 2003;170:1034–1042. doi: 10.4049/jimmunol.170.2.1034. [DOI] [PubMed] [Google Scholar]
- 36.Wang C. C., Ng C. P., Lu L., Atlashkin V., Zhang W., Seet L. F., Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev. Cell. 2004;7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Sengelov H., Kjeldsen L., Borregaard N. Control of exocytosis in early neutrophil activation. J. Immunol. 1993;150:1535–1543. [PubMed] [Google Scholar]
- 38.Palmer M., Harris R., Freytag C., Kehoe M., Tranum-Jensen J., Bhakdi S. Assembly mechanism of the oligomeric streptolysin O pore: the early membrane lesion is lined by a free edge of the lipid membrane and is extended gradually during oligomerization. EMBO J. 1998;17:1598–1605. doi: 10.1093/emboj/17.6.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Benna J., Park J. W., Ruedi J. M., Babior B. M. Cell-free activation of the respiratory burst oxidase by protein kinase C. Blood Cells Mol. Dis. 1995;21:201–206. doi: 10.1006/bcmd.1995.0023. [DOI] [PubMed] [Google Scholar]
- 40.Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl. Acad. Sci. U.S.A. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlsson A., Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid. Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 42.Wu X. S., Rao K., Zhang H., Wang F., Sellers J. R., Matesic L. E., Copeland N. G., Jenkins N. A., Hammer J. A., III Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 43.Reeves E. P., Lu H., Jacobs H. L., Messina C. G., Bolsover S., Gabella G., Potma E. O., Warley A., Roes J., Segal A. W. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 44.Palfrey H. C., Artalejo C. R. Secretion: kiss and run caught on film. Curr. Biol. 2003;13:R397–R399. doi: 10.1016/s0960-9822(03)00320-8. [DOI] [PubMed] [Google Scholar]
- 45.Neeft M., Wieffer M., de Jong A. S., Negroiu G., Metz C. H., van L. A., Griffith J., Krijgsveld J., Wulffraat N., Koch H., et al. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol. Biol. Cell. 2005;16:731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lominadze G., Powell D. W., Luerman G. C., Link A. J., Ward R. A., McLeish K. R. Proteomic analysis of human neutrophil granules. Mol. Cell. Proteomics. 2005;4:1503–1521. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C., Yang J., Jacobs J. D., Jennings L. K. Interaction of myeloperoxidase with vascular NAD(P)H oxidase-derived reactive oxygen species in vasculature: implications for vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2563–H2572. doi: 10.1152/ajpheart.00435.2003. [DOI] [PubMed] [Google Scholar]
- 48.Zhang R., Brennan M. L., Fu X., Aviles R. J., Pearce G. L., Penn M. S., Topol E. J., Sprecher D. L., Hazen S. L. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA, J. Am. Med. Assoc. 2001;286:2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 49.Brandford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]