Abstract
Interactions between E-cadherin, β-catenin and PTP1B (protein tyrosine phosphatase 1B) are crucial for the organization of AJs (adherens junctions) and epithelial cell–cell adhesion. In the present study, the effect of acetaldehyde on the AJs and on the interactions between E-cadherin, β-catenin and PTP1B was determined in Caco-2 cell monolayers. Treatment of cell monolayers with acetaldehyde induced redistribution of E-cadherin and β-catenin from the intercellular junctions by a tyrosine phosphorylation-dependent mechanism. The PTPase activity associated with E-cadherin and β-catenin was significantly reduced and the interaction of PTP1B with E-cadherin and β-catenin was attenuated by acetaldehyde. Acetaldehyde treatment resulted in phosphorylation of β-catenin on tyrosine residues, and abolished the interaction of β-catenin with E-cadherin by a tyrosine kinase-dependent mechanism. Protein binding studies showed that the treatment of cells with acetaldehyde reduced the binding of β-catenin to the C-terminal region of E-cadherin. Pairwise binding studies using purified proteins indicated that the direct interaction between E-cadherin and β-catenin was reduced by tyrosine phosphorylation of β-catenin, but was unaffected by tyrosine phosphorylation of E-cadherin-C. Treatment of cells with acetaldehyde also reduced the binding of E-cadherin to GST (glutathione S-transferase)–PTP1B. The pairwise binding study showed that GST–E-cadherin-C binds to recombinant PTP1B, but this binding was significantly reduced by tyrosine phosphorylation of E-cadherin. Acetaldehyde increased the phosphorylation of β-catenin on Tyr-331, Tyr-333, Tyr-654 and Tyr-670. These results show that acetaldehyde induces disruption of interactions between E-cadherin, β-catenin and PTP1B by a phosphorylation-dependent mechanism.
Keywords: acetaldehyde, adherens junction, intestinal epithelium, protein tyrosine phosphatase 1B (PTP1B), tyrosine kinase
Abbreviations: AJ, adherens junction; GST, glutathione S-transferase; LC/MS/MS, liquid chromatography tandem MS; PTP1B and PTP1D, protein tyrosine phosphatases 1B and 1D; p-Tyr, phosphotyrosine; TER, transepithelial electrical resistance; TFA, trifluoroacetic acid; TJ, tight junction; ZO-1, zonula occludens 1 protein
INTRODUCTION
Acetaldehyde, a highly mutagenic and carcinogenic molecule, produced in the intestinal lumen by oxidation of ethanol [1–3], is suggested to play a role in the alcohol-induced disruption of epithelial junctions and an increased risk for gastrointestinal cancers [4]. A significant body of evidence indicates that acetaldehyde is accumulated in the colonic lumen due to enteric microbial fermentation [1,5]. Intracolonic acetaldehyde level is dramatically increased by alcohol consumption [3,5–8]. While 0.37 mM acetaldehyde has been detected in the saliva of alcoholics [2], greater than 1 mM concentration of acetaldehyde was recorded in the colonic lumen of rats after alcohol administration [7]. Many dietary and pharmacological agents may further increase the levels of intracolonic acetaldehyde [6]. Therefore colonic mucosa of alcoholics is exposed to a high concentration of acetaldehyde. Our recent studies indicated that acetaldehyde (0.1–0.6 mM) disrupts TJs (tight junctions) and increases paracellular permeability to endotoxins in an intestinal epithelial monolayer by a tyrosine kinase-dependent mechanism [8–12]. Acetaldehyde also induced redistribution of E-cadherin and β-catenin from the intercellular junctions into the intracellular compartments [11,12].
Epithelial AJ (adherens junction) is organized by the interaction between E-cadherin and β-catenin, interaction between β-catenin and α-catenin, and binding of α-catenin to the actin cytoskeleton [13]. The protein–protein interactions in AJ are regulated by intracellular signal transduction that involves protein kinases and protein phosphatases. Interaction of β-catenin with the intracellular domain of N-cadherin [14] and E-cadherin [15,16] is regulated by tyrosine phosphorylation of β-catenin. Phosphorylation of β-catenin on tyrosine residues results in the loss of cadherin-based cell–cell adhesion in neural cells [14,17–19], and dephosphorylation of β-catenin promotes assembly of E-cadherin, β-catenin and α-catenin into cell–cell adhesion complexes in human keratinocytes [20].
Tyrosine phosphorylation of β-catenin and the interaction between β-catenin and N-cadherin is regulated by PTP1B (protein tyrosine phosphatase 1B) in neural cells [14]. The interaction of PTP1B with E-cadherin in epithelial junctions is unknown. In neural cells, PTP1B associates directly with the cytoplasmic domain of N-cadherin [14,17,21]. PTP1B is suggested to dephosphorylate β-catenin on tyrosine residues and maintain it in a dephosphorylated state, which is essential for the interaction of β-catenin with N-cadherin and promotion of cadherin-based cell–cell adhesion. Overexpression of dominant-negative PTP1B(C215S) results in hyperphosphorylation of β-catenin on tyrosine residues and loss of N-cadherin function in neural cells [14,17]. In Caco-2 cell monolayers, acetaldehyde-induced disruption of TJ is associated with an inhibition of PTPase (protein tyrosine phosphatase) activity and increased protein tyrosine phosphorylation [10].
In the present study, we determined the effect of acetaldehyde on the PTP1B–E-cadherin–β-catenin complex in Caco-2 cell monolayers. The results of the present study show that: (i) acetaldehyde reduces E-cadherin and β-catenin-associated PTPase activity and dissociates PTP1B from the E-cadherin–β-catenin complex, (ii) acetaldehyde dissociates E-cadherin–β-catenin complex by a tyrosine kinase-dependent mechanism, (iii) acetaldehyde and tyrosine phosphorylation of β-catenin reduces the interaction of cellular β-catenin with recombinant GST (glutathione S-transferase)–E-cadherin-C, the C-terminal region of human E-cadherin, (iv) acetaldehyde and tyrosine phosphorylation of E-cadherin reduces interaction between E-cadherin and PTP1B, and (v) acetaldehyde increases phosphorylation of β-catenin on Tyr-331, Tyr-333, Tyr-654 and Tyr-670 in Caco-2 cells.
EXPERIMENTAL
Cell culture
Caco-2 cells, purchased from the American Type Culture Collection (Rockville, MD, U.S.A.), were maintained under standard cell culture conditions at 37 °C in a medium containing 10% (v/v) fetal bovine serum. Cells were grown on polycarbonate membranes in Transwells (6.5 or 24 mm; Costar, Cambridge, MA, U.S.A.), and experiments were performed on 12–14 days (6.5 mm) or 19–21 days (24 mm) after seeding.
Acetaldehyde treatment
Acetaldehyde was administered by exposing cell monolayers in PBS (Dulbecco's saline containing 1.2 mM CaCl2, 1 mM MgCl2, 10 mM glucose and 0.6% BSA) to vapour-phase acetaldehyde as described previously [10] to achieve an acetaldehyde concentration of 400 μM in the buffer bathing the cell monolayer. Genistein (100 μM) was administered to both the apical and basal compartments for 60 min prior to the administration of acetaldehyde. Genistein continued to be present during the acetaldehyde treatment.
Measurement of TER (transepithelial electrical resistance)
TER was measured as described by Hidalgo et al. [22] using a Millicell-ERS electrical resistance system (Millipore, Bedford, MA, U.S.A.). TER was calculated as Ω·cm2 by multiplying it by the surface area of the monolayer (0.33 cm2 for 6.5 mm wells). The resistance of the supporting membrane in Transwells (which is usually ∼30 Ω·cm2) was subtracted from all readings prior to calculations.
Unidirectional flux of inulin
Cell monolayers in Transwells were incubated under different experimental conditions in the presence of FITC–inulin (50 μg/ml) in the basal well. Apical and basal media (100 μl) were withdrawn and fluorescence was measured in a FLX800 fluorescence microplate reader (BioTek Instruments, Winooski, VT, U.S.A.). The flux into the apical well was calculated as the percentage of total fluorescence administered into the basal well per h per cm2 of surface area.
Immunofluorescence microscopy
Cell monolayers incubated with acetaldehyde for various times were fixed in acetone/methanol (1:1) at 0 °C for 5 min and blocked in 3% (w/v) non-fat milk in TBST (20 mM Tris, pH 7.2, and 150 mM NaCl). Cells were incubated with primary antibodies [rabbit polyclonal anti-β-catenin or anti-ZO-1 (zonula occludens 1 protein)] for 1 h. After washing, cell monolayers were incubated with secondary antibodies (Cy3-conjugated anti-rabbit IgG) for 1 h. The fluorescence was visualized using a Zeiss LSM 5 laser scanning confocal microscope, and images from x–y sections (1 μm) were collected. Images from different sections were stacked using the software, Image J [NIH (National Institutes of Health), Bethesda, MD, U.S.A.], and processed by Adobe Photoshop (Adobe Systems, San Jose, CA, U.S.A.).
Immunoprecipitation
After acetaldehyde treatment, cell monolayers (24 mm) were washed with ice-cold 20 mM Tris (pH 7.4) and the proteins were extracted in lysis buffer N (20 mM Tris, pH 7.4, containing 150 mM NaCl, 0.5% Nonidet P40, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin A and 0.1 mM PMSF) at 4 °C for 30 min. Each cell monolayer was extracted in 0.75 ml of lysis buffer N, and extracts from two monolayers were pooled for each value. Extracts were centrifuged at 3000 g for 10 min at 4 °C, and the supernatant containing 1.0–1.5 mg of protein/ml was incubated with 2 μg of rabbit polyclonal anti-E-cadherin, anti-β-catenin, anti-occludin, anti-PTP1B and anti-PTP1D antibodies or pre-immune IgG at 4 °C overnight. Immunocomplexes were isolated by precipitation using Protein A–Sepharose (for 1 h at 4 °C). Washed beads were suspended in PTPase buffer (50 mM Hepes, pH 7.2, 60 mM NaCl, 60 mM KCl, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin A and 0.1 mM PMSF) for PTPase assay or heated in 20 μl of Laemmli's sample buffer at 100 °C for 5 min. Extracts were immunoblotted for E-cadherin, β-catenin, ZO-1, PTP1B or PTP1D as described below.
For determination of tyrosine phosphorylation, proteins were extracted under denatured conditions in lysis buffer D (0.3% SDS in 10 mM Tris buffer, pH 7.4, containing 1 mM vanadate and 0.33 mM PMSF). p-Tyr (phosphotyrosine) was immunoprecipitated using biotin-conjugated anti-p-Tyr antibodies and the immunocomplexes were precipitated by using streptavidin–agarose. Immunocomplexes were then immunoblotted for β-catenin and E-cadherin.
PTPase assay
PTPase activity was measured as described previously [23]. Anti-E-cadherin and anti-β-catenin immunocomplexes, suspended in 30 μl of PTPase buffer, were incubated with 5 μl of phosphopeptide substrate, TSTEPQpYQPGENL (5 μg), at 30 °C for 10 min. Free phosphate released during the reaction was measured by using Malachite Green reagent in a 96-well microtitre plate and a plate reader (Spectra MAX 190; Molecular Devices, Sunnyvale, CA, U.S.A.). PTPase activity in complexes prepared using pre-immune IgG was used as control activity and subtracted from the activities associated with specific immunocomplexes.
Immunoblot analysis
Proteins were separated by SDS/PAGE and transferred to nitrocellulose PVDF membranes. Membranes were blotted for E-cadherin, β-catenin, PTP1B or PTP1D using mouse monoclonal anti-E-cadherin, anti-β-catenin, anti-PTP1B and anti-PTP1D antibodies in combination with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG antibodies. The blot was developed using the ECL® (enhanced chemiluminescence) method (Amersham, Arlington Heights, IL, U.S.A.). Specific bands from different blots (inverted X-ray film images) were quantified by densitometric analysis using Image J software. Density was measured as pixels per unit area. Density values for all bands were normalized to one of the values for control sample or the group that showed the highest density. This is indicated in the legend for the corresponding Figure.
Preparation of recombinant proteins
The cDNA construct, pCDNA3E-Cadherin, was kindly provided by Dr David Rimm (Yale University, New Haven, CT, U.S.A.) [24]. The C-terminal region (151 amino acids) of human E-cadherin (E-cadherin-C) was PCR-amplified using the above construct as template and the DNA primers (upper, TATAGGATCCAGGAGAGCGGTGGTCAAAG and lower, TATAGCGGCCGCCTAGTCGTCCTCGCCGCC). The PCR products were cloned into pET-41 (EMD Biosciences, San Diego, CA, U.S.A.). Full-length human β-catenin was PCR-cloned using a cDNA library prepared from Caco-2 cells and the DNA primers (upper, TTTTGGATCCATGGCTACTCAAGCTGAT and lower, TATACAGCTGTTACAGGTCAGTATCAAA). PCR products were sequenced and inserted into pGEX2T vector (Amersham Biosciences, Pittsburgh, PA, U.S.A.). E-cadherin-C and β-catenin were prepared as GST fusion proteins in the non-phosphorylated form in Escherichia coli BL21 cells and purified using GSH–agarose as described previously [25]. Tyrosine phosphorylation of GST–E-cadherin-C and GST–β-catenin was induced by incubation with c-Src (2 μg/ml) and ATP in kinase buffer (50 mM Hepes, pH 7.4, containing 0.1 mM EDTA, 0.2% 2-mercaptoethanol, 3 mM MgCl2 and 100 μM ATP) at 30 °C for 3 h. For pairwise binding assays, GST from GST–E-cadherin-C or GST–β-catenin was cleaved by incubation with thrombin and the free GST was cleared by incubation with GSH–agarose.
GST pull-down assay
Caco-2 cells, incubated with or without 0.25 or 0.5 mM acetaldehyde, were lysed in 0.2% Triton X-100 in PBS containing 1 mM sodium orthovanadate, 10 mM sodium fluoride and 20 mM sodium pyrophosphate and 0.1 mM PMSF and centrifuged to prepare cell extract. GST–E-cadherin, GST–β-catenin or GST-PTP1B (10 μg) was incubated with 500 μl of Caco-2 cell extract at 4 °C on a rocker for 16 h. GST–E-cadherin, GST–β-catenin and GST–PTP1B were pulled down by incubation for 1 h with GSH–agarose beads. The beads were washed with PBS and heated in 25 μl of sample buffer at 100 °C for 5 min. The supernatant was immunoblotted for E-cadherin or β-catenin using mouse monoclonal anti-E-cadherin and β-catenin antibodies. Similarly, binding of tyrosine-phosphorylated GST–β-catenin to cellular E-cadherin was performed. To determine the non-specific binding, GST was used in place of GST–E-cadherin and GST–β-catenin.
Pairwise binding assay
To determine the direct interaction between β-catenin and the C-terminal region of E-cadherin, GST–E-cadherin-C (0.3 μg) was incubated with recombinant, thrombin-cleaved β-catenin (0.1–1.0 μg) in PBS containing 0.2% Triton X-100, 1 mM vanadate and 10 mM sodium fluoride for 3 h at 30 °C on a rocker. GST–E-cadherin-C was pulled down by binding to 20 μl of 50% (w/v) GSH–agarose slurry at 30 °C for 1 h. The amounts of β-catenin bound to the GSH–agarose pull-down were determined by immunoblot analysis. Both non-phosphorylated and tyrosine-phosphorylated GST–E-cadherin-C were used in this binding assay. Direct interaction between PTP1B and non-phosphorylated or tyrosine-phosphorylated E-cadherin was investigated by a similar method using thrombin-cleaved PTP1B and GST–E-cadherin-C that was pre-incubated with c-Src in the presence or absence of ATP as described above.
MS analysis
Isolation of β-catenin
Caco-2 cells in 75 mm flasks were incubated with or without 400 μM acetaldehyde for 5 h. Cells were lysed in lysis buffer D, and centrifuged at 3000 g for 15 min. β-Catenin was immunoprecipitated from the supernatant by incubation with rabbit polyclonal anti-β-catenin antibody for 16 h at 4 °C. β-Catenin–antibody complexes were precipitated by Protein A–Sepharose. Washed beads were heated in 25 μl of sample buffer, and proteins were separated by SDS/PAGE and stained with Coomassie Blue. The protein band corresponding to 92 kDa was excised and used for in-gel trypsin digestion.
In-gel trypsin digestion
The gel pieces were crushed and dried in a Speedvac. The dry powder was resuspended in 10% (v/v) acetonitrile in 10 mM NH4HCO3 (pH 8.5) and incubated at 37 °C overnight with Tos-Phe-CH2Cl (tosylphenylalanylchloromethane; ‘TPCK’)-treated trypsin (enzyme to substrate molar ratio of 1:10). The digest was filtered through 0.45 μm filters. The clear supernatant was freeze-dried to dryness. Air-dried samples were equilibrated in an aqueous solution containing 0.1% TFA (trifluoroacetic acid) and desalted by passing through C-18 ZipTip (Millipore, Bedford, MA, U.S.A.) according to the manufacturer's protocol. Peptides extracted from the ZipTip were subjected to sequence determination by LC/MS/MS (liquid chromatography tandem MS) analyses.
LC/MS/MS analysis
Sequence analysis of tryptic peptides was performed by injecting 3 μl of the ZipTip-purified sample on to a capillary C-18 LC column online with a Finnigan LCQDECA (Thermoquest, San Jose, CA, U.S.A.) ion-trap mass analyser that is equipped with a nano-ESI (electrospray ionization) source. The capillary C-18 column was prepared in-house using New Objective Pico Frit (360 μm outer diameter, 75 μm inner diameter, 15 μm tip and 10.4 cm length) and Magic C18AQ packing material (5 μm beads, 200 Å pores; 1 Å=0.1 nm). The peptides were fractionated using 0.1% formic acid in water as solvent A and 90% acetonitrile as solvent B. The acquired spectra were visualized using Qual-browser in the X-Calibur software suite (Thermo Scientific). Raw data thus obtained were analysed against a human protein database generated from the SwissProt database using the Sequest software suite (Sequest Technologies, Lisle, IL, U.S.A.).
Chemicals
Cell culture media and related reagents were purchased from Gibco BRL (Grand Island, NY, U.S.A.). Protease inhibitors, vanadate, GSH–agarose, α-cyano-4-hydroxycinnamic acid, 2-mercaptoethanol, genistein, streptavidin–agarose and Protein A–Sepharose were purchased from Sigma Chemical Company (St. Louis, MO, U.S.A.). All other chemicals were of analytical grade and purchased either from Sigma Chemical Company or Fisher Scientific (Tustin, CA, U.S.A.). Trypsin was purchased from Promega (Madison, WI, U.S.A.), and acetonitrile and TFA were purchased from Aldrich (Milwaukee, WI, U.S.A.). Phospho-peptide substrate was custom synthesized by Sigma-GenoSys (St. Louis, MO, U.S.A.). Active c-Src was purchased from Upstate USA (Lake Placid, NY, U.S.A.).
Antibodies
Rabbit polyclonal anti-E-cadherin and anti-PTP1B antibodies were purchased from Zymed Laboratories (South San Francisco, CA, U.S.A.). Mouse monoclonal anti-E-cadherin, anti-β-catenin and anti-PTP1B and biotin-conjugated anti-p-Tyr antibodies were purchased from Transduction Laboratories (Lexington, KY, U.S.A.). Rabbit polyclonal anti-β-catenin was purchased from Chemicon International (Temecula, CA, U.S.A.). Cy3-conjugated anti-rabbit IgG was purchased from Sigma–Aldrich Chemical Company (St. Louis, MO, U.S.A.), and Alexa Flour® 488-conjugated anti-mouse IgG was purchased from Molecular Probes (Eugene, OR, U.S.A.).
Statistics
Comparison between two groups was made by the Student's t tests for grouped data. The significance in all tests was derived at 95% or greater confidence level.
RESULTS
Acetaldehyde disrupts AJ and TJ
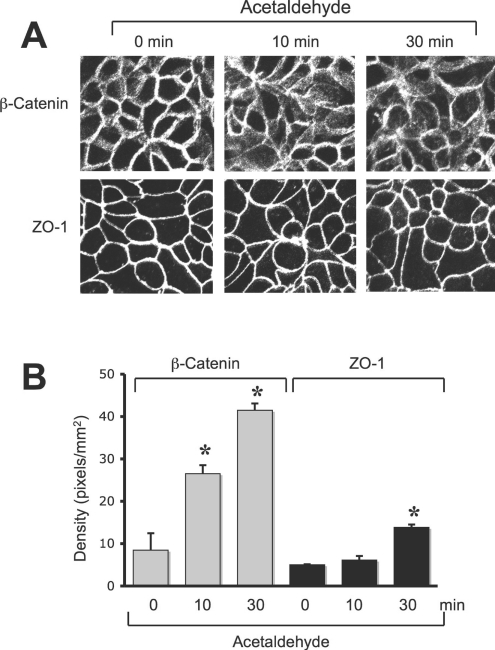
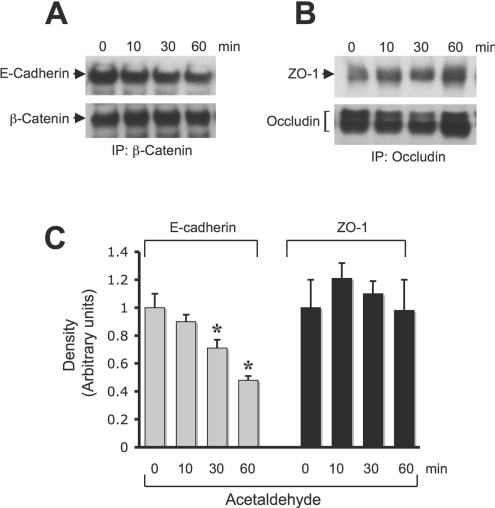
Previous studies demonstrated that acetaldehyde disrupts both AJ and TJ by disrupting the junctional localization and interaction between occludin and ZO-1, and E-cadherin and β-catenin. Although AJ does not directly form a physical barrier to macromolecules, it indirectly regulates the integrity of TJ and the epithelial barrier function. Disruption of AJ by calcium depletion also results in disruption of TJ [26]. In the present study, we evaluated the temporal effect of acetaldehyde on the AJ and TJ by analysing the distribution of β-catenin and ZO-1 by immunofluorescence confocal microscopy. Results show that the junctional distribution of β-catenin was disrupted at 10 min after administration of acetaldehyde (Figure 1A), while the junctional distribution of ZO-1 was unaffected at this time (Figure 1A). Densitometric analysis of intracellular fluorescence demonstrated that there was a significant increase in β-catenin in the intracellular compartment, while that of ZO-1 was unaffected at 10 min (Figure 1B); however, at 30 min, ZO-1 in the intracellular compartment was also significantly increased. Co-immunoprecipitation of E-cadherin with β-catenin was significantly reduced at 30 min after acetaldehyde administration (Figures 2A and 2C), while co-immunoprecipitation of ZO-1 with occludin was not significantly altered until 60 min (Figures 2B and 2C).
Figure 1. Acetaldehyde disrupts AJ and TJ.
(A) Caco-2 cell monolayers were incubated with 400 μM acetaldehyde for various time periods. Cell monolayers were fixed in acetone/methanol and stained for β-catenin or ZO-1 by the immunofluorescence method. Fluorescence images were collected using a confocal microscope. (B) Fluorescence in the intracellular compartments was evaluated using Image J software. Values are means±S.E.M. (n=10). Asterisks indicate the values that are significantly (P<0.05) different from corresponding values for the 0 min group.
Figure 2. Acetaldehyde reduces co-immunoprecipitation of E-cadherin with β-catenin and ZO-1 with occludin.
(A) Caco-2 cell monolayers were incubated with acetaldehyde for various time periods. Proteins were extracted under native conditions and β-catenin was immunoprecipitated. Immunocomplexes were then immunoblotted for β-catenin and E-cadherin. (B) Occludin was immunoprecipitated from protein extracts of cells exposed to acetaldehyde for various time periods followed by immunoblot analysis for ZO-1 and occludin. (C) Densitometric analysis. The density of the inverted bands was measured in terms of pixels/mm2. Values for density of E-cadherin and ZO-1 were normalized to the density value at 0 min for corresponding protein. Values are means±S.E.M. (n=3). Asterisks (*) indicate values that are significantly (P<0.05) different from the values for the corresponding 0 min time-point.
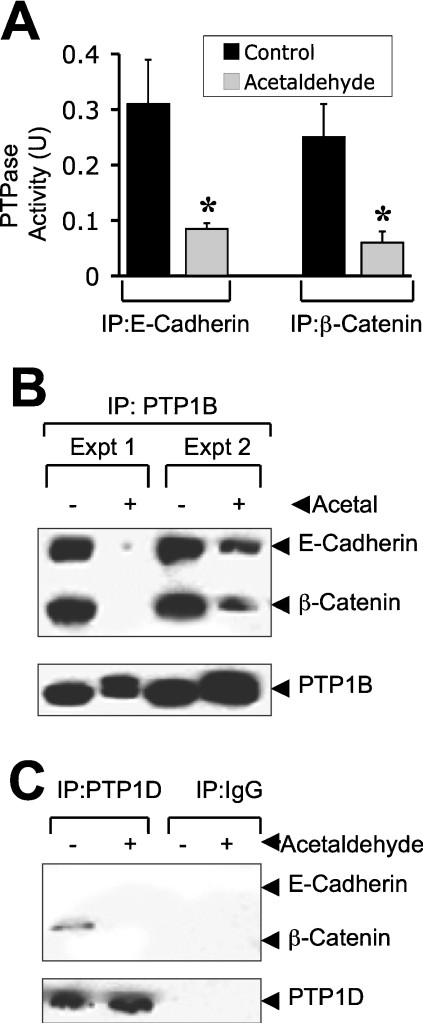
Acetaldehyde dissociates PTP1B from the E-cadherin–β-catenin complex
PTP1B binds to the cytoplasmic domain of N-cadherin and maintains β-catenin in a dephosphorylated state in neural cells [14]. We evaluated the effect of acetaldehyde on the association of PTP1B with the E-cadherin–β-catenin complex in Caco-2 cells. Immunoprecipitation of E-cadherin and β-catenin followed by assay for PTPase activity showed that PTPase activity is associated with E-cadherin and β-catenin (Figure 3A). Treatment of Caco-2 cells with acetaldehyde significantly reduced the PTPase activity associated with immunocomplexes of E-cadherin and β-catenin (Figure 3A). Immunoprecipitation of PTP1B followed by immunoblot analysis for E-cadherin and β-catenin also showed that E-cadherin and β-catenin co-immunoprecipitate with PTP1B, and this was dramatically reduced by the treatment of cells with acetaldehyde (Figure 3B). β-Catenin was also detected in immunocomplexes of PTP1D, which was reduced by acetaldehyde (Figure 3C). However, no E-cadherin was detected in anti-PTP1D immunocomplexes. This suggests that PTP1D may bind to a different pool of β-catenin, which is not relevant to E-cadherin–β-catenin complexes of AJ. Complexes prepared using pre-immune IgG showed no trace of E-cadherin or β-catenin (Figure 3C). The distorted appearance of PTP1B bands in acetaldehyde-treated cells was caused by phosphorylation of PTP1B. Treatment of immunocomplexes with alkaline phosphatase prior to immunoblot analysis compressed it into a sharp band (results not shown). The intensity of dephosphorylated bands indicates that the total amount of PTP1B was unaffected by acetaldehyde.
Figure 3. Acetaldehyde dissociates PTP1B from E-cadherin–β-catenin complex.
Caco-2 cell monolayers were incubated with (Acetal) or without (Control) acetaldehyde (400 μM) for 5 h. (A) E-cadherin and β-catenin were immunoprecipitated from native protein extracts and assayed for PTPase activity. Values are means±S.E.M. (n=4). Asterisks indicate the values that are significantly (P<0.05) different from the corresponding control values. Control assay was conducted under similar conditions, but using pre-immune IgG instead of specific antibodies. These values were subtracted from values for anti-E-cadherin and anti-β-catenin immunocomplexes. (B) Following incubation of cells with or without acetaldehyde, PTP1B was immunoprecipitated and immunocomplexes were immunoblotted for E-cadherin, β-catenin and PTP1B. Samples from two different experiments were run in the above blot. (C) Following incubation with or without acetaldehyde, immunoprecipitation was conducted using anti-PTP1D antibody or pre-immune IgG. These immunocomplexes were immunoblotted for E-cadherin, β-catenin and PTP1D. Immunoprecipitation of occludin followed by immunoblot analysis for PTP1B showed no interaction of PTP1B with occludin (results not shown) and serves as a negative control.
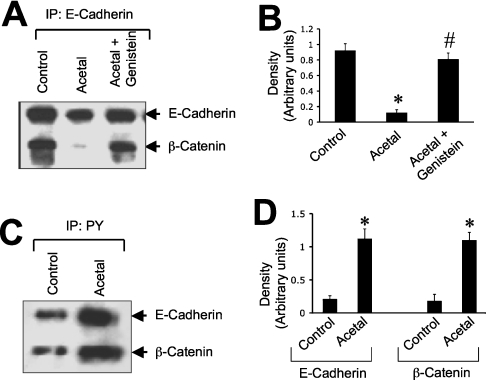
Acetaldehyde reduces interaction between E-cadherin and β-catenin by a tyrosine kinase-dependent mechanism
To determine the effect of acetaldehyde on the interaction between E-cadherin and β-catenin, we evaluated the effect of acetaldehyde on co-immunoprecipitation of E-cadherin and β-catenin. β-Catenin was co-immunoprecipitated with E-cadherin in control cell monolayers, but this was dramatically reduced by acetaldehyde treatment (Figure 4A). Acetaldehyde-induced loss of co-immunoprecipitation of E-cadherin and β-catenin was completely prevented by the pretreatment of cells with genistein (Figures 4A and 4B). Genistein, by itself, produced no significant effect on the interaction between E-cadherin and β-catenin (results not shown).
Figure 4. Acetaldehyde disrupts the E-cadherin–β-catenin complex by a tyrosine kinase-dependent mechanism.
Caco-2 cell monolayers were pre-incubated with or without genistein (100 μM) for 1 h prior to incubation with (Acetal) or without (Control) 400 μM acetaldehyde for 5 h. (A) E-cadherin from native protein extracts was immunoprecipitated followed by immunoblot analysis for β-catenin and E-cadherin. (B) Densitometric analysis of β-catenin bands in (A). The ratio of density of β-catenin bands to density of E-cadherin bands is presented. Values are means±S.E.M. from three independent experiments. Asterisk and ‘#’ indicate the values that are significantly (P<0.05) different from the values for control and acetaldehyde groups respectively. (C) Denatured protein extracts from control and acetaldehyde-treated cells were immunoprecipitated for p-Tyr followed by immunoblot analysis for E-cadherin and β-catenin. (D) Densitometric analysis of E-cadherin and β-catenin bands in (C). Values are means±S.E.M. from three independent experiments. Asterisks indicate the values that are significantly (P<0.05) different from the values for the corresponding control group.
Loss of interaction of PTP1B with the E-cadherin–β-catenin complex is likely to increase tyrosine phosphorylation of β-catenin in acetaldehyde-treated cells. Tyrosine phosphorylation was evaluated by immunoprecipitation of p-Tyr followed by immunoblot analysis for β-catenin and E-cadherin. A significant level of tyrosine-phosphorylated β-catenin was detected in the control cell monolayer, which was increased severalfold by the treatment of cells with acetaldehyde (Figures 4C and 4D). Acetaldehyde treatment also increased the tyrosine phosphorylation of E-cadherin (Figures 4C and 4D).
Regulated interaction between E-cadherin and β-catenin in acetaldehyde-treated cells
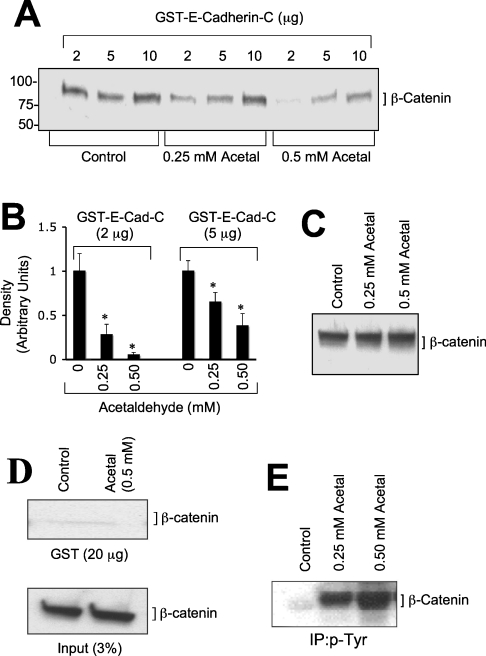
The regulated interaction between E-cadherin and β-catenin was determined by GST pull-down assay using recombinant GST–E-cadherin-C (C-terminal 151 amino acids), GST–β-catenin and detergent-soluble protein extracts from Caco-2 cells that were incubated with or without different concentrations of acetaldehyde. GST–E-cadherin-C pulled down β-catenin from control cell extracts (Figure 5A); this binding was significantly reduced by acetaldehyde in a dose-dependent manner (Figures 5A and 5B). The total amounts of β-catenin present in untreated and acetaldehyde-treated cells were not different from each other (Figure 5C). Incubation of cell extracts with GST did not pull-down β-catenin (Figure 5D). β-Catenin in the acetaldehyde-treated cell extracts was tyrosine-phosphorylated in a dose-dependent manner (Figure 5E). Incubation of GST–β-catenin with cell extracts pulled down E-cadherin, and this binding was significantly low in extracts prepared from acetaldehyde-treated cells (Figures 6A and 6B). Tyrosine-phosphorylated GST–β-catenin failed to pull-down E-cadherin from both untreated and acetaldehyde-treated cell extracts (Figures 6A and 6B).
Figure 5. Acetaldehyde prevents β-catenin binding to the C-terminal region of E-cadherin.
(A) Different concentrations of GST–E-cadherin-C (C-terminal 151 amino acids of human E-cadherin) were incubated at 4 °C for 16 h with detergent-soluble protein extracts from Caco-2 cells that were pre-incubated with or without (control) different concentrations of acetaldehyde. GSH–agarose pull-down was immunoblotted for β-catenin. (B) Densitometric analyses of β-catenin bands in (A). Values are means±S.E.M. (n=3). Asterisks indicate the values that are significantly different (P<0.05) from the corresponding control values. (C) Total protein extracts from Caco-2 cells (3% of that used for GST pull-down assay in A) were immunoblotted for β-catenin. (D) GST (20 μg) was incubated for 16 h at 4 °C with detergent-soluble protein extracts from control or acetaldehyde-treated cells. GSH–agarose pull-down and the protein extracts were immunoblotted for β-catenin. (E) p-Tyr was immunoprecipitated from denatured protein extracts from Caco-2 cells that were incubated with or without (control) different concentrations of acetaldehyde and immunocomplexes were immunoblotted for β-catenin.
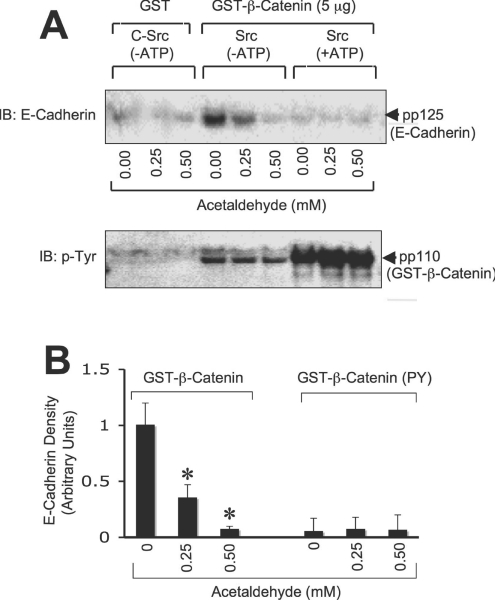
Figure 6. Acetaldehyde prevents E-cadherin binding to GST–β-catenin.
(A) GST–β-catenin, non-phosphorylated or tyrosine-phosphorylated by c-Src, was incubated at 4 °C for 16 h with detergent-soluble protein extracts from Caco-2 cells that were pre-incubated with or without different concentrations of acetaldehyde. GSH–agarose pull-down was immunoblotted for E-cadherin. Lower panel: immunoblot analysis of the same experiment for p-Tyr showing tyrosine phosphorylation of GST–β-catenin where it was incubated with c-Src in the presence of ATP. (B) Densitometric analyses of E-cadherin bands in (A). Values are means±S.E.M. (n=3). Asterisks indicate the values that are significantly different (P<0.05) from the corresponding control values.
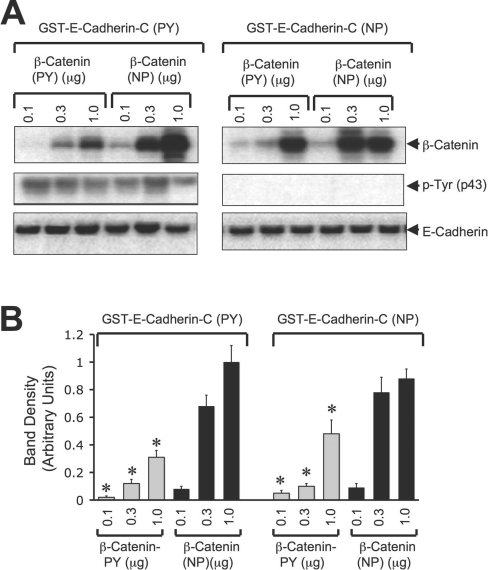
To determine the effect of tyrosine phosphorylation on the direct interaction between E-cadherin and β-catenin, pairwise binding studies were conducted using recombinant GST–E-cadherin-C (C-terminal 150 amino acids) and β-catenin (full length). GST–E-cadherin-C and β-catenin were also tyrosine-phosphorylated by incubation with c-Src and ATP. Incubation of GST–E-cadherin-C with recombinant β-catenin showed a direct interaction of β-catenin with the C-terminal tail of E-cadherin (Figure 7A). The binding of GST–E-cadherin-C to tyrosine-phosphorylated β-catenin was lower than the binding to non-phosphorylated β-catenin (Figures 7A and 7B). Incubation of tyrosine-phosphorylated GST–E-cadherin-C also showed a dose-dependent binding of β-catenin, and it was significantly low for tyrosine-phosphorylated β-catenin (Figures 7A and 7B). However, the binding of β-catenin to tyrosine-phosphorylated GST–E-cadherin-C was not different from the binding to non-phosphorylated GST–E-cadherin-C (Figures 7A and 7B).
Figure 7. Direct interaction between β-catenin and the C-terminal region of E-cadherin is attenuated by tyrosine phosphorylation.
(A) Non-phosphorylated (NP) GST–E-cadherin-C (incubated with c-Src in the absence of ATP) or tyrosine-phosphorylated (PY) GST–E-cadherin-C (incubated with c-Src in the presence of ATP) (0.3 μg) was incubated with varying concentrations of thrombin-cleaved non-phosphorylated or tyrosine-phosphorylated β-catenin. GSH–agarose pull-down was immunoblotted for β-catenin, p-Tyr and E-cadherin. (B) Experiments in (A) were repeated twice and β-catenin bands were evaluated by densitometric analysis. Density values were normalized to the corresponding values for E-cadherin bands and one of the values for binding between GST–E-cadherin-C (NP) and 1 μg of β-catenin (NP). Values are means±S.E.M. (n=3). Asterisks indicate the values that are significantly (P<0.05) different from the corresponding values for β-catenin (NP).
Tyrosine phosphorylation of E-cadherin reduces its interaction with PTP1B
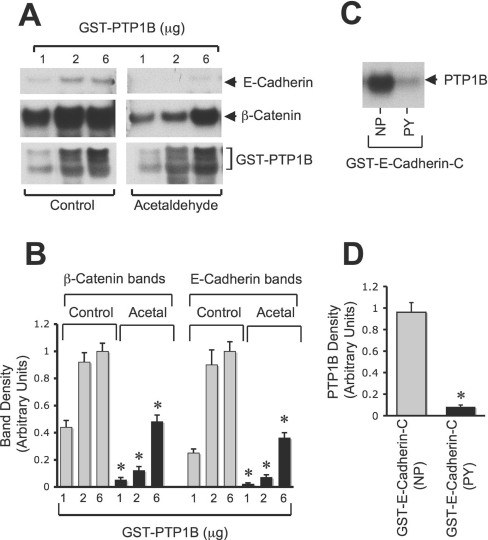
Although it is well established that PTP1B interacts with N-cadherin in a regulated manner [14,17,21], there is no evidence to indicate that PTP1B interacts with E-cadherin. Results presented in Figure 4(C) demonstrate that acetaldehyde elevates tyrosine phosphorylation of E-cadherin. However, results presented in Figure 7 indicate that tyrosine phosphorylation of E-cadherin has no influence on its interaction with β-catenin. Therefore we evaluated the effect of tyrosine phosphorylation of E-cadherin on its interaction with PTP1B. Incubation of GST–PTP1B with protein extracts from untreated and acetaldehyde-treated cells showed that GST–PTP1B does bind to E-cadherin and β-catenin in control cell extract (Figure 8A). However, the binding of E-cadherin and β-catenin to GST–PTP1B was significantly low in acetaldehyde-treated cell extract (Figure 8B). A pairwise binding study using thrombin-cleaved PTP1B with non-phosphorylated or tyrosine-phosphorylated GST–E-cadherin-C demonstrated that PTP1B directly interacts with the C-terminal region of E-cadherin (Figure 8C). Src-mediated tyrosine phosphorylation of E-cadherin significantly reduced the interaction between PTP1B and GST–E-cadherin-C (Figures 8C and 8D).
Figure 8. Acetaldehyde dissociates interaction of E-cadherin with PTP1B.
(A) Various concentrations of GST–PTP1B were incubated with cell extracts from untreated (Control) or acetaldehyde-treated (Acetal) cell monolayers as described in the Experimental section. GSH–agarose pull-down was immunoblotted for E-cadherin, β-catenin and PTP1B. (B) β-Catenin and E-cadherin bands in experiments in (A) were evaluated by densitometric analysis. Density values were normalized to one of the values for the corresponding control at 6 μg of PTP1B. Values are means±S.E.M. (n=3). Asterisks indicate the values that are significantly (P<0.05) different from the corresponding values for Control. (C) Incubation of 2 μg of non-phosphorylated (NP) or tyrosine-phosphorylated (PY) GST–E-cadherin-C with thrombin-cleaved PTP1B (0.25 μg) was performed. GSH–agarose pull-down was immunoblotted for PTP1B. (D) PTP1B bands in (C) were evaluated by densitometric analysis. Density values were normalized to one of the values for GST–E-cadherin-C (NP). Values are means±S.E.M. (n=3). The asterisk indicates the values that are significantly (P<0.05) different from the corresponding value for NP.
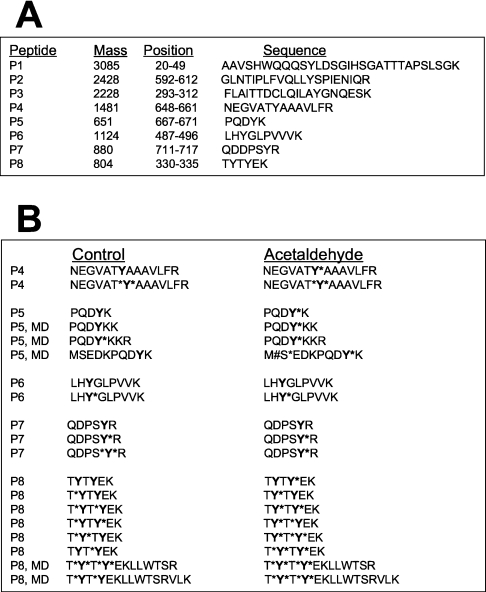
Phosphorylation of β-catenin on Tyr-331, Tyr-333, Tyr-654 and Tyr-670
Phosphorylation of β-catenin on Tyr-654 has previously been shown to reduce its interaction with E-cadherin [15,16]. To determine the site of phosphorylation in β-catenin, we immunoprecipitated β-catenin from control and acetaldehyde-treated cells and the in-gel trypsin digest was subjected to MS. Relatively equal amounts of β-catenin were isolated from the control and acetaldehyde-treated cells, as confirmed by SDS/PAGE and Coomassie Blue staining. Analysis of the β-catenin fragments using ExPASy's peptide mass software [26a] identified eight different peptides (P1–P8) containing tyrosine residues (Figure 9A), and the masses of the phosphorylated forms of these peptides were calculated. LC/MS/MS and Sequest analyses detected several forms of each peptide due to misdigestion by trypsin, phosphorylation of serine or threonine residue and oxidation of methionine residue. These peptides for control and acetaldehyde-treated cells are listed in Figure 9(B). LC/MS/MS analysis detected phosphorylated forms of peptides P4–P8, showing the phosphorylation of β-catenin on residues Tyr-331, Tyr-333, Tyr-489, Tyr-654, Tyr-670 and Tyr-716. Two different peptides related to P4 were detected in β-catenin from control and the acetaldehyde-treated cells. Both peptide forms were found to be tyrosine-phosphorylated in β-catenin isolated from the acetaldehyde-treated cells, while only one of the peptides was tyrosine-phosphorylated in β-catenin isolated from the control cells. This tyrosine residue corresponds to Tyr-654 of β-catenin, the phosphorylation of which is known to prevent interaction of β-catenin with E-cadherin [15,16]. Similarly, peptides P5 and P8 are hyper-phosphorylated on tyrosine residues in β-catenin isolated from the acetaldehyde-treated cells (Figure 9B). These tyrosine residues correspond to Tyr-331, Tyr-333 and Tyr-670 of β-catenin.
Figure 9. Sequest analysis of ion fragmentation spectra from trypsin fragments of β-catenin from control and acetaldehyde-treated cells.
Caco-2 cells were incubated for 5 h with or without 400 μM acetaldehyde. β-Catenin in proteins extracted under denaturing conditions was immunoprecipitated and separated by SDS/PAGE. The gel was stained with Coomassie Blue. The protein band corresponding to a 92 kDa protein was excised and subjected to in-gel trypsin digestion followed by LC/MS/MS analysis. Sequences of β-catenin fragments were determined by Sequest analysis of ion fragmentation spectra for different peptides. (A) The masses of tyrosine-containing trypsin fragments of β-catenin were determined by using ExPASy's peptide mass software. (B) Variants of each peptide formed due to phosphorylation of a serine or threonine residue (S*, T*), misdigestion (MD) by trypsin and methionine oxidation (M#) are listed. Residue with an asterisk on its right indicates that the residue is phosphorylated.
DISCUSSION
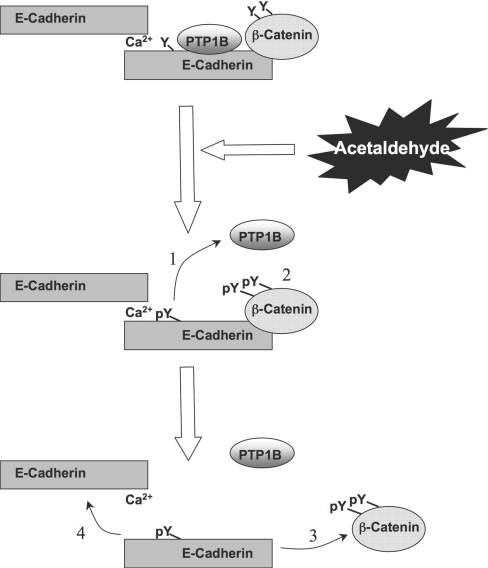
Assembly of AJ and cell–cell adhesion in epithelial cell monolayers is mediated by the organization of E-cadherin and β-catenin. In the present study, we show that acetaldehyde, an oxidative product of ethanol, disrupts PTP1B–E-cadherin-β–catenin complex in an intestinal epithelium by a tyrosine kinase-dependent mechanism. These results imply a role for acetaldehyde in the disruption of AJ and the E-cadherin-based cell–cell adhesion in the intestinal epithelium by altering the phosphorylation of β-catenin. Acetaldehyde induces dissociation of PTP1B from E-cadherin–β-catenin complex and increases the tyrosine phosphorylation of β-catenin, leading to the disruption of interaction between E-cadherin and β-catenin and the homophilic interaction between the extracellular domains of E-cadherin (Figure 10).
Figure 10. Diagrammatic model showing acetaldehyde-induced loss of interaction between PTP1B–E-cadherin–β-catenin.
Cadherin-based cell–cell adhesion is mediated by interaction of E-cadherin with β-catenin. PTP1B binds to the intracellular domain of E-cadherin and dephosphorylates β-catenin on tyrosine residues. Treatment of cell monolayers with acetaldehyde induces inhibition and dissociation of PTP1B from E-cadherin (1), which results in increased tyrosine phosphorylation of β-catenin and E-cadherin (2). Tyrosine phosphorylation of β-catenin results in the loss of its interaction with E-cadherin (3), and loss of interaction between E-cadherin and β-catenin leads to a loss of homophilic interaction between extracellular domains of E-cadherin (4).
Redistribution of E-cadherin and β-catenin in acetaldehyde-treated cell monolayers indicated that acetaldehyde disrupts AJ and cadherin-based cell–cell adhesion as early as 10 min after treatment. However, during this time the distribution of occludin and ZO-1 was not significantly affected. These results suggest that the effect of acetaldehyde on the AJ precedes its effect on the TJ. This was further confirmed by the loss of co-immunoprecipitation of E-cadherin with β-catenin before the loss of co-immunoprecipitation of ZO-1 with occludin. These results suggest that acetaldehyde-induced disruption of TJ may be mediated by the disruption of AJ. It is known that disruption of AJ by chelation of extracellular calcium leads to disruption of TJ [26]. The E-cadherin–β-catenin complex functions as a suppressor of carcinogenesis and tumour invasion. The loss of cadherin-based cell–cell adhesion plays a crucial role in carcinogenesis [27]. Evidence indicates that acetaldehyde increases the risk for gastrointestinal cancer in alcoholics [4]. Therefore the disruption of AJ and cadherin-based cell–cell adhesion may play an important role in the mechanism of acetaldehyde-induced increase in risk for carcinogenesis.
Co-immunoprecipitation of E-cadherin and β-catenin with PTP1B indicates that PTP1B interacts with the E-cadherin–β-catenin complex in Caco-2 cell monolayers. Acetaldehyde treatment results in the dissociation of PTP1B from E-cadherin and β-catenin. PTPases such as PTPmu [28] and PTP LAR [29] have been shown to interact with the E-cadherin–β-catenin complex. Although PTP1B was shown to interact with N-cadherin [14,15,18], evidence of its interaction with E-cadherin has not been found. The present study demonstrates that PTP1B does interact with the E-cadherin–β-catenin complex in Caco-2 cells, and that acetaldehyde disrupts this interaction of PTP1B with E-cadherin. It is likely that PTP1B interacts directly with E-cadherin, as it has been previously shown that PTP1B interacts directly with N-cadherin [13,14,17]. Previous studies showed that PTP1B and β-catenin bind to two adjacent regions of the N-cadherin sequence [21].
PTP1B bound to N-cadherin plays a role in the dephosphorylation of β-catenin on tyrosine residues [14]. The expression of dominant-negative PTP1B(C215S) increases the tyrosine phosphorylation of β-catenin and disrupts the interaction between E-cadherin and β-catenin [17]. Additionally, previous studies have also demonstrated that tyrosine phosphorylation of β-catenin prevents its interaction with E-cadherin, leading to disruption of cadherin-based cell–cell adhesion [15,16]. The present study shows that acetaldehyde-induced dissociation of PTP1B from E-cadherin–β-catenin complex is accompanied by an increase in tyrosine phosphorylation of β-catenin, and a loss of co-immunoprecipitation of β-catenin with E-cadherin. Loss of co-immunoprecipitation of β-catenin with E-cadherin by acetaldehyde was completely prevented by genistein, a tyrosine kinase inhibitor. Therefore dissociation of PTP1B may be responsible for acetaldehyde-induced tyrosine phosphorylation of β-catenin and disruption of the E-cadherin–β-catenin complex. Tyrosine phosphorylation of β-catenin and the loss of cadherin-based cell–cell adhesion are likely to play a crucial role in carcinogenesis and tumour metastasis [30]. Cervical cancer is associated with the reduced expression of E-cadherin and β-catenin [31]. β-Catenin was found to be highly tyrosine-phosphorylated in gastric cancer [32] and cervical cancer [31]. Therefore tyrosine phosphorylation and loss of interaction between E-cadherin and β-catenin and the disruption of AJ may play a crucial role in the acetaldehyde-induced promotion of carcinogenesis and tumour metastasis.
The loss of interaction between E-cadherin and β-catenin by acetaldehyde treatment was confirmed by GST pull-down assay. Interaction of GST–E-cadherin-C with β-catenin in acetaldehyde-treated cells was significantly lower than that in untreated cell extracts, indicating that the modification of β-catenin is responsible for the loss of interaction between E-cadherin and β-catenin in acetaldehyde-treated cells. This was further confirmed by a pairwise binding assay using non-phosphorylated and tyrosine-phosphorylated recombinant E-cadherin-C and β-catenin. While tyrosine phosphorylation of β-catenin attenuated its interaction with E-cadherin-C, tyrosine phosphorylation of E-cadherin-C did not reduce its interaction with β-catenin. These results demonstrate that it is the tyrosine phosphorylation of β-catenin rather than tyrosine phosphorylation of E-cadherin that mediates the acetaldehyde-induced loss of interaction between E-cadherin and β-catenin. The acetaldehyde-induced tyrosine phosphorylation of β-catenin may be mediated by the inhibition [10] and the dissociation of PTP1B from the E-cadherin–β-catenin complex.
The present study demonstrates that acetaldehyde treatment also results in a dramatic increase in tyrosine phosphorylation of E-cadherin. GST pull-down assay using non-phosphorylated and tyrosine-phosphorylated GST–E-cadherin-C demonstrates that tyrosine phosphorylation of GST–E-cadherin-C does not influence its interaction with β-catenin. However, both the GST pull-down assay and the pairwise binding assay indicate that acetaldehyde treatment and tyrosine phosphorylation of E-cadherin reduce interaction between E-cadherin and PTP1B. The loss of interaction between E-cadherin and PTP1B by tyrosine phosphorylation of E-cadherin suggests that such a mechanism may exist in acetaldehyde-treated cells and contribute to the acetaldehyde-induced tyrosine phosphorylation of β-catenin.
LC/MS/MS and Sequest analyses indicate that β-catenin in untreated Caco-2 cells is phosphorylated on Tyr-331, Tyr-333, Tyr-654 and Tyr-670, and the phosphorylation of these residues appears to be enhanced by acetaldehyde treatment. Previous studies showed that phosphorylation of β-catenin on Tyr-654 results in the loss of its interaction with E-cadherin [15,16]. Therefore it is likely that increased phosphorylation of Tyr-654 in β-catenin is involved in the acetaldehyde-induced disruption of the E-cadherin–β-catenin complex. It is not clear, at the moment, if phosphorylation of β-catenin on Tyr-331, Tyr-333 or Tyr-670 also plays a similar role in the disruption of the interaction between E-cadherin and β-catenin. It is however possible that the phosphorylation of Tyr-331, Tyr-333 or Tyr-670 may play a role in the regulation of interaction of β-catenin with α-catenin, actin or other AJ proteins.
The present study therefore shows that acetaldehyde disrupts the AJ in Caco-2 cell monolayers by disrupting the interaction between PTP1B and the E-cadherin–β-catenin complex and by inducing a loss of interaction between β-catenin and E-cadherin by a tyrosine phosphorylation-dependent mechanism. These mechanisms may play a role in the acetaldehyde-mediated disruption of epithelial barrier and promotion of carcinogenesis and tumour metastasis.
Acknowledgments
This study was supported by NIH grants AA12307 and DK55532 (to R. R.) and DRR14593 (to D. M. D.).
References
- 1.Salaspuro M. P. Acetaldehyde, microbes, and cancer of the digestive tract. Crit. Rev. Clin. Lab. Sci. 2003;40:183–208. doi: 10.1080/713609333. [DOI] [PubMed] [Google Scholar]
- 2.Homann N., Tillonen J., Meurman J. H., Rintamaki H., Lindqvist C., Rautio M., Jousimies-Somer H., Salaspuro M. Increased salivary acetaldehyde levels in heavy drinkers and smokers: a microbiological approach to oral cavity cancer. Carcinogenesis. 2000;21:663–668. doi: 10.1093/carcin/21.4.663. [DOI] [PubMed] [Google Scholar]
- 3.Visapaa J. P., Tillonen J., Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol. 2002;37:322–326. doi: 10.1093/alcalc/37.4.322. [DOI] [PubMed] [Google Scholar]
- 4.Poschl G., Seitz H. K. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 5.Salaspuro M. Bacteriocolonic pathway for ethanol oxidation: characteristics and implications. Ann. Med. 1996;28:195–200. doi: 10.3109/07853899609033120. [DOI] [PubMed] [Google Scholar]
- 6.Tillonen J., Vakevainen S., Salaspuro V., Zhang Y., Rautio M., Jousimies-Somer H., Kindros K., Salaspuro M. Metronidazole increases intracolonic but not peripheral blood acetaldehyde in chronic ethanol-treated rats. Alcohol. Clin. Exp. Res. 2000;24:570–575. [PubMed] [Google Scholar]
- 7.Koivisto T., Salaspuro M. Aldehyde dehydrogenases of the rat colon: comparison with other tissues of the alimentary tract and the liver. Alcohol. Clin. Exp. Res. 1996;20:551–555. doi: 10.1111/j.1530-0277.1996.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 8.Rao R. K., Seth A., Sheth P. Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am. J Physiol. Gastroinstest. Liver Physiol. 2004;286:G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 9.Rao R. K. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol. Clin. Exp. Res. 1998;22:1724–1730. [PubMed] [Google Scholar]
- 10.Atkinson K. J., Rao R. K. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol. Gastroinstest. Liver Physiol. 2001;280:G1280–G1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- 11.Sheth P., Seth A., Thangavel M., Basuroy S., Rao R. K. Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol. Clin. Exp. Res. 2004;28:797–804. doi: 10.1097/01.alc.0000125358.92335.90. [DOI] [PubMed] [Google Scholar]
- 12.Seth A., Basuroy S., Sheth P., Rao R. K. L-glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am. J. Physiol. Gastroinstest. Liver Physiol. 2004;287:G510–G517. doi: 10.1152/ajpgi.00058.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lilien J., Balsamo J., Arregui C., Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev. Dyn. 2002;224:18–29. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- 14.Balsamo J., Leung T., Ernst H., Zanin M. K., Hoffman S., Lilien J. Regulated binding of PTP1B-like phosphatase to N-cadherin: control of cadherin- mediated adhesion by dephosphorylation of beta-catenin. J. Cell Biol. 1996;134:801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roura S., Miravet S., Piedra J., Garcia de Herreros A., Dunach M. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 16.Piedra J., Martinez D., Castano J., Miravet S., Dunach M., Garcia de Herreros A. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J. Biol. Chem. 2001;276:20436–20443. doi: 10.1074/jbc.M100194200. [DOI] [PubMed] [Google Scholar]
- 17.Balsamo J., Arregui C., Leung T., Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin–actin linkage. J. Cell Biol. 1998;143:523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinch M. S., Clark G. J., Der C. J., Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J. Cell Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao R. K., Basuroy S., Rao V. U., Karnaky K. J., Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin–ZO-1 and E-cadherin–beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem. J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu P., O'Keefe E. J., Rubenstein D. S. Tyrosine phosphorylation of human keratinocyte beta-catenin and plakoglobin reversibly regulates their binding to E-cadherin and alpha-catenin. J. Invest. Dermatol. 2001;117:1059–1067. doi: 10.1046/j.0022-202x.2001.01523.x. [DOI] [PubMed] [Google Scholar]
- 21.Xu G., Arregui C., Lilien J., Balsamo J. PTP1B modulates the association of beta-catenin with N-cadherin through binding to an adjacent and partially overlapping target site. J. Biol. Chem. 2002;277:49989–49997. doi: 10.1074/jbc.M206454200. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo I. J., Raub T. J., Borchardt R. T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 23.Harder K. W., Owen P., Wong L. K., Aebersold R., Clark-Lewis I., Jirik F. R. Characterization and kinetic analysis of the intracellular domain of human protein tyrosine phosphatase beta (HPTP beta) using synthetic phosphopeptides. Biochem. J. 1994;298:395–401. doi: 10.1042/bj2980395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimm D. L., Morrow J. S. Molecular cloning of human E-cadherin suggests a novel subdivision of the cadherin super family. Biochem. Biophys. Res. Commun. 1994;200:1754–1761. doi: 10.1006/bbrc.1994.1656. [DOI] [PubMed] [Google Scholar]
- 25.Kale G., Naren A. P., Sheth P., Rao R. K. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem. Biophys. Res. Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 26.Gumbiner B., Stevenson B., Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R. D., Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidel B., Brae S., Adler G., Welch D., Mince A. E- and N-cadherin differ with respect to their associated p120ctn is forms and their ability to suppress invasive growth in pancreatic cancer cells. Ontogeny. 2004;23:5532–5542. doi: 10.1038/sj.onc.1207718. [DOI] [PubMed] [Google Scholar]
- 28.Brady-Kalona S. M., Rimm D. L., Tanks N. K. Receptor protein tyrosine phosphatase Stump associates with cadherins and catenins in vivo. J. Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller T., Choidas A., Reichmann E., Ullrich A. Phosphorylation and free pool of beta-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J. Biol. Chem. 1999;274:10173–10183. doi: 10.1074/jbc.274.15.10173. [DOI] [PubMed] [Google Scholar]
- 30.Hirohashi S., Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575–581. doi: 10.1111/j.1349-7006.2003.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon H. S., Park W. I., Choi E. A., Chung H. W., Kim S. C. The expression and tyrosine phosphorylation of E-cadherin/catenin adhesion complex, and focal adhesion kinase in invasive cervical carcinomas. Int. J. Gynecol. Cancer. 2003;13:640–646. doi: 10.1046/j.1525-1438.2003.13396.x. [DOI] [PubMed] [Google Scholar]
- 32.Ono H., Takeuchi Y., Ukegawa J., Kusayanagi S., Mitamura K., Imawari M. Increased tyrosine kinase activities may lead to the phenotypic differences of gastric cancer cells. Anticancer Res. 2004;24:699–705. [PubMed] [Google Scholar]