Abstract
Syphilis serodiagnosis relies on a combination of nonspecific screening tests (antilipoidal antibodies) and Treponema pallidum-specific tests (anti-T. pallidum antibodies). We studied a group of six recombinant T. pallidum antigens for their sensitivities and specificities with sera from individuals with syphilis (n = 43), relapsing fever (n = 8), Lyme disease (n = 8), and leptospirosis (n = 9) and from uninfected individuals (n = 15). Three recombinant proteins, Tp0155, Tp0483, and Tp0751, demonstrated sensitivity values that ranged from 28 to 42%. In contrast, three other recombinant proteins exhibited the following sensitivity and specificity values: Tp0453, 100% sensitivity and 100% specificity; Tp92 (Tp0326), 98% sensitivity and 97% specificity; and Gpd (Tp0257), 91% sensitivity and 93% specificity. Tp0453, Tp92, and Gpd also were recognized by sera from individuals with early primary syphilis that were nonreactive with the antilipoidal Venereal Disease Research Laboratory test. The reactivities of syphilis patient sera with Tp0453, Tp92, and Gpd were proportional to the titers of these sera with the treponemal test MHA-TP (microhemagglutination assay for T. pallidum). Thus, the recombinant T. pallidum antigens Tp0453, Tp92, and Gpd show promise as diagnostic antigens in the enzyme-linked immunosorbent assay-based assay.
Syphilis is a sexually transmitted disease caused by infection with the spirochete bacterium Treponema pallidum subsp. pallidum. Primary syphilis is characterized by a chancre, typically containing large numbers of spirochetes. Secondary syphilis presents as a generalized rash in which each characteristic lesion contains a high bacterial burden. Upon resolution of the secondary stage of syphilis, the disease enters a latent, asymptomatic phase. This stage of syphilis can typically be divided into an early latent phase (estimated onset of infection within the last 2 years) and a late latent phase (estimated duration of infection of >2 years). In the preantibiotic era, approximately 30% of those infected progressed to tertiary syphilis, where manifestations included neuropsychiatric illness, syphilitic gummas, and bone and cardiovascular involvement.
Syphilis diagnosis during the early primary stage can be accomplished by dark-field microscopy of primary chancre samples for the presence of spirochetes. Following the resolution of the primary chancre and in clinics lacking dark-field microscopy, the mainstay of syphilis diagnosis is a variety of serologic tests. The most common screening tests are the rapid plasma reagin and Venereal Disease Research Laboratory (VDRL) tests, both of which test for the presence of antilipoidal antibodies. Because neither of these tests assays for syphilis-specific antibodies, there are problems associated with both their specificity and their sensitivity. In early primary disease antilipoidal antibodies may not have developed, and in late syphilis (late latent and tertiary) up to 30% of individuals may lack antilipoidal antibodies (27). In addition, because a variety of conditions (e.g., lupus and increased age) lead to antilipoidal antibodies and false-positive results, a confirmatory test is often required. Confirmatory tests include FTA-Abs (fluorescent treponemal antibody absorption test), MHA-TP (microhemagglutination assay for T. pallidum), and TPHA (T. pallidum hemagglutination assay), which use crude T. pallidum antigens (12); tests using whole T. pallidum antigen extracts; and a variety of T. pallidum recombinant protein tests (6-9, 12, 16, 18-23, 27-30, 32).
There are several reasons to evaluate additional recombinant antigens for use in serologic testing for syphilis. First, recombinant protein tests are not widely used and there is no general agreement as to which protein antigens are best for sensitivity and specificity. Second, the whole genome of T. pallidum strain Nichols has been elucidated and new protein-encoding open reading frames (ORFs) are available for testing (5). Finally, surface-exposed proteins may be superior in sensitivity to the proteins currently used because of their immediate exposure to the immune system.
The purpose of this study was to screen recombinant proteins for sensitivity by using sera collected from individuals with syphilis. Recombinant proteins selected for screening included Gpd (Tp0257) (3, 26) and Tp92 (Tp0326) (4), which were previously identified from a serologic screen as being reactive with syphilis patient sera, and proteins predicted by computer analyses of the genome to be putative outer membrane proteins. Since other spirochetal diseases such as Lyme disease, relapsing fever, and leptospirosis would be expected to have antigens most similar to T. pallidum, we used sera collected from individuals with these infections to test the specificity of the recombinant antigens for syphilis serodiagnosis.
MATERIALS AND METHODS
Reagents and serum samples.
Human syphilis patient sera were obtained from the collection of the Ludwig Boltzmann Institute for Dermato-Venerological Serodiagnostics, Vienna, Austria. The sera were tested for syphilis serology titers by the VDRL (Dade Behring, Marburg, Germany) and the MHA-TP (Fujirebio, Tokyo, Japan) tests (22). The criteria used to establish the stage of syphilis were as follows: primary, typical chancre present; secondary, generalized typical rash and positive syphilis serology test; early latent, positive syphilis serology tests and a positive immunoglobulin M test to T. pallidum antigens (Mercia Syphilis M enzyme-linked immunosorbent assay [ELISA]; Microgen Bioproducts, Camberley, United Kingdom) with no history of treatment and no clinical manifestations; and neurosyphilis, reactive cerebrospinal fluid (CSF) values plus TPHA index values greater than 70 (13). The TPHA index value was equal to the CSF MHA-TP divided by the albumin quotient (CSF albumin divided by serum albumin) and also divided by 1,000. Syphilis stage diagnoses were confirmed by dermatologists and/or neurologists. None of the subjects had been treated for syphilis at the time when the serum was obtained.
Sera from individuals with relapsing fever were obtained from the collection of Rocky Mountain Laboratories, Hamilton, Mont., and from the Centers for Disease Control and Prevention, Fort Collins, Colo. The criteria for relapsing fever diagnosis were compatible clinical history, exposure to tick-borne relapsing fever in eastern Washington or northern Idaho, and high seropositivity (greater than 1:2,048) in the Western blot for Borrelia hermsii HS1. Lyme disease patient sera were obtained from the Centers for Disease Control and Prevention collection (Fort Collins, Colo.). The criteria for the diagnosis of Lyme disease were residence in an area of endemicity, clinical manifestations consistent with Lyme disease, and more than five reactive bands by Western blot analysis of Borrelia burgdorferi. Sera from individuals with severe leptospirosis were obtained from the collection of the Oswaldo Cruz Foundation (Salvador, Bahia, Brazil) from individuals identified in Salvador in the convalescent phase of leptospirosis with clinical histories consistent with leptospirosis. These individuals had laboratory-confirmed diagnoses according to microagglutination test criteria of a fourfold rise in agglutination titers or a reciprocal agglutination titer greater than 1:800. Convalescent-phase sera were obtained 14 to 28 days after hospitalization of these individuals for leptospirosis. In the city of Salvador, the etiologic agent is Leptospira interrogans serovar Copenhageni (11). Sera from uninfected controls were obtained from laboratory personnel in Seattle, Wash.
This study was approved by the Human Subjects Institutional Review Board of the University of Washington, as well as the Human Subjects Institutional Review Boards at the Ludwig Boltzmann Institute for Dermato-Venerological Serodiagnostics; the Centers for Disease Control and Prevention, Fort Collins, Colo.; the Oswaldo Cruz Foundation; and the National Institutes of Health, Rocky Mountain Laboratories, Hamilton, Mont.
Computer analyses.
Computer analyses were performed on the published T. pallidum genome (5) to identify ORFs predicted to encode putative outer membrane proteins. Such proteins have the potential to reside on the bacterial surface and thus are the most likely to possess cellular functions such as host cell attachment. Sequence analysis tools used included the TMpred program (http://www.ch.embnet.org/software/TMPRED_form.html) for transmembrane topology analysis, the PSORT program (http://www.psort.nibb.ac.jp) (15) for signal sequence and cellular location predictions, and the Prosite database (http://www2.ebi.ac.uk/ppsearch) for identification of characteristic protein motifs.
Preparation of recombinant proteins.
ORFs encoding computer-predicted putative outer membrane proteins were PCR amplified from T. pallidum subsp. pallidum (Nichols strain) genomic DNA with primers designed from the coding sequence of each gene (Table 1). Proteins were expressed without the N-terminal signal sequence, with the exception of Gpd (Tp0257), where the signal sequence was retained (Table 2). Where possible, the full-length ORFs were expressed. However, Tp0155 and Tp0751 were toxic to Escherichia coli when expressed as full-length proteins, and as a result N-terminal fragments of these proteins were instead expressed (Table 2). PCR products representing the portion of the ORF encoding the amino acids listed in Table 2 were ligated in frame with expression plasmids. All but one were expressed using the pRSET T7 expression plasmid in the E. coli expression strain BL21(DE3)/pLysS (Invitrogen, Carlsbad, Calif.). Tp0326 (Tp92) was expressed in the pBAD TOPO TA expression plasmid in the E. coli strain TOP10 (Invitrogen).
TABLE 1.
ORF-specific primers used to amplify fragments for recombinant expressiona
| ORF | Sense primer | Antisense primer | Amplicon detail
|
|
|---|---|---|---|---|
| Size (bp) | Portion of ORF | |||
| Tp0155 | 5′-GGATCCAACCATTGACACCTGCC | 5′-GAATTCTGCAGCTGAATTATAGAAC | 428 | 5′ end |
| Tp0257 (Gpd) | 5′-GGATCCTGCGGGGAACATATTGTGTG | 5′-GAATTCATACGGGGCGGGTTTGCC | 1,067 | Full |
| Tp0326 (Tp92) | 5′-CAGGCAAACGACAATTGG | 5′-CAAATTATTTACCGTGAACG | 2,448 | Full |
| Tp0453 | 5′-GGATCCCGTGGAAGGCATCAGTAG | 5′-GAATTCCGAACTTCCCTTTTTGGAG | 758 | Full |
| Tp0483 | 5′-GGATCCAGGAACTCGTCCACGTATC | 5′-GAATTCGTTATGAAAGCGATAGCCG | 1,058 | Full |
| Tp0751 | 5′-GGATCCGGGACACCGCCGCACAC | 5′-GAATTCCTTGCGGTGTGTGTGCGC | 360 | 5′ end |
Restriction sites incorporated into the primers are indicated by underlining.
TABLE 2.
Expressed recombinant T. pallidum proteins
| Recombinant protein | PSORT outer membrane predictions (% probability that predicted protein resides in outer membrane) | Amino acids expressed | Fragment of protein, with or without signal sequence (SS) |
|---|---|---|---|
| Tp0155 | 86 | 46-187 | N terminus, no SS |
| Gpd (Tp0257) | Lipoprotein | 1-356 | Full-length, with SS |
| Tp92 (Tp0326) | 85 | 22-837 | Full-length, no SS |
| Tp0453 | 78 | 30-287 | Full-length, no SS |
| Tp0483 | 69 | 22-374 | Full-length, no SS |
| Tp0751 | 92 | 54-173 | N terminus, no SS |
Expression and purification of the resulting N-terminal six-histidine-tagged recombinant proteins were performed as previously described (4). The expressed proteins were renatured by dialysis based upon the renaturation protocol described by Qi et al. (17), with the dialysis modification described by Zhang et al. (31). This procedure has been shown to produce recombinant proteins that most closely resemble native T. pallidum proteins (31). Briefly, 0.5% Zwittergent 3-12 (Calbiochem, San Diego, Calif.) was added to the expressed proteins prior to dialysis against 100 mM Tris (pH 8.0)-200 mM NaCl-10 mM EDTA. Protein quantitation of each of the recombinant proteins was performed with the bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.).
ELISAs.
Ninety-six-well plates (Maxisorp F9; Costar) were coated overnight at 4°C with 50 μl of the recombinant T. pallidum proteins per well in phosphate-buffered saline (PBS), pH 7.4, with 0.1% sodium dodecyl sulfate at concentrations of 2 μg/ml for Gpd, Tp92, and Tp0453 and 4 μg/ml for Tp0155, Tp0483, and Tp0751. Plates were blocked at room temperature for 2 h with 1× PBS-4% milk. Human sera were diluted 1:200 (Gpd assays) or 1:100 (all other assays) in dilution buffer (1× PBS-4% milk-0.2% Triton X-100). In preliminary studies using serial dilutions (data not shown), a dilution of 1:100 was found to provide optimal sensitivity and specificity for all antigens except Gpd, which was optimal at a 1:200 dilution. The diluted sera were adsorbed overnight at 4°C with a 0.5% (vol/vol) lysate of E. coli expressing an irrelevant Trypanosoma cruzi recombinant protein (SA85-1.1) in pRSET (10). This adsorption step was omitted from the sera tested for reactivity to Gpd since preliminary experiments with Gpd showed that this step had no effect on background reactivity. Samples were spun at 4°C at 12,000 × g for 10 min, and 50 μl of each serum was added to triplicate wells and incubated for 1 h at room temperature. After washing, 50 μl of a 1:3,000 dilution of goat anti-human (gamma specific) F(ab′)2 peroxidase (Sigma-Aldrich, St. Louis, Mo.) was applied and incubated at room temperature for 1 h. Plates were developed for 30 min at room temperature with 100 μl of tetramethylbenzidine-H2O2 substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) per well, and the absorbance at 600 nm was measured.
Statistics.
The cutoff absorbance values for assigning negative or positive results for each recombinant antigen were defined as the mean plus two times the standard deviation of the absorbance of the uninfected sera. The cutoff values were calculated from the values obtained from the 15 uninfected control sera for Gpd, Tp92, and Tp0453 or from the values from four separate ELISAs of 15 pooled uninfected control sera for Tp0155, Tp0483, and Tp0751. The cutoff values for the recombinant protein ELISAs were as follows: 0.0723 for Tp0155, 0.0667 for Gpd (Tp0257), 0.0576 for Tp92 (Tp0326), 0.0476 for Tp0453, 0.0517 for Tp0483, and 0.0431 for Tp0751. The negative sera were defined as those that yielded absorbance values less than or equal to the cutoff, while the positive sera were defined as those that gave absorbance values greater than this value. Differences between groups were measured by chi-square analysis, and significance was set as P < 0.05.
RESULTS
T. pallidum recombinant proteins.
Six recombinant T. pallidum proteins were tested for their reactivities with sera from individuals with syphilis. Gpd and Tp92 had previously been reported to be strongly immunoreactive with T. pallidum-infected rabbit sera. The ORFs Tp0453, Tp0155, Tp0483, and Tp0751 were predicted from the T. pallidum subsp. pallidum Nichols genome as having a greater than 69% likelihood (Table 2) of encoding outer membrane proteins. These ORFs were selected since outer membrane proteins may be recognized by antibodies earlier in infection and may stimulate persistent immune responses late in infection.
Sensitivities of the T. pallidum recombinant proteins in detecting sera from individuals with syphilis.
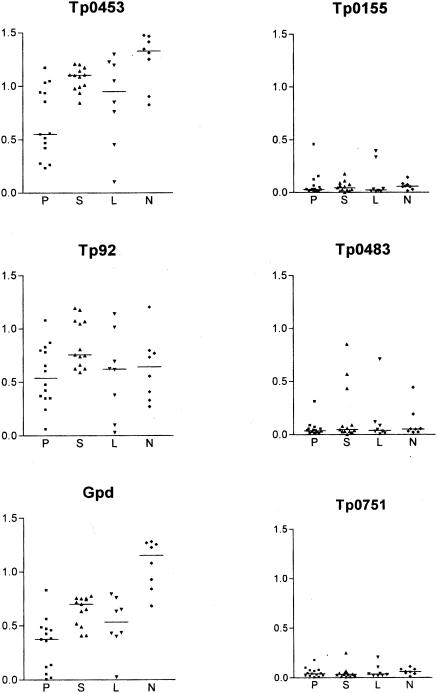
The recombinant proteins varied greatly in their recognition by antibodies in sera from individuals with syphilis as judged by ELISA (Fig. 1). Tp92, Tp0453, and Gpd were recognized by almost all of the sera from individuals with syphilis, but many of the syphilis patient sera failed to recognize Tp0155, Tp0483, and Tp0751. The same general pattern of reactivity was seen with sera from individuals with primary, secondary, and early latent forms of syphilis (Fig. 1). Tp0453 was recognized by all of the syphilis patient sera (n = 43). Reactivity to Tp92 and Gpd was nearly as complete, with only 1 and 4 of 43 sera, respectively, failing to show reactivity above background. By chi-square analysis, the difference in sensitivity between Tp0453 and Tp92 was not significant but the difference in sensitivity between Tp0453 and Gpd was significant (P < 0.05). Significantly fewer syphilis patient sera reacted with Tp0155 (12 of 43), Tp0483 (18 of 43), and Tp0751 (18 of 43). The absorbance values of sera reactive against Tp0155, Tp0483, and Tp0751 were significantly lower than the values obtained with Tp92, Gpd, or Tp0453 (Fig. 1). These results suggest that the T. pallidum proteins Tp92, Tp0453, and Gpd elicit higher antibody levels in syphilis infection and further suggest that Tp0453 and Tp92 are the most sensitive antigens tested in this study.
FIG. 1.
Reactivities of sera from patients at different stages of syphilis to the recombinant T. pallidum proteins. The y axis shows the mean ELISA absorbance values at 600 nm for each serum sample from individuals with syphilis tested with each recombinant protein. The sera on the x axis are grouped by stage of syphilis: P, primary (n = 14); S, secondary (n = 13); L, latent (n = 8); and N, neurosyphilis (n = 8). The overall mean absorbance of each group is represented by a horizontal line.
Specificity of immunoreactivity to the T. pallidum recombinant proteins.
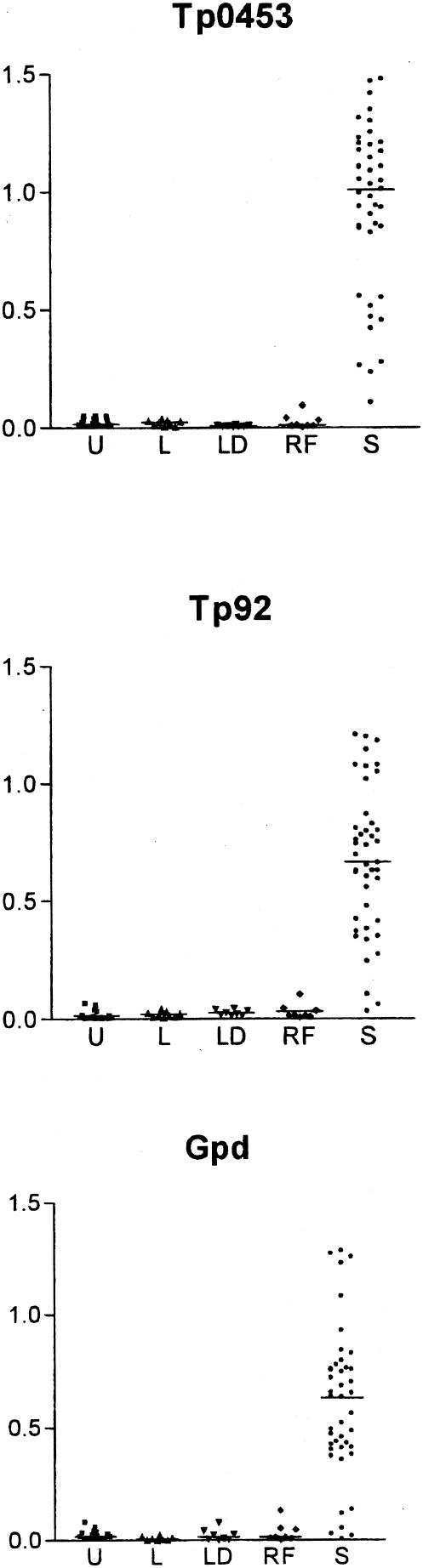
Sera collected from uninfected individuals (n = 15) and individuals with other spirochetal diseases, specifically leptospirosis (n = 9), relapsing fever (n = 8), and Lyme disease (n = 8), were used to determine the specificity of immunoreactivity to Tp92, Tp0453, and Gpd. As shown in Fig. 2, none of the uninfected controls had a significant reactivity to Tp92 or Tp0453, and of the individuals with spirochetal diseases, only one relapsing fever patient serum sample had a marginally positive response to Tp92 (optical density = 0.104, mean plus two times the standard deviation of uninfected controls = 0.0576). More false-positive reactions were demonstrated with Gpd, with one serum from each of the uninfected individual and Lyme disease and relapsing fever patient samples showing absorbance values which were above the cutoff for negativity. Thus, the sensitivities and specificities of these recombinant protein antigens were as follows: Tp0453, 100% sensitivity and 100% specificity; Tp92, 98% sensitivity and 97% specificity; and Gpd, 91% sensitivity and 93% specificity.
FIG. 2.
Reactivities of syphilis patient sera and control sera from uninfected individuals and individuals with other spirochetal diseases to the recombinant T. pallidum proteins. The y axis shows the mean ELISA absorbance values at 600 nm for reactivities of the sera to the recombinant Tp0453, Tp92, and Gpd proteins. On the x axis the sera are grouped as follows: U, uninfected laboratory personnel sera (n = 15); L, leptospirosis patient sera (n = 9); LD, Lyme disease patient sera (n = 8); RF, relapsing fever patient sera (n = 8); and S, syphilis patient sera (n = 43). The overall mean absorbance of each group is represented by a horizontal line.
Comparison of immunoreactivity with the T. pallidum recombinant proteins with that for present syphilis diagnostic tests.
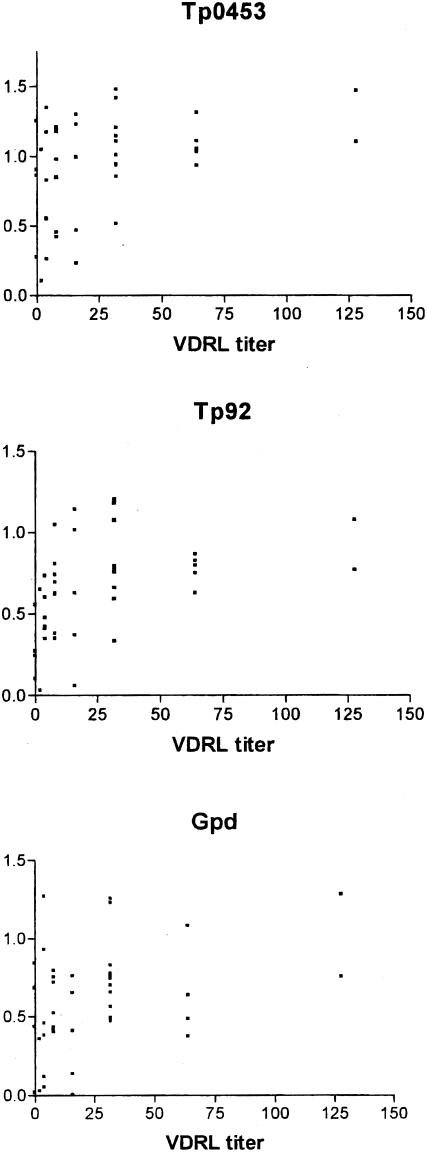
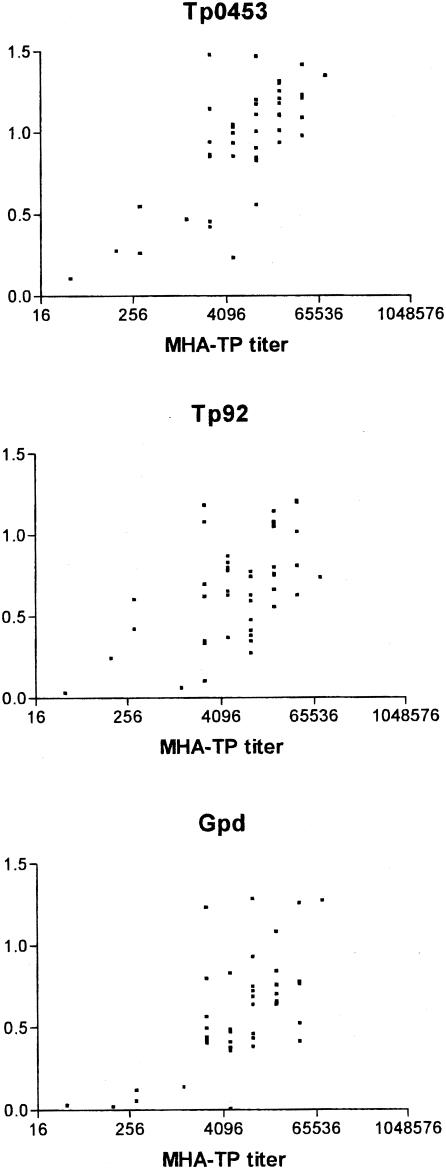
The VDRL lipoidal antigen test is often used to screen sera for syphilis seroreactivity. The ELISA-based assay to detect reactivity to Tp0453 was more sensitive than the VDRL test in detecting syphilis seropositivity (Fig. 3). Four serum samples that had a negative VDRL result demonstrated reactivity against recombinant Tp0453 (Fig. 3); these sera were from individuals with early primary syphilis. In general, the absorbance values from the Tp0453, Tp92, and Gpd ELISAs did not correlate well with the VDRL titer (Fig. 3), reflecting the nontreponemal nature of the VDRL assay. In contrast, the immunoreactivity observed with Tp0453, Tp92, and Gpd ELISAs correlated well with the MHA-TP titer (Fig. 4), which is based on crude T. pallidum antigen preparations and represents a T. pallidum-specific serodiagnostic test.
FIG. 3.
Relationship of the reactivities of the syphilis patient sera to the VDRL test and to recombinant T. pallidum proteins. The mean absorbance values of the syphilis patient sera at 600 nm for reactivities of the recombinant Tp0453, Tp92, and Gpd proteins are shown on the y axis. The x axis displays the value for the VDRL test, shown as the reciprocal of the highest dilution tested that gave reactivity in the test. The four values at 0 along the x axis were nonreactive or of equivocal reactivity in the VDRL test with undiluted sera of individuals with early primary syphilis.
FIG. 4.
Relationship of the reactivities of the syphilis patient sera to the MHA-TP test and to recombinant T. pallidum proteins. The mean absorbance values at 600 nm for reactivities of the recombinant Tp0453, Tp92, and Gpd proteins are shown on the y axis. The x axis displays the value for the MHA-TP test (microhemagglutination assay with T. pallidum crude antigen), shown as the reciprocal of the highest dilution tested that gave reactivity in the test on a log2 scale.
DISCUSSION
Recombinant antigens have shown promise for syphilis serodiagnosis. A variety of T. pallidum proteins have been tested including TpN44.5 (TmpA, Tp0768), TpN15 (Tp0171), TpN17 (Tp0435), and TpN47 (Tp0574) (20, 29, 30, 32). These antigens are sometimes used in combination in commercial tests. These tests have often been shown to identify individuals with active syphilis as well as those who have been treated successfully. From screening many hundreds of serum samples, it has been determined that the sensitivity and specificity of tests employing some of these antigens can be as high as 99.7% (7, 20, 29, 30, 32). However, not all those with early syphilis are detected with the use of these antigens (27), and more sensitive and specific recombinant T. pallidum proteins would be useful for syphilis seroscreening.
In this report, we have tested a variety of additional recombinant proteins for their potential suitability as antigens for the serodiagnosis of syphilis. We demonstrate that the putative outer membrane protein Tp0453 has excellent sensitivity for sera from individuals with early syphilis. In addition, the lack of reactivity of this protein with sera from 15 uninfected individuals and 25 individuals with other spirochetal infections, including leptospirosis, Lyme disease, and relapsing fever, demonstrates its specificity.
With the group of sera tested, Tp0453 appeared to have an advantage in sensitivity and specificity compared with Tp92 and Gpd. More extensive testing with larger numbers of sera collected from uninfected individuals and individuals with syphilis will need to be performed to demonstrate if Tp0453 is superior to Tp92, as the slight differences in observed sensitivity and specificity did not reach statistical significance. To define specificity, some reports have screened hundreds or thousands of blood donors for reactivity with commercially available recombinant T. pallidum antigen tests (7, 20, 30, 32). These screens have identified donor sera that were reactive with T. pallidum recombinant antigens and were also positive with other syphilis-specific serologic tests. These sera were probably from individuals who were infected with T. pallidum, but since clinical histories are not available interpretation of these studies is difficult. Nonetheless, before the Tp0453 and Tp92 antigens can be definitively demonstrated to be helpful in serodiagnosis, larger numbers of sera will need to be tested by a high-throughput commercial serological assay.
Using recombinant T. pallidum antigens to test for syphilis seroreactivity has advantages over lipoidal antigen-based and crude T. pallidum antigen tests. Lipoidal antigen-based screening misses up to 30% of sera from individuals with very early and late syphilis (27). In the group of sera that we tested, there were four individuals with early primary syphilis who had no reactivity in the VDRL lipoidal antigen-based screening test, yet had good reactivity with Tp0453 and Tp92 antigens (Fig. 3). These individuals were also positive with the crude T. pallidum antigen test MHA-TP. Overall, the MHA-TP results correlated well with the Tp0453 and Tp92 results. However, there is a significant advantage in preparation of recombinant antigens over preparation of crude T. pallidum antigen. Recombinant T. pallidum antigens can be produced economically and in large quantities in in vitro E. coli culture, but crude T. pallidum antigens must be extracted from treponemes grown within the rabbit animal model. In addition, there is the potential for false-positive reactions with antigens present in the crude T. pallidum antigen extracts from rabbit tissues.
Computer analyses predict that Gpd is a lipoprotein (26), and [14C]palmitate labeling studies performed by Shevchenko et al. confirm that the protein is lipid modified (25). Similar to Gpd, many of the recombinant antigens used to date for syphilis serodiagnosis are lipoproteins, namely, TpN44.5, TpN15, TpN17, and TpN47. Lipoprotein antigens stimulate a strong immune response which is thought to be due to the ability of lipoproteins to activate antigen-presenting cells through Toll-like 2 receptors (1, 2). Though some of these lipoprotein antigens were originally believed to reside on the surface of the bacterium, these antigens are now thought to be concealed in the periplasm of intact T. pallidum (24). Presumably the antibody response to the internal lipoprotein antigens is first stimulated during the widespread phagocytosis and destruction of T. pallidum that occur during the clearance stages of early syphilis (14). Though the lipoprotein Gpd showed better sensitivity than did the nonlipoprotein antigens Tp0155, Tp0483, and Tp0751, 4 of 43 (9%) syphilis patient sera, each of which was from individuals with early primary infection, failed to react with Gpd. By contrast, 100% of syphilis patient sera reacted with Tp0453 and 98% reacted with Tp92. In the early stage of infection prior to destruction of spirochetes, antigens that are surface exposed would be visible to the immune system in intact organisms and therefore would have an advantage over internal antigens. By computer models, Tp0453 and Tp92 are predicted to reside in the outer membrane and thus may be surface exposed. This may explain the superior ability of Tp0453 and Tp92 compared to that of Gpd in the detection of antibodies in individuals with early syphilis. Further, after initial clearance in early syphilis, small numbers of presumably intact treponemes remain and may cause late manifestations of disease. Antigens that are surface exposed would have a theoretical advantage in that they are continuously exposed to the immune system and continually stimulate robust antibody responses. This hypothesis was not tested in this study, and future studies should include testing Tp0453 and Tp92 for reactivity with sera from individuals with late syphilis and low antibody responses to T. pallidum.
Tp92 and Gpd are proteins that have homologues in a number of gram-negative bacteria (3, 4, 26). This may explain the reactivity of sera from some individuals with nonsyphilis spirochetal infections to these molecules. Tp0453, which has no homologues in other bacteria, exhibited no cross-reactivity with serum samples from individuals with other spirochete diseases. Further studies need to be performed, but the superior sensitivity and specificity of Tp0453 show promise for seroscreening of syphilis.
Acknowledgments
We thank Tom Schwan, Rocky Mountain Laboratories, National Institutes of Health, Hamilton, Mont.; Albert Ko, Weill-Cornell Medical College, New York, N.Y.; and the Oswaldo Cruz Foundation, Salvador, Bahia, Brazil, for providing serum samples for this study. We also thank Susannah Weyte for assistance with recombinant protein production.
This research was supported by the U.S. Public Health Service NIH/NIAID grants AI43456, AI51334, AI34616, and AI42143.
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 3.Cameron, C. E., C. Castro, S. A. Lukehart, and W. C. Van Voorhis. 1998. Function and protective capacity of Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase. Infect. Immun. 66:5763-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, C. E., S. A. Lukehart, C. Castro, B. Molini, C. Godornes, and W. C. Van Voorhis. 2000. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J. Infect. Dis. 181:1401-1413. [DOI] [PubMed] [Google Scholar]
- 5.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, C. Fujii, S. Garland, B. Hatch, K. Horst, L. Watthey, J. Weidman, H. O. Smith, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 6.Gerber, A., S. Krell, and J. Morenz. 1996. Recombinant Treponema pallidum antigens in syphilis serology. Immunobiology 196:535-549. [DOI] [PubMed] [Google Scholar]
- 7.Hagedorn, H. J., A. Kraminer-Hagedorn, K. De Bosschere, F. Hulstaert, H. Pottel, and M. Zrein. 2002. Evaluation of INNO-LIA syphilis assay as a confirmatory test for syphilis. J. Clin. Microbiol. 40:973-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ijsselmuiden, O. E., G. Beelaert, L. M. Schouls, B. Tank, E. Stolz, and G. van der Groen. 1989. Line immunoassay and enzyme-linked line immunofiltration assay for simultaneous detection of antibody to two treponemal antigens. Eur. J. Clin. Microbiol. Infect. Dis. 8:716-721. [DOI] [PubMed] [Google Scholar]
- 9.Ijsselmuiden, O. E., L. M. Schouls, E. Stolz, G. N. Aelbers, C. M. Agterberg, J. Top, and J. D. Van Embden. 1989. Sensitivity and specificity of an enzyme-linked immunosorbent assay using the recombinant DNA-derived Treponema pallidum protein TmpA for serodiagnosis of syphilis and the potential use of TmpA for assessing the effect of antibiotic therapy. J. Clin. Microbiol. 27:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn, S. J., and M. Wleklinski. 1997. The surface glycoproteins of Trypanosoma cruzi encode a superfamily of variant T cell epitopes. J. Immunol. 159:4444-4451. [PubMed] [Google Scholar]
- 11.Ko, A. I., R. M. Galvao, C. M. Ribeiro Dourado, W. D. Johnson, Jr., L. W. Riley, et al. 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820-825. [DOI] [PubMed] [Google Scholar]
- 12.Larsen, S. A., E. A. Hambie, D. E. Pettit, M. W. Perryman, and S. J. Kraus. 1981. Specificity, sensitivity, and reproducibility among the fluorescent treponemal antibody-absorption test, the microhemagglutination assay for Treponema pallidum antibodies, and the hemagglutination treponemal test for syphilis. J. Clin. Microbiol. 14:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luger, A. F., B. L. Schmidt, and M. Kaulich. 2000. Significance of laboratory findings for the diagnosis of neurosyphilis. Int. J. STD AIDS 11:224-234. [DOI] [PubMed] [Google Scholar]
- 14.Lukehart, S. A., S. A. Baker-Zander, R. M. C. Lloyd, and S. Sell. 1992. Immunology and pathogenesis of syphilis, p. 141-163. In T. C. Quinn, J. I. Gallin, and A. S. Fauci (ed.), Sexually transmitted diseases. Raven Press, New York, N.Y.
- 15.Nakai, K., and M. Kanehisa. 1991. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins 11:95-110. [DOI] [PubMed] [Google Scholar]
- 16.Peterson, K. M., J. B. Baseman, and J. F. Alderete. 1986. Isolation of a Treponema pallidum gene encoding immunodominant outer envelope protein P6, which reacts with sera from patients at different stages of syphilis. J. Exp. Med. 164:1160-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi, H. L., J. Y. Tai, and M. S. Blake. 1994. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect. Immun. 62:2432-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radolf, J. D., E. B. Lernhardt, T. E. Fehniger, and M. A. Lovett. 1986. Serodiagnosis of syphilis by enzyme-linked immunosorbent assay with purified recombinant Treponema pallidum antigen 4D. J. Infect. Dis. 153:1023-1027. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez, I., E. L. Alvarez, C. Fernandez, and A. Miranda. 2002. Comparison of a recombinant-antigen enzyme immunoassay with Treponema pallidum hemagglutination test for serological confirmation of syphilis. Mem. Inst. Oswaldo Cruz 97:347-349. [DOI] [PubMed] [Google Scholar]
- 20.Sambri, V., A. Marangoni, M. A. Simone, A. D'Antuono, M. Negosanti, and R. Cevenini. 2001. Evaluation of recomWell Treponema, a novel recombinant antigen-based enzyme-linked immunosorbent assay for the diagnosis of syphilis. Clin. Microbiol. Infect. 7:200-205. [DOI] [PubMed] [Google Scholar]
- 21.Sato, N. S., M. H. Hirata, R. D. Hirata, L. C. Zerbini, E. P. Silveira, C. S. de Melo, and M. Ueda. 1999. Analysis of Treponema pallidum recombinant antigens for diagnosis of syphilis by western blotting technique. Rev. Inst. Med. Trop. Sao Paulo 41:115-118. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt, B. L., M. Edjlalipour, and A. Luger. 2000. Comparative evaluation of nine different enzyme-linked immunosorbent assays for determination of antibodies against Treponema pallidum in patients with primary syphilis. J. Clin. Microbiol. 38:1279-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schouls, L. M., O. E. Ijsselmuiden, J. Weel, and J. D. Van Embden. 1989. Overproduction and purification of Treponema pallidum recombinant-DNA-derived proteins TmpA and TmpB and their potential use in serodiagnosis of syphilis. Infect. Immun. 57:2612-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellati, T. J., D. A. Bouis, M. J. Caimano, J. A. Feulner, C. Ayers, E. Lien, and J. D. Radolf. 1999. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 163:2049-2056. [PubMed] [Google Scholar]
- 25.Shevchenko, D. V., T. J. Sellati, D. L. Cox, O. V. Shevchenko, E. J. Robinson, and J. D. Radolf. 1999. Membrane topology and cellular location of the Treponema pallidum glycerophosphodiester phosphodiesterase (GlpQ) ortholog. Infect. Immun. 67:2266-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stebeck, C. E., J. M. Shaffer, T. W. Arroll, S. A. Lukehart, and W. C. Van Voorhis. 1997. Identification of the Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase homologue. FEMS Microbiol. Lett. 154:303-310. [DOI] [PubMed] [Google Scholar]
- 27.Young, H. 1998. Syphilis. Serology. Dermatol. Clin. 16:691-698. [DOI] [PubMed] [Google Scholar]
- 28.Young, H., G. Aktas, and A. Moyes. 2000. Enzywell recombinant enzyme immunoassay for the serological diagnosis of syphilis. Int. J. STD AIDS 11:288-291. [DOI] [PubMed] [Google Scholar]
- 29.Young, H., A. Moyes, I. de Ste. Croix, and A. McMillan. 1998. A new recombinant antigen latex agglutination test (Syphilis Fast) for the rapid serological diagnosis of syphilis. Int. J. STD AIDS 9:196-200. [DOI] [PubMed] [Google Scholar]
- 30.Young, H., A. Moyes, L. Seagar, and A. McMillan. 1998. Novel recombinant-antigen enzyme immunoassay for serological diagnosis of syphilis. J. Clin. Microbiol. 36:913-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, H. H., D. R. Blanco, M. M. Exner, E. S. Shang, C. I. Champion, M. L. Phillips, J. N. Miller, and M. A. Lovett. 1999. Renaturation of recombinant Treponema pallidum rare outer membrane protein 1 into a trimeric, hydrophobic, and porin-active conformation. J. Bacteriol. 181:7168-7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zrein, M., I. Maure, F. Boursier, and L. Soufflet. 1995. Recombinant antigen-based enzyme immunoassay for screening of Treponema pallidum antibodies in blood bank routine. J. Clin. Microbiol. 33:525-527. [DOI] [PMC free article] [PubMed] [Google Scholar]