Abstract
Traditionally, the dependence of enzyme activity on temperature has been described by a model consisting of two processes: the catalytic reaction defined by ΔGDaggercat, and irreversible inactivation defined by ΔGDaggerinact. However, such a model does not account for the observed temperature-dependent behaviour of enzymes, and a new model has been developed and validated. This model (the Equilibrium Model) describes a new mechanism by which enzymes lose activity at high temperatures, by including an inactive form of the enzyme (Einact) that is in reversible equilibrium with the active form (Eact); it is the inactive form that undergoes irreversible thermal inactivation to the thermally denatured state. This equilibrium is described by an equilibrium constant whose temperature-dependence is characterized in terms of the enthalpy of the equilibrium, ΔHeq, and a new thermal parameter, Teq, which is the temperature at which the concentrations of Eact and Einact are equal; Teq may therefore be regarded as the thermal equivalent of Km. Characterization of an enzyme with respect to its temperature-dependent behaviour must therefore include a determination of these intrinsic properties. The Equilibrium Model has major implications for enzymology, biotechnology and understanding the evolution of enzymes. The present study presents a new direct data-fitting method based on fitting progress curves directly to the Equilibrium Model, and assesses the robustness of this procedure and the effect of assay data on the accurate determination of Teq and its associated parameters. It also describes simpler experimental methods for their determination than have been previously available, including those required for the application of the Equilibrium Model to non-ideal enzyme reactions.
Keywords: enzyme activity, Equilibrium Model, kinetics, stability, thermal-dependence
Abbreviations: Eact, active enzyme; Einact, inactive enzyme; pNAA, p-nitroacetanilide; pNPP, p-nitrophenylphosphate; Teq, temperature at which the concentrations of Eact and Einact are equal; Topt, temperature optimum
INTRODUCTION
The effect of temperature on enzyme activity has been described by two well-established thermal parameters: the Arrhenius activation energy, which describes the effect of temperature on the catalytic rate constant, kcat, and thermal stability, which describes the effect of temperature on the thermal inactivation rate constant, kinact. Anomalies arising from this description have been resolved by the development [1] and validation [2] of a new model (the Equilibrium Model) that more completely describes the effect of temperature on enzyme activity by including an additional mechanism by which enzyme activity decreases as the temperature is raised. In this model, the active form of the enzyme (Eact) is in reversible equilibrium with an inactive (but not denatured) form (Einact), and it is the inactive form that undergoes irreversible thermal inactivation to the thermally denatured state (X):
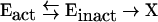 |
Figure 1 shows the most obvious graphical effect of the Model, which is a temperature optimum (Topt) at zero time (Figures 1A and 1B), matching experimental observations [2]. In contrast, the ‘Classical Model’, which assumes a simple two-state equilibrium between an active and a thermally-denatured state (Eact→X), and can be described in terms of only two parameters (the Arrhenius activation energy and the thermal stability), shows that when the data are plotted in three dimensions there is no Topt at zero time (Figure 1C).
Figure 1. The temperature-dependence of enzyme activity.
(A) Experimental data for alkaline phosphatase. The enzyme was assayed as described by Peterson et al. [2], and the data were smoothed as described here in the Experimental section; the data are plotted as rate (μM·s−1) against temperature (K) against time during assay (s). (B) The result of fitting the experimental data for alkaline phosphatase to the Equilibrium Model. Parameter values derived from this fitting are: ΔG‡cat, 57 kJ·mol−1; ΔG‡inact, 97 kJ·mol−1; ΔHeq, 86 kJ·mol−1; Teq, 333 K [2]. (C) The result of running a simulation of the Classical Model using the values of ΔG‡cat and ΔG‡inact derived from the fitting described above. The experimental data itself cannot be fitted to the Classical Model.
In addition to the obvious differences in the graphs representing the two models, it has been observed experimentally that at any temperature above the maximum enzyme activity, the loss of activity attributable to the shift in the Eact/Einact equilibrium is very fast (<1 s) relative to the loss of activity due to thermal denaturation (shown in Figure 1 by the lines of rate against time) [2]. This and other evidence to date [2] suggest that the phenomenon described by the model (i.e. the Eact/Einact equilibrium) arises from localized conformational changes rather than global changes in structure. However, the extent of the conformational change, and the extent to which it could be described as a partial unfolding, is not yet established.
The equilibrium between the active and inactive forms of the enzyme can be characterized in terms of the enthalpy of the equilibrium, ΔHeq, and a new thermal parameter, Teq, which is the temperature at which the concentrations of Eact and Einact are equal; Teq can therefore be regarded as the thermal equivalent of Km. Teq has both fundamental and technological significance. It has important implications for our understanding of the effect of temperature on enzyme reactions within the cell and of enzyme evolution in response to temperature, and will possibly be a better expression of the effect of environmental temperature on the evolution of the enzyme than thermal stability. Teq thus provides an important new parameter for matching an enzyme's properties to its cellular and environmental function. Teq must also be considered in engineering enzymes for biotechnological applications at high temperatures [3]. Enzyme engineering is frequently directed at stabilizing enzymes against denaturation; however, raising thermal stability may not enhance high temperature activity if Teq remains unchanged.
The detection of the reversible enzyme inactivation, which forms the basis of the Equilibrium Model, requires careful acquisition and processing of assay data due to the number of conflicting influences that arise when increasing the temperature of an enzyme assay. Determination of Teq to date has used continuous assays, because this method produces progress curves directly and obviates the need to perform separate activity and stability experiments, and has utilized enzymes whose reactions are essentially irreversible (far from reaction equilibrium), do not show any substrate or product inhibition and remain saturated with substrate throughout the assay. However, there are a large number of enzymes that do not fit these criteria, narrowing the potential utility of determining Teq. The present paper describes methods for the reliable determination of Teq under ideal or non-ideal enzyme reaction conditions, using either continuous or discontinuous assays, and outlines the assay data required for accurate determination of Teq and the thermodynamic constants (ΔG‡cat, ΔG‡inact and ΔHeq) associated with the model [2]; it also introduces a method of fitting progress curves directly to the Equilibrium Model and determines the robustness of the data-fitting procedures. The results show directly how the Equilibrium Model parameters are affected by the data.
The methods described in the present paper allow the determination of the new parameters ΔHeq and Teq, required for any description of the way in which temperature affects enzyme activity. In addition, they facilitate the straightforward and simultaneous determination of ΔG‡cat and ΔG‡inact under relatively physiological conditions. They therefore have the potential to be of considerable value in the pure and applied study of enzymes.
EXPERIMENTAL
Materials
Aryl-acylamidase (aryl-acylamide amidohydrolyase; EC 3.5.1.13) from Pseudomonas fluorescens, β-lactamase (β-lactamhydrolase; EC 3.5.2.6) from Bacillus cereus and pNPP (p-nitrophenylphosphate) were purchased from Sigma–Aldrich. pNAA (p-nitroacetanilide) was obtained from Merck, wheat germ acid phosphatase [orthophosphoric-monoester phosphohydrolase (acid optimum); EC 3.1.3.2] from Serva Electrophoresis and nitrocefin from Oxoid. All other chemicals used were of analytical grade.
Instrumentation
All enzymic activities were measured using a Thermospectronic™ Helios γ-spectrophotometer equipped with a Thermospectronic™ single-cell Peltier-effect cuvette holder. This system was networked to a computer installed with Vision32™ (version 1.25, Unicam) software including the Vision Enhanced Rate program capable of recording absorbance changes over time intervals down to 0.125 s.
Temperature control
The temperature of each assay was recorded directly, using a Cole-Parmer Digi-Sense® thermocouple thermometer accurate to ±0.1% of the reading and calibrated using a Cole–Parmer NIST (National Institute of Standards and Technology)-traceable high-resolution glass thermometer. The temperature probe was placed inside the cuvette adjacent to the light path during temperature equilibration before the initiation of the reaction and again immediately after completion of each enzyme reaction. Measurements of temperature were also taken at the top and bottom of the cuvette to check for temperature gradients. Where the temperature measured before and after the reaction differed by more than 0.1 °C, the reaction was repeated.
Assay conditions
Assays at high temperature (and over any wide temperature range) can sometimes pose special problems and may need additional care [4–6]. Quartz cuvettes were used in all experiments for their relatively quick temperature equilibration and heat-retaining capacity. Where required, a plastic cap was fitted to the cuvette to prevent loss of solvent due to evaporation (at higher temperatures), or a constant stream of a dry inert gas (e.g. nitrogen) was blown across the cuvette to prevent condensation at temperatures below ambient. Buffers were adjusted to the appropriate pH value at the assay temperature, using a combination electrode calibrated at this temperature. Where very low concentrations of enzyme were used, salts or low concentrations of non-ionic detergents were added to prevent loss of protein to the walls of the cuvette.
Substrate concentrations were maintained at not less than 10 times the Km to ensure that the enzyme remained saturated with substrate for the assay duration. Where these concentrations could not be maintained (e.g. because of substrate solubility), tests were conducted to confirm that there was no decrease in rate over the assay period arising from substrate depletion. In addition, Km values over the full temperature range examined were determined. Since Km values for enzymes tend to rise with temperature [7,8], in some cases dramatically, this is particularly important. Any decrease in rate at higher temperatures that is caused by an increase in Km at higher temperatures is a potential source of large errors.
Assay reactions were initiated by the rapid addition of a few microlitres of chilled enzyme, so that the addition had no significant effect on the temperature of the solution inside the cuvette.
Enzyme assays
Aryl-acylamidase activity was measured by following the increase in absorbance at 382 nm (ϵ382=18.4 mM−1·cm−1) corresponding to the release of p-nitroaniline from the pNAA substrate [9]. Reaction mixtures contained 0.1 M Tris/HCl, pH 8.6, 0.75 mM pNAA and 0.003 units of enzyme. One unit is defined as the amount of enzyme required to catalyse the hydrolysis of 1 μmol of pNAA per min at 37 °C.
Acid phosphatase activity was measured discontinuously using pNPP as substrate [10]. Reaction mixtures (1 ml) contained 0.1 M sodium acetate, pH 5.0, 10 mM pNPP and 8 μ-units of enzyme. The assay was stopped using 0.5 ml of 1 M NaOH. The amount of p-nitrophenol released was measured at 410 nm (ϵ410=18.4 mM−1·cm−1). One unit is defined as the amount of enzyme that hydrolyses 1 μmol of pNPP to p-nitrophenol per min at 37 °C.
β-Lactamase activity was measured by following the increase in absorbance at 485 nm (ϵ485=20.5 mM−1·cm−1) associated with the hydrolysis of the β-lactam ring of nitrocefin [11]. Reaction mixtures contained 0.05 M sodium phosphate, pH 7.0, 1 mM EDTA, 0.1 mM nitrocefin and 0.003 units of enzyme. One unit is defined as the amount of enzyme that will hydrolyse the β-lactam ring of 1 μmol of cephalosporin per min at 25 °C.
Protein determination
Protein concentrations claimed by the manufacturers (determined by Biuret) were checked using the far-UV method of Scopes [12].
Data capture and analysis
For each enzyme, reaction-progress curves at a variety of temperatures were collected; the time interval was set so that an absorbance reading was collected every 1 s. Three progress curves were collected at each temperature; where the slope for these triplicates deviated by more than 10%, the reactions were repeated.
When required, the initial (zero time) rate of reaction for each assay triplicate was determined using the linear search function in the Vision32™ rate program.
Although earlier determinations of ΔG‡cat, ΔG‡inact, ΔHeq and Teq used initial parameter estimates derived from the calculation of rates from progress curves (described in [2]), more recent analysis of results indicates that the method described below is simpler and equally accurate.
Using the values for ΔG‡cat (80 kJ·mol−1), ΔG‡inact (95 kJ·mol−1), ΔHeq (100 kJ·mol−1) and Teq (320 K) described in the original paper [1] as initial parameter estimates (deemed to be ‘typical’ or ‘plausible’ values for each of the parameters) and the concentration of protein in each assay (expressed in mol·l−1), the experimental data were fitted to the Equilibrium Model using MicroMath® Scientist® for Windows software (version 2.01, MicroMath Scientific Software).
The values for each parameter were first ‘improved’ by Simplex searching [13,14]. The experimental data were then fitted to the Equilibrium Model using the parameters derived from the Simplex search, employing an iterative non-linear minimization of least squares. This minimization utilizes Powell's algorithm [15] to find a local minimum, possibly a global minimum, of the sum of squared deviations between the experimental data and the model calculations.
In each case, the fitting routine was set to take minimum and maximum iterative step-sizes of 1×10−12 and 1 respectively. The sum of squares goal (the termination criterion for the fitting routine) was set to 1×10−12.
The S.D. values in the Tables refer to the fit of the data to the model. On the basis of the variation between the individual triplicate rates from which the parameters are derived for all the enzymes we have assayed so far, we find that the experimental errors in the determination of ΔG‡cat, ΔG‡inact and Teq are less than 0.5%, and less than 6% in the determination of ΔHeq.
A stand-alone Matlab® [version 7.1.0.246 (R14) Service Pack 3; Mathworks] application, enabling the facile derivation of the Equilibrium Model parameters from a Microsoft® Office Excel file of experimental progress curves (product concentration against time) can be obtained on CD from R.M.D. This application is suitable for computers running Microsoft® Windows XP, and is for non-commercial research purposes only.
RESULTS AND DISCUSSION
The Equilibrium Model has four data inputs: enzyme concentration, temperature, concentration of product and time. From the last two, an estimate of the rate of reaction (in M·s−1) can be obtained. In describing the effect of temperature on catalytic activity, the rate of the catalytic reaction is the measurement of interest. The quantitative expression of the dependence of rate on temperature, T, and time, t, is given by eqn (1):
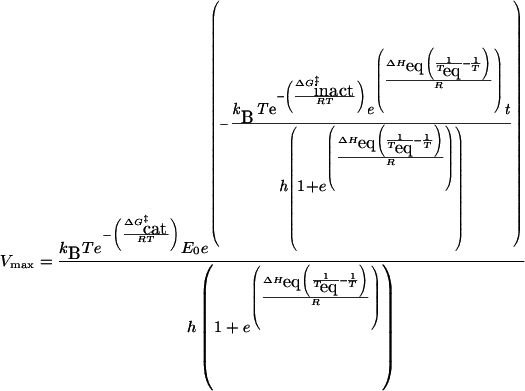 |
(1) |
where kB is Boltzmann's constant and h is Planck's constant. This is the expression that we have used in our proposal [1] and validation [2] of the Equilibrium Model to date. Experimentally, however, rates are rarely measured directly; rather, product concentration is determined at increasing times, either by continuous or discontinuous assay, giving a series of progress curves. The quantitative expression relating the product concentration, time and temperature for the Equilibrium Model can be obtained by integrating eqn (1), giving eqn (2):
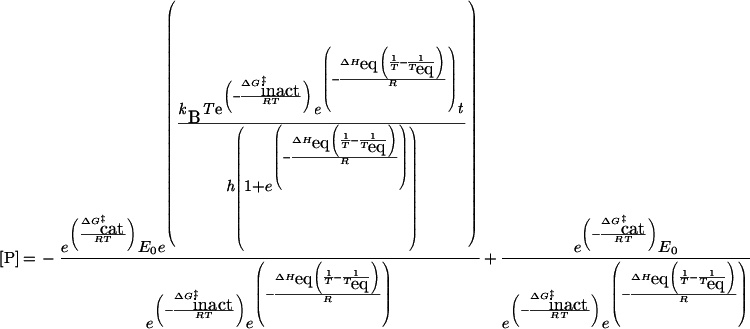 |
(2) |
We find that data processed as enzyme rates using eqn (1), or as product concentration changes using eqn (2), give essentially the same results. However, since eqn (2) involves a more direct measurement, the experimental protocol used in the present study involves measuring progress curves of product concentration against time at different temperatures and fitting these data to eqn (2).
Robustness of the fitted constants
If the enzyme preparation used in the determination of Teq is not pure, then overestimation of the enzyme concentration is likely. Few methods of determining protein concentration give answers that are correct in absolute terms; apart from any limitations in terms of sensitivity and interferences, most are based on a comparison with a standard of uncertain equivalence to the enzyme under investigation. The determination of enzyme concentration is thus a potential source of error.
To determine how dependent the fitted constants are on the accuracy of the enzyme concentration, data for β-lactamase [2] were fitted against the experimentally determined progress curves with the enzyme concentration reduced 2-, 5- and 10-fold compared with that determined experimentally (Table 1). It is evident that errors in determining enzyme concentration have little effect upon parameter determination, except, of course, in respect of ΔG‡cat, which is reduced as the model attempts to relate the reduced enzyme concentration to the observed rates of reaction. Even with changing the enzyme concentration 5-fold, errors in the values for ΔG‡inact, ΔHeq and Teq are small.
Table 1. The effect of input data accuracy on parameter values.
The experimental data for β-lactamase were used to generate the Equilibrium Model parameters as described in the Experimental section. Changes were then made to the experimentally determined enzyme concentration to determine the dependence of the fitted constants on the accuracy of the protein concentration. Parameter values are ±S.D.
| Parameter | Determined [E0] | [E0] reduced 2-fold | [E0] reduced 5-fold | [E0] reduced 10-fold |
|---|---|---|---|---|
| ΔG‡cat (kJ·mol−1) | 68.9±0.01 | 67.1±0.01 | 64.8±0.01 | 63.0±0.01 |
| ΔG‡inact (kJ·mol−1) | 93.7±0.08 | 93.6±0.07 | 93.4±0.07 | 93.4±0.07 |
| ΔHeq (kJ·mol−1) | 138.2±1.1 | 139.4±1.1 | 140.2±1.1 | 144.2±1.1 |
| Teq (K) | 325.6±0.1 | 326.2±0.1 | 327.0±0.1 | 327.6±0.1 |
| [E0] (M) | 5.5×10−9 | 2.75×10−9 | 1.1×10−9 | 0.55×10−9 |
Data sampling requirements: sampling rate
The increasing need for automation in enzyme assays has led to the development of instruments that use sampling techniques to assay enzymes at different times. Additionally, some assays are difficult to carry out continuously. It is therefore important to know whether the fitting procedures described herein are sufficiently robust to deal with discontinuous data collection. To determine the sampling requirement, progress curves for the reaction catalysed by aryl-acylamidase were collected in triplicate at 1 s intervals over a 25 min period at a variety of temperatures. Progress curves were then manipulated by the successive removal of a proportion of the data points to determine the effect of sampling rate on the fitting of the data to the Equilibrium Model and on the resulting parameters (Table 2). Using the 1 s sampling interval as a reference, the absolute values of ΔG‡cat, ΔG‡inact, ΔHeq and Teq are essentially the same at all sampling rates (up to a 150 s interval), despite the increase in the S.D. values as the sampling interval increases. The results indicate that discontinuous enzyme assays can be used for the determination of Teq. The minimum number of points per progress curve required to give accurate values for the parameters will depend upon the length of the assay and the curvature of the progress curve, but, as expected, the larger number of data points arising from continuous assays give more accurate results. The results also show that accuracy is not dominated by a requirement for ‘early’ data, taken very soon after zero time, and that the S.D. provides a good guide to the accuracy of the parameters.
Table 2. Data sampling requirements: the effect of sampling rate on parameter values.
Progress curves for aryl-acylamidase, collected over 25 min and at ten different temperatures, were used to generate the Equilibrium Model parameters as described in the Experimental section. Experimental data points were then successively removed to give the effect of reduced frequency of data points to determine the effect of various sampling rates on the final parameter values. Parameter values are means±S.D.
| Sampling interval (s)… | 1 | 5 | 20 | 60 | 150 | |
|---|---|---|---|---|---|---|
| Parameter | Data points per progress curve… | 1500 | 300 | 75 | 25 | 10 |
| ΔG‡cat (kJ·mol−1) | 74.4±0.01 | 74.4±0.02 | 74.4±0.03 | 74.4±0.06 | 74.4±0.09 | |
| ΔG‡inact (kJ·mol−1) | 94.5±0.04 | 94.5±0.09 | 94.5±0.18 | 94.5±0.31 | 94.5±0.48 | |
| ΔHeq (kJ·mol−1) | 138.5±0.6 | 138.5±1.4 | 138.5±2.8 | 138.7±4.8 | 138.8±7.4 | |
| Teq (K) | 310.0±0.1 | 310.0±0.1 | 310.0±0.2 | 310.0±0.3 | 310.0±0.5 |
The results presented above imply that the parameters can be obtained accurately from as few as ten data points (sampling only every 150 s in the case of the 1500 s aryl-acylamidase assays). We would expect the ‘data sampling’ shown in Table 2 to be a satisfactory proxy for a discontinuous assay. However, this was confirmed using another enzyme. Acid phosphatase was incubated with the substrate pNPP for a total assay duration of 30 min, and the reaction was sampled in triplicate every 60 s, stopped with NaOH, and the absorbance was read at 410 nm. Three progress curves (absorbance against time) at each temperature were generated from the triplicate absorbance values obtained when the reaction was stopped. Product concentrations (expressed in mol·l−1) were then calculated for each absorbance reading, and the data set was fitted to the Equilibrium Model as described previously and compared with data obtained in a continuous assay [2]. Taking experimental error into account, the parameter values generated from fitting these data (Table 3) indicate no significant difference between the two methods, except in the case of ΔG‡inact. The increased value of the errors on each parameter determined using the discontinuous data indicate that, as expected, continuous assays give more accurate results.
Table 3. Final parameter values for an enzyme employing a discontinuous assay.
Acid phosphatase was assayed discontinuously over a period of 30 min with a sampling rate of 60 s and at 5 °C intervals from 20 to 80 °C (13 temperature points). The results of fitting data for the same enzyme over the same temperature range and using the same intervals, but using a continuous assay (effective sampling rate of 1 s) have been included for comparison [2]. The progress curves generated for both methods were fitted to the Equilibrium Model and the parameters generated as described in the Experimental section. Parameter values are means±S.D.
| Parameter | Discontinuous assay | Continuous assay |
|---|---|---|
| ΔG‡cat (kJ·mol−1) | 79.0±0.02 | 79.1±0.01 |
| ΔG‡inact (kJ·mol−1) | 96.1±0.23 | 94.5±0.04 |
| ΔHeq (kJ·mol−1) | 146.0±2.2 | 142.5±0.5 |
| Teq (K) | 333.6±0.5 | 336.9±0.1 |
Data sampling requirements: temperature range
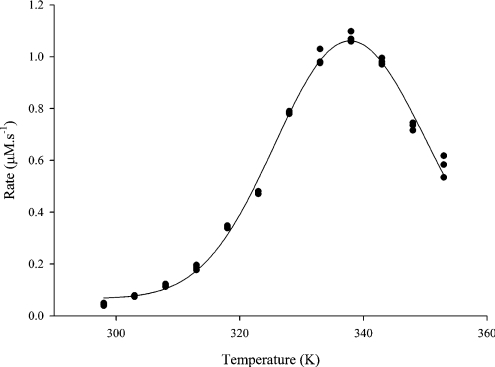
Progress curves at 12 temperatures were collected for acid phosphatase [2]. Analysis of the initial rate of reaction (i.e. at zero time) shows three points above the temperature at which maximum product is formed (Figure 2). By sequentially truncating the data set from the highest or the lowest temperature point and re-fitting the resulting data sets, we gain some insight into the dependence of the fitting routine and accurate estimation of the parameters on the data points above and below the Topt (Table 4).
Figure 2. The effect of temperature on the initial (zero-time) rate of reaction of acid phosphatase.
Acid phosphatase was assayed continuously as described by Peterson et al. [2]. For each triplicate progress curve, the initial rate of reaction was determined using the linear search function in the programme, Vision32™. The data are plotted as rate (μM·s−1) against temperature (K).
Table 4. Data sampling requirements: the effect of temperature range on parameter values.
A full set of experimental data for acid phosphatase was used to generate the Equilibrium Model parameters as described in the Experimental section. Temperature points above the Topt (Figure 2) were sequentially truncated from the complete data set to determine the influence of data points above the Topt on the final parameter values. Temperature points were also sequentially truncated from the lowest temperature point to the highest from the complete data set (12 temperature points) to determine how many points, in total, below the Topt are required for the accurate determination of Teq and the other thermodynamic parameters. In this case, each data set included all temperature points above the Topt. Parameter values are means±S.D.
| Truncated from highest temperature point | Truncated from lowest temperature point | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Minus three temperature points (nine points) | Minus two temperature points (ten points) | Minus one temperature point (11 points) | Full data set (12 points) | Minus two temperature points (ten points) | Minus four temperature points (eight points) | Minus six temperature points (six points) |
| ΔG‡cat (kJ·mol−1) | 78.8±0.01 | 79.1±0.01 | 79.1±0.01 | 79.1±0.01 | 79.1±0.01 | 79.0±0.01 | 79.3±0.02 |
| ΔG‡inact (kJ·mol−1) | 94.3±0.03 | 94.6±0.05 | 94.6±0.05 | 94.5±0.04 | 94.5±0.05 | 94.5±0.05 | 94.2±0.06 |
| ΔHeq (kJ·mol−1) | 108.5±0.5 | 148.8±0.7 | 146.5±0.5 | 142.5±0.5 | 142.8±0.6 | 142.1±0.7 | 149.8±0.8 |
| Teq (K) | 337.3±0.1 | 336.8±0.1 | 336.8±0.1 | 336.9±0.1 | 337.0±0.1 | 336.9±0.1 | 338.3±0.2 |
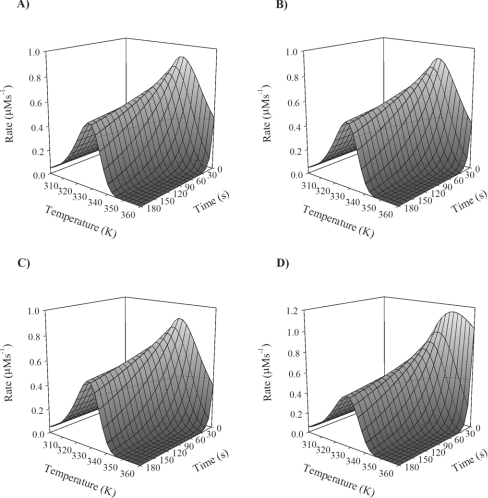
For data truncated from the highest temperature point, the values of ΔG‡cat, ΔG‡inact and Teq do not vary greatly with the various data treatments. However, for the fit excluding the last three temperature points, there is a substantial loss in accuracy for the Equilibrium Model parameter, ΔHeq. This difference is not reflected in the S.D. values. Figure 3, which illustrates the differences in each fitting of the truncated data sets to the Equilibrium Model presented as a three-dimensional plot of rate (μM·s−1) against temperature (K) against time (s), shows the reason for this. The plots indicate that when only one or two data points are removed, there is little difference in the shape of the plot when the data are simulated in three dimensions, but without a data point above the Topt, the equilibrium model effectively relapses towards the Classical Model (Figure 1), with a sharp decline in ΔHeq, even though a reasonable value for Teq has been obtained. These results suggest that it is possible to obtain acceptable estimates of the parameters with only one temperature point above the Topt.
Figure 3. Data sampling requirements: the effect of data points beyond Topt.
Acid phosphatase was assayed as described by Peterson et al. [2]. Temperature points above the Topt (see Figure 2) were sequentially truncated from the complete data set to determine the influence of data points above the Topt on the final parameter values. Illustrated here are the results plotted as rate (μM·s−1) against temperature (K) against time (s) for the fit of acid phosphatase data to the Equilibrium Model using (A) the full data set, (B) the data set excluding the last data point, (C) the data set excluding the last two data points, and (D) the data set excluding the last three data points.
All the foregoing discussion is based on an ab initio presumption that the temperature-dependence of enzyme activity is described by the Equilibrium Model. Of the 50 or so enzymes studied in detail by us, all follow the model. However, data that do not show clear evidence of a Topt when initial (zero time) rates are plotted against temperature may in fact be fitted equally well by the simpler Classical Model. In this situation, it would be foolhardy to carry out the procedure described in the present paper. It must therefore be stressed that if only one or two points above the Topt are determined, the measured initial rates at those temperatures must be sufficiently lower than that at Topt for the assumption of the Equilibrium Model to be justified. Ideally, two or more rate measurements above Topt showing a clear trend of falling rates should be obtained to apply the Equilibrium Model with confidence.
For data sequentially truncated from the lowest temperature point to the highest, a trend in the parameter values was seen (Table 4). Parameter values were maintained close to the ‘complete’ data set level down to eight points; below eight points, values moved outside the S.D. limits, but were still relatively close in all cases.
The results of this data manipulation suggest that data at eight temperatures with two points above Topt (showing a clear downwards trend) are sufficient to yield parameter values for ΔG‡cat, ΔG‡inact, ΔHeq and Teq with reasonable precision.
Enzymes operating under ‘non-ideal’ conditions: the use of initial rates
To use data from progress curves collected over extended periods of time for valid fitting to the Equilibrium Model requires that any decrease in activity observed is due solely to thermal factors and not to some other process. This means that the enzyme and its reaction be ‘ideal’; that is, the enzyme is not product inhibited, the reaction is essentially irreversible and the enzyme operates at Vmax for the entire assay. To date, the enzymes that we have fitted to the Equilibrium Model have been chosen to meet, or come very close to meeting, these criteria over the 3–5 min duration of the assay.
However, many enzyme reactions are necessarily assayed under non-ideal conditions. For example, the reaction may be sufficiently reversible that the back reaction contributes to the observed rate during the assay and/or the products of the reaction may be inhibitors of the enzyme. Application of the Equilibrium Model to these non-ideal enzyme reactions can usually be achieved by restricting assays to the initial rate of reaction. Setting t=0 in eqn (1) gives eqn (3) below. Using this, it is possible to fit the experimental data for zero time (i.e. initial rates) to the Equilibrium Model to determine ΔG‡cat, ΔHeq and Teq, although the time-dependent thermal denaturation parameter, ΔG‡inact, cannot be determined. At t=0,
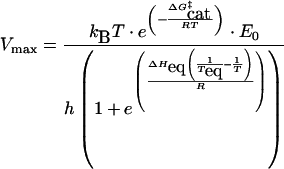 |
(3) |
Another circumstance where ‘non-ideality’ may occur is when the decrease of rate during the assay is partially due to substrate depletion. If the enzyme is saturated at the start of the assay, lowering the enzyme concentration or increasing the sensitivity of the assay may remove this problem. In either case, using initial rates will allow the equilibrium model to be applied. However, if insufficient substrate is present at zero time to saturate the enzyme, either because of, e.g., solubility limitations, or as a result of increases in Km [7,8] as the temperature is altered, then considerable errors may arise. Even here, it may be possible, if the Km is known at each temperature, to obtain reasonable approximations of the initial rates at saturation by calculating the degree of saturation using the relationship v/Vmax=S/(Km+S), and applying the appropriate corrections.
To simulate the determination of the Equilibrium Model parameters for an enzyme that operates under non-ideal conditions, initial rates of reaction were calculated from each progress curve in the β-lactamase data set [2] and fitted to the modified zero-time version of the Equilibrium Model using the Scientist® software (Table 5). No significant differences in any of the parameters determined this way were found, suggesting that this manner of determination is potentially as accurate as fitting the entire time course to the Equilibrium Model for the determination of ΔG‡cat, ΔHeq and Teq.
Table 5. The use of initial rate data to determine parameters associated with the Equilibrium Model.
To simulate the determination of the Equilibrium Model parameters for an enzyme that operates under non-ideal conditions, initial rates of reaction were calculated from each progress curve in the β-lactamase data set [2] using the linear search function in the programme, Vision32™, and fitted to the Equilibrium Model via eqn (3). Parameters calculated for the complete data set (entire time course) have been included for comparison [2]. Parameter values are means±S.D.
| Parameter | Progress curves | Initial rates |
|---|---|---|
| ΔG‡cat (kJ·mol−1) | 68.9±0.01 | 68.9±0.22 |
| ΔG‡inact (kJ·mol−1) | 93.7±0.08 | − |
| ΔHeq (kJ·mol−1) | 138.2±1.1 | 132.2±12.4 |
| Teq (K) | 325.6±0.1 | 325.6±1.3 |
Conclusions
To date, determination of the parameters associated with the Equilibrium Model for individual enzymes has involved continuous assays with collection of data at 1 s intervals over 5 min periods at 2–3 °C temperature intervals over at least a 40 °C range, with each temperature run being carried out in triplicate; i.e. processing approx. 15000 data points gathered in approx. 50 experimental runs [2]. Using a simple technique of fitting the raw data (product concentration against time) to the Equilibrium Model, we have shown that data collection (and thus labour) can be reduced considerably without compromising the accuracy of the derived parameters. Accurate results require preferably more than one data point taken above Topt and more than eight temperature points in total. Major errors in enzyme determination affect only the determination of ΔG‡cat. Although continuous assays will give the most accurate results, ΔG‡cat, ΔG‡inact, ΔHeq and Teq can be determined accurately using discontinuous assays. Among other things, this will allow the determination of the parameters of enzymes from extreme thermophiles; since Topt for these enzymes may be above 100 °C, and since few continuous assay methods are practical at such temperatures, most such assays will have to be discontinuous [4]. Finally, we have demonstrated that the use of initial, zero-time rates enables the ready determination of the Equilibrium Model parameters (except ΔG‡inact) of most non-ideal enzyme reactions.
The method described here enables the determination of the new fundamental enzyme thermal parameters arising from the Equilibrium Model. It should be noted that the Equilibrium Model itself enables an accurate description of the effect of temperature on enzyme activity, but does not purport to describe the molecular basis of this behaviour. Evidence so far ([2], and M. E. Peterson, C. K. Lee, C. Monk and R. M. Daniel, unpublished work) suggests that the conformational changes between the active and inactive forms of the enzyme described by the model are local rather than global, and possibly quite slight. The model, and the work described here, provides the foundation, and one of the tools needed to determine the molecular basis of these newly described properties of enzymes. The focus of future work must now be to apply the appropriate physicochemical techniques to determine the precise nature of this proposed structural change.
Acknowledgments
This work was supported by the Royal Society of New Zealand's International Science and Technology Linkages Fund, and the Marsden Fund.
References
- 1.Daniel R. M., Danson M. J., Eisenthal R. The temperature optima of enzymes: a new perspective on an old phenomenon. Trends Biochem. Sci. 2001;26:223–225. doi: 10.1016/s0968-0004(01)01803-5. [DOI] [PubMed] [Google Scholar]
- 2.Peterson M. E., Eisenthal R., Danson M. J., Spence A., Daniel R. M. A new, intrinsic, thermal parameter for enzymes reveals true temperature optima. J. Biol. Chem. 2004;279:20717–20722. doi: 10.1074/jbc.M309143200. [DOI] [PubMed] [Google Scholar]
- 3.Eisenthal R., Peterson M. E., Daniel R. M., Danson M. J. The thermal behaviour of enzymes: implications for biotechnology. Trends Biotechnol. 2006;24:289–292. doi: 10.1016/j.tibtech.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Daniel R. M., Danson M. J. Assaying activity and assessing thermostability of hyperthermophilic enzymes. Methods Enzymol. 2001;334:283–293. doi: 10.1016/s0076-6879(01)34476-2. [DOI] [PubMed] [Google Scholar]
- 5.Tipton K. F. Principles of enzymes assays and kinetic studies. In: Eisenthal R., Danson M. J., editors. Enzyme Assays, a Practical Approach. Oxford: Oxford University Press; 1992. pp. 1–58. [Google Scholar]
- 6.John R. A. Photometric Assays. In: Eisenthal R., Danson M. J., editors. Enzyme Assays, a Practical Approach. Oxford: Oxford University Press; 1992. pp. 59–92. [Google Scholar]
- 7.Thomas T. M., Scopes R. K. The effects of temperature on the kinetics and stability of mesophilic and thermophilic 3-phosphoglycerate kinases. Biochem. J. 1998;330:1087–1095. doi: 10.1042/bj3301087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang Z., Tsigos I., Bouriotis V., Klinman J. P. Impact of protein flexibility on hydride-transfer parameters in thermophilic and psychrophilic alcohol dehydrogenases. J. Am. Chem. Soc. 2004;126:9500–9501. doi: 10.1021/ja047087z. [DOI] [PubMed] [Google Scholar]
- 9.Hammond P. M., Price C. P., Scawen M. D. Purification and properties of aryl acylamidase from Pseudomonas fluorescens ATCC 39004. Eur. J. Biochem. 1983;132:651–655. doi: 10.1111/j.1432-1033.1983.tb07413.x. [DOI] [PubMed] [Google Scholar]
- 10.Hollander V. P. Acid phosphatases. In: Boyer P. D., editor. The Enzymes, vol. 4. New York: Academic Press; 1971. pp. 449–498. [Google Scholar]
- 11.O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1972;1:283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scopes R. K. Analysis – Measurement of protein and enzyme activity. In: Cantor C. R., editor. Protein Purification: Principles and Practice. 3rd edn. San Diego: Springer Verlag; 1994. pp. 44–50. [Google Scholar]
- 13.Deming S. N., Morgan S. L. Simplex optimization of variables in analytical chemistry. Anal. Chem. 1973;45:278A–283A. [Google Scholar]
- 14.Caceci M. S., Cacheris W. P. Fitting curves to data: the simplest algorithm is the answer. Byte. 1984;9:340–362. [Google Scholar]
- 15.Powell M. J. D. A hybrid method for non-linear equations. In: Rabinowitz P., editor. Numerical Methods for Nonlinear Algebraic Equations. New York: Gordon and Breach Science Publishers; 1970. pp. 87–144. [Google Scholar]