Abstract
Objectives
Biochemical markers reflecting the degradation of the type II collagen helical (Helix‐II) and type II collagen C telopeptides (CTX‐II) have been developed.
Aim
To investigate the association of rapidly destructive hip osteoarthritis with urinary Helix‐II and urinary CTX‐II.
Patients and methods
12 patients (mean age 70 years) meeting the criteria for rapidly destructive hip osteoarthritis and 28 patients with slowly progressive hip osteoarthritis (mean age 63 years) defined as <0.20 mm joint space loss/year were included in a case–control study. In each patient, urinary Helix‐II and CTX‐II were measured at the end of the follow‐up period, with retrospective evaluation of x rays.
Results
Helix‐II levels were 41% (p = 0.002) higher in the 40 patients with hip osteoarthritis than in 75 healthy controls. Increased Helix‐II levels were associated with decreased minimum joint space width of the hip (r = −0.57, p = 0.001). Mean urinary Helix‐II levels were 71% higher in rapidly destructive than in slowly progressive disease (mean (standard deviation (SD)) ng/mmol Cr: 396 (160) v 232 (118) ng/mmol; p = 0.002). When levels of Helix‐II and CTX‐II in the highest tertile were both included in a multivariate logistic regression model, high Helix‐II level (OR; (95% CI) 5.73 (1.01 to 32.8)) after adjustment for age and body mass index and high CTX‐II level (6.67 (1.14 to 39.0)) were, independently of each other, associated with a rapidly destructive disease.
Conclusion
Increased urinary Helix‐II levels are associated with rapidly destructive hip osteoarthritis, independently of urinary CTX‐II. Measurement of Helix‐II, alone or in combination with CTX‐II, could be useful for the clinical investigation of patients with hip osteoarthritis.
One of the hallmarks of osteoarthritis is cartilage loss leading to joint destruction. Plain radiography, the reference technique for assessing the severity of joint destruction, provides direct information on bones but not on cartilage, and is characterised by limited sensitivity. Magnetic resonance imaging, which provides direct information on changes in the different joint tissues, is currently being optimised in osteoarthritis.1 Conventional markers such as C reactive protein show inflammation in some patients but provide little information on joint damage. Given the limitations of currently available investigations, there has been considerable interest in exploring the potential of specific biological markers that reflect quantitative and dynamic variations in joint tissue remodelling.2,3 Among the different potential clinical uses of biochemical markers, their ability to predict disease progression is of particular relevance as progression shows considerable variation across people with osteoarthritis, and the predictive capacity of clinical and radiological indices is poor.4
Biochemical markers are molecules or fragments of connective tissue matrices which are released into biological fluids during the process of tissue turnover and can be measured by immunoassays. Several biochemical markers of joint tissues have been described and their use in patients with osteoarthritis has been reviewed recently.3,4 As articular cartilage loss constitutes the hallmark of osteoarthritis and as type II collagen is the most abundant protein of the cartilage matrix, biochemical markers of type II collagen breakdown represent one of the most attractive approaches to assess disease progression.
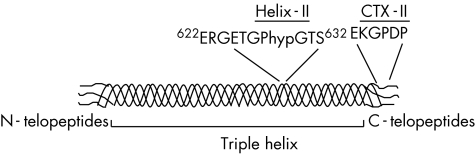
Type II collagen is formed by the association of three identical α1 chains in triple helix except at the two ends, in the N and C telopeptides (fig 1). Degradation of type II collagen involves different enzymatic processes including primarily the collagenases, which cleave the triple helical region of type II collagen at a single site between residues 778 and 776, generating two fragments representing ¾ and ¼, respectively, of the intact collagen molecule.5 These fragments of triple helix carry neoepitopes that have been used to develop type II collagen antibodies.6,7 More recently, immunoassays detecting degradation fragments of the type II collagen C telopeptide (CTX‐II)8,9 or the type II collagen helical peptide (Helix‐II)10,11 have been developed (fig 1). Increased urinary levels of CTX‐II and Helix‐II, arising from the C‐telopeptide and helical regions, respectively, have been reported in patients with knee osteoarthritis and early rheumatoid arthritis.10,12 Levels of CTX‐II were found to be associated with radiological progression in early patients with rheumatoid arthritis,13,14 knee osteoarthritis12,15 and hip osteoarthritis.16 In patients with rheumatoid arthritis, urinary CTX‐II and Helix‐II were, independently of each other, associated with radiological progression and their combination was more efficient than one marker alone in identifying patients at risk of disease progression.10 The potential role of Helix‐II as a prognostic factor of disease progression in patients with osteoarthritis has not yet been investigated.
Figure 1 Schematic representation of type II collagen and localisation of the sequence of the biochemical markers Helix‐II and CTX‐II. Type II collagen is formed by the association of three identical α1 chains in a triple helicoidal domain, except at two ends in the so‐called N and C telopeptides. Helix‐II is a degradation fragment of the triple helix, whereas CTX‐II is a degradation fragment of the C telopeptides.
The aims of our study were to investigate (1) the association of urinary Helix‐II with rapidly progressive disease in hip osteoarthritis and (2) whether Helix‐II and CTX‐II are, independently of each other, associated with disease progression.
Patients and methods
Patients with hip osteoarthritis and healthy controls
This case–control study was conducted in 40 patients (23 women, 17 men, mean age (standard deviation (SD)) 64 (12) years; range 44–82 years) who met the American College of Rheumatology criteria for primary hip osteoarthritis17 as described previously.18 Between 1996 and 1999, 150 patients consulted our outpatient clinic for symptomatic hip osteoarthritis. Of these, 62 had two hip x rays at a 1‐year interval and urine samples at the second x ray available. The other 88 patients were not included in the study, as a second x ray could not be obtained after at least 1 year for different reasons including no follow‐up visit at the hospital, absence of follow‐up x ray or hip replacement. Among the 62 patients for whom two hip x rays were available, 12 fulfilled the criteria of rapidly progressive disease and 28 were selected because of a very low joint space narrowing progression. The remaining 22 patients had an annual joint space loss of between 0.2 and 1 mm.
Rapidly progressive hip osteoarthritis was defined by the following five criteria: (1) severe hip pain; (2) symptom onset within the past 2 years; (3) annual rate of joint space loss >1 mm; (4) erythrocyte sedimentation rate of <20 mm/h; and (5) absence of detectable inflammation of crystal induced joint disease, according to the criteria proposed by Lequesne et al19 and used in a previously published study.18 Examination of the hip arthroplasty specimens confirmed the diagnosis of osteoarthritis in all 12 patients.
The 28 patients with slowly progressive hip osteoarthritis had a joint space loss of ⩽0.20 mm during the previous year, 0.20 mm being the threshold of detection of relevant change in joint space width with the radiological method used in our study.20 All patients underwent at least two pelvic x rays 1 year apart, showing a hip joint space narrowing progression of <0.2 mm/year (mean −0.13 mm; from −0.20 to +0.13 mm), allowing them to be considered as slow progressors.
At entry, all patients underwent a full clinical history and examination to obtain the following information: height, weight, body mass index (BMI) and disease duration. Patients of the two groups were on various drug regimens of analgesics and non‐steroidal anti‐inflammatory drugs, but none of the patients presented clinically detectable disease or treatment including bisphosphonates, which may interfere with current levels of the urinary markers investigated in this study. Furthermore, all women were postmenopausal and all patients were without treatment which could interfere with bone metabolism including oestrogen replacement.
In each patient with hip osteoarthritis, all clinical parameters, radiographic data used in this analysis and urine samples were collected once on a single study day. In patients with rapidly progressive hip osteoarthritis, the study day was the day when the diagnosis of rapidly progressive disease was established. Patients with a slowly progressive disease were receiving follow‐up at 6‐month intervals. The study day occurred after at least 1 year of follow‐up to establish that the annual rate of joint space loss was <0.20 mm and urine samples were collected at the time of the second hip x ray.
Healthy subjects included 47 postmenopausal women and 28 men aged from 44 to 90 years (mean (SD) 63 (11) years). All subjects were apparently healthy, with no treatment that could interfere with bone or joint metabolism including hormone replacement treatment in postmenopausal women.
Radiological evaluation
An anterioposterior x ray of the pelvis was taken in patients with hip osteoarthritis on the day of urine collection. A standardised procedure was used: patient in the standing position with the feet turned inward 20°, focal distance 100 cm and beam aligned on the upper edge of the pubic symphysis. Joint space width (JSW) at the narrowest point of the two hips was measured using a digitised image analysis method that has been validated previously.20,21 The most affected hip was considered as the target hip. Measurements were taken by the same experienced observer. Briefly the x ray to be analysed was digitised at a resolution of 600×1200 ppi (giving a pixel size of 0.004 mm) and 4096 grey levels. Subtraction and magnification were carried out to obtain a very clear outline of the joint space. The joint space contours were delineated with the mouse on the superior convex margin of the femoral head and the inferior margin of the acetabulum at an angle, whose summit was the centre of the femoral head (automatically given by the computer from three peripheral points drawn using the mouse) and that included the whole superior joint space from the internal boundary to the external end of the acetabulum. Interbone distance at the narrowest point of the joint space was automatically given by the computer. The reproducibility of JSW assessment was assessed by repeated measurements of 50 hip x rays. The intraclass coefficient of correlation was 0.99. The root‐mean‐square standard deviation of minimal JSW was 0.013, giving a coefficient of variation (CV) of 0.57%.
Biochemical markers of cartilage degradation
Fast second morning void urine samples were collected in plastic containers. After mixing the whole collection, aliquots of urine were transferred into plastic tubes and frozen at −70°C without acidification. Samples were obtained in controls according to the same procedure. All urine samples were kept frozen at −70°C until assayed.
Urinary CTX‐II was measured by a competitive ELISA (Cartilaps, Nordic Bioscience, Herlev, Denmark) based on a mouse monoclonal antibody raised against the EKGPDP sequence of human CTX‐II.9 This sequence is found exclusively in type II collagen and not in the other collagens including type I or other structural proteins. Intra‐assay and interassay CVs are <8% and 15%, respectively.
Urinary Helix‐II was measured by a competitive ELISA (Syncart) using a polyclonal antibody raised against the 622–632 peptide from the α1 chain of human type II collagen sequence. This sequence is found exclusively in human type II collagen, and not in the other collagens including type I and type III.10 Intra‐assay and interassay CVs are <12 and 15%, respectively.
Urinary CTX‐II and Helix‐II levels were corrected for variation in urine dilution by urinary creatinine measured by standard colorimetric assay.
Statistical analyses
All data are expressed in mean (SD) unless otherwise specified. Distribution of urinary CTX‐II and Helix‐II was investigated using the Shapiro–Wilk test. The distribution of both markers was abnormal in both controls and patients with hip osteoarthritis (p<0.001 and p<0.05 for CTX‐II and Helix‐II, respectively). Consequently, levels of these two biochemical markers were transformed logarithmically before analyses. After logarithmic transformation, both markers showed a normal distribution (p = 0.87 and p = 0.41 for CTX‐II and Helix‐II, respectively). Differences in urinary Helix‐II and CTX‐II between controls and patients with hip osteoarthritis and between patients with rapidly progressive and slowly progressive hip osteoarthritis were assessed by unpaired Student's t test. Correlations were evaluated by linear regression analyses. To identify patients with hip osteoarthritis with a high rate of cartilage degradation, patients were also categorised in tertiles of urinary Helix‐II and CTX‐II as suggested previously.10,16 Logistic regression analysis was carried out to investigate the association between levels of urinary Helix‐II and CTX‐II and rapidly destructive disease, which was entered in the model as a binary outcome variable. Urinary CTX‐II and Helix‐II levels were included in the model as dichotomous variables (tertile 3 v tertiles 1 and 2), and age and BMI were included as covariates.
Results
When patients with rapidly destructive and slowly progressive hip osteoarthritis were considered as a single group, patients with hip osteoarthritis did not differ from controls for age, sex distribution and BMI (table 1). In patients with hip osteoarthritis, there was no significant association between age and urinary Helix‐II (r = 0.18, p = 0.26) or urinary CTX‐II (r = 0.11, p = 0.50). When BMI was considered as a continuous variable, there was also no significant correlation between BMI and urinary Helix‐II (r = 0.20, p = 0.21) or CTX‐II (r = 0.19, p = 0.24). To further investigate the influence of BMI on the levels of biochemical markers, patients were stratified in two groups according to whether they had a BMI <25 kg/m2or >25 kg/m2 as suggested previously.22 The average age of the 23 patients with a BMI ⩾25 kg/m2 was slightly, but not significantly higher than that of the other 17 patients (mean (SD) 65 (8) v 61 (14) years, p = 0.22). Urinary Helix‐II levels in patients with BMI ⩾25 kg/m2 were significantly higher than in those with BMI <25 kg/m2 (333 (165) v 211 (98) ng/mmol Cr, p = 0.019). Urinary CTX‐II levels were also slightly higher in patients with a high BMI compared with the other subjects, although the difference did not reach significance (517 (261) v 439 (172) ng/mmol Cr, p = 0.36). In patients with hip osteoarthritis, urinary Helix‐II level modestly correlated with urinary CTX‐II level (r = 0.32, p = 0.04). Compared with controls, the 40 patients with hip osteoarthritis had increased urinary Helix‐II (mean (SD) 281 (151) v 200 (113) ng/mmol Cr, p = 0.004) and increased urinary CTX‐II (485 (230) v 335 (120), p<0.001; table 1) levels. When patients with a rapidly and slowly progressive hip osteoarthritis were considered separately, both groups had significantly higher urinary levels of Helix‐II (p<0.001 and p = 0.045 for rapidly and slowly progressive hip osteoarthritis, respectively) and urinary CTX‐II (p<0.001 for both rapidly and slowly progressive hip osteoarthritis) than controls.
Table 1 Characteristics of patients with hip osteoarthritis and controls.
| Parameters | Hip OA (n = 40) | Controls (n = 75) | p Value overall hip OA v controls | ||
|---|---|---|---|---|---|
| Rapidly destructive (n = 12) | Slowly progressive (n = 28) | p Value rapidly v slowly progressive | |||
| Sex (F/M) | 7/5 | 16/12 | 0.79 | 47/28 | 0.98 |
| Age (years) | 70 (8) | 63 (9) | 0.03 | 63 (11) | 0.66 |
| BMI (kg/m2) | 27.5 (3.4) | 25.6 (4.2) | 0.13 | 26.1 (3.6) | 0.95 |
| Bilateral/unilateral hip OA | 6/6 | 7/21 | 0.07 | — | |
| Minimal joint space width at the end of follow‐up (x ray, mm) | 0.63 (0.67) | 1.73 (1.24) | 0.008 | — | |
| Urinary Helix‐II (ng/mmol Cr) | 396 (160) | 232 (118) | 0.002 | 200 (113) | 0.004 |
| Urinary CTX‐II (ng/mmol Cr) | 612 (118) | 441 (221) | 0.015 | 335 (120) | <0.001 |
BMI, body mass index; CTX‐II, type II collagen C telopeptides; F, female; Helix‐II, type II collagen helical; M, male; OA, osteoarthritis;—, not determined.
Results are shown as mean (SD).
As shown in table 1, patients with a rapidly destructive hip osteoarthritis were significantly older and had as expected a more severe joint space narrowing than patients with slowly progressive osteoarthritis, but did not differ for sex. Although BMI was also higher in the rapidly progressive hip osteoarthritis than in the slowly progressive group (mean 27.5 v 25.6 kg/m2, p = 0.13), the difference did not reach significance probably because of the limited number of patients (table 1). The proportion of patients with bilateral disease was higher (50 v 25%) in those with a rapidly destructive disease, although the difference did not reach significance (p = 0.07).
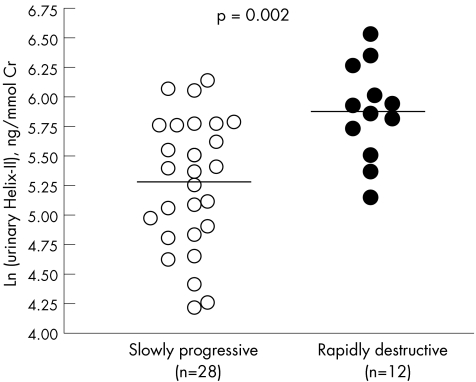
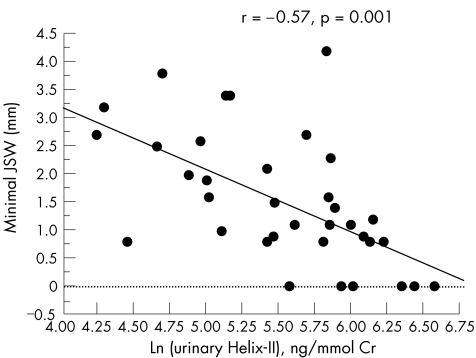
Patients with rapidly progressive hip osteoarthritis had on average 71% higher urinary Helix‐II levels (p = 0.002) than those with a slowly progressive disease (table 1, fig 2). As previously reported in this population,18 urinary CTX‐II levels were also 40% (p = 0.015) higher in patients with rapidly progressive than in those with slowly progressive hip osteoarthritis. In the whole population of patients with hip osteoarthritis, increased urinary Helix‐II (r = −0.57, p = 0.001) and urinary CTX‐II (r = −0.48, p = 0.003) levels were significantly associated with decreased minimal joint space width assessed by x rays of the most affected hip carried out on the day diagnosis was established and urine samples collected (fig 3).
Figure 2 Individual values of urinary Helix‐II in patients with hip osteoarthritis with a rapidly or slowly progressive disease. The plain lines represent the mean value in each group.
Figure 3 Relationship between urinary Helix‐II levels and minimal joint space width (JSW) in patients with hip osteoarthritis. The graph shows the correlation between logarithmically transformed urinary Helix‐II levels (x axis) and minimum joint space width of the hip (y axis).
The 40 patients with hip osteoarthritis were then separated into low and high levels of urinary Helix‐II and CTX‐II, using as a cut‐off the highest tertile. In univariate logistic regression analysis, when urinary Helix‐II or CTX‐II was individually included as a dichotomous variable in the model (highest tertile v tertiles 1 and 2), levels of each marker in the highest tertile were significantly associated with an increased odds ratio (OR) of rapidly progressive disease (table 2). When age and BMI were included as covariables in the model, levels of urinary Helix‐II and urinary CTX‐II in the highest tertile remained significantly associated with increased odds of rapidly destructive disease (table 2). As there was no significant interaction between the two biochemical markers (p = 0.56) with respect to rapidly destructive disease, both Helix‐II and CTX‐II were included as dichotomous variables (highest tertile v tertiles 1 and 2 for both markers) in the same logistic regression model. In this multivariable model, both urinary Helix‐II and CTX‐II levels remained significantly associated with rapidly destructive disease, even after adjustment for age and BMI (table 2). Combination of the two markers in the same model allowed the percentage of patients with rapidly or slowly progressive hip osteoarthritis correctly predicted to increase from 10% to 13%, compared with the use of a single marker (table 2).
Table 2 Odds ratio of rapidly progressive hip osteoarthritis disease for levels of urinary Helix‐II and CTX‐II in the highest tertile and percentage of patients correctly predicted.
| Marker in the highest tertile | Odds ratio (95% CI) of rapidly destructive disease | Patients correctly predicted (%) | |
|---|---|---|---|
| Non‐adjusted | Adjusted for age and BMI | ||
| Model 1 | 75 | ||
| Urinary Helix‐II alone (>330 ng/mmol Cr) | 9.20 (1.97 to 42.9) | 7.59 (1.53 to 37.7) | |
| Model 2 | 72 | ||
| Urinary CTX‐II alone (>490 ng/mmol Cr) | 7.33 (1.63 to 32.9) | 8.73 (1.68 to 45.4) | |
| Model 3 | 85 | ||
| Urinary Helix‐II | 7.67 (1.15 to 40.6) | 5.73 (1.01 to 32.8) | |
| Urinary CTX‐II in the same model | 6.00 (1.14 to 40.6) | 6.67 (1.14 to 39.0) | |
BMI, body mass index; CTX‐II, type II collagen C telopeptide; Helix‐II, type II collagen helical.
In each model, rapidly destructive disease was used as a binary outcome variable.
Discussion
In this study, we found that among patients with hip osteoarthritis, those with a rapidly progressive hip osteoarthritis had 71% higher levels of urinary Helix‐II than patients with a slowly progressive disease. We report for the first time that two biochemical markers reflecting different biological processes of type II collagen degradation, namely Helix‐II and CTX‐II, were associated, independently of each other, with rapidly progressive hip osteoarthritis.
Several in vitro and in vivo data indicate that urinary Helix‐II specifically reflects type II collagen degradation. The antibody used in the assay is raised against the 622–632 peptide from the α1 chain of human type II collagen sequence peptide, which is found exclusively in type II collagen and not in the other collagens, including type I and type III collagens or other structural proteins.10 Immunohistochemistry experiments using Helix‐II antibody have shown strong immunostaining in the fibrillated zones but not in the adjacent normal areas of articular cartilage in patients with knee osteoarthritis.23 Immunoreactive Helix‐II fragments have also been detected in the culture medium of human articular cartilage stimulated by interleukin 1 and oncostatin M (unpublished data), indicating that this assay recognises peptides directly released from the degradation of cartilage. In vitro incubation of human hip articular cartilage with recombinant enzymes believed to be involved in arthritic joint destruction and including the matrix‐metalloproteases 3 and 7 and cathepsins L and S, generates immunoreactive Helix‐II fragments.24 In vivo, urinary levels of Helix‐II have been shown to be markedly increased in patients with early rheumatoid arthritis, but not in patients with Paget's disease of bone, levels correlating with the progression of joint destruction.10 Thus, biochemical, in vitro and in vivo data strongly indicate that Helix‐II specifically reflects cartilage destruction.
In this study, we found that urinary Helix‐II and CTX‐II levels were not significantly associated with age in patients with hip osteoarthritis. CTX‐II levels have previously been reported to increase slightly with age in both apparently healthy men and women.15,22 However, in the study of Mouritzen et al,22 this increase was observed mainly after the menopause in women and was not significant after the age of 55 years in both women and men. In the study of Reijman et al,15 the slight increase with age was attributed to an increasing prevalence of osteoarthritis with age. Because in our study 80% of patients were >55 years old, all with hip osteoarthritis, we may not have been able to detect this slight age‐related increase in CTX‐II. Mourizten et al22 also reported that urinary CTX‐II was on average 25% (p<0.001) higher in 273 patients with a BMI ⩾25 kg/m2 compared with the 236 patients with a BMI <25 kg/m2. In our study of patients with hip osteoarthritis, we also found that patients with a BMI ⩾25 kg/m2 had on average CTX‐II values 18% higher than the other patients, although the difference did not reach statistical significance, probably because of the limited number of patients. Urinary Helix‐II levels were significantly higher in patients with a BMI ⩾25 kg/m2 compared with the others, suggesting that this new cartilage degradation marker is associated with a well‐known risk factor for knee osteoarthritis development.
This is the first study reporting increased urinary Helix‐II in patients with hip osteoarthritis. This finding is in agreement with the increase in this biochemical marker in patients with knee osteoarthritis,10 and with the elevation of other type II collagen fragments, including CTX‐II, in knee and hip osteoarthritis.9,10,12,16,18 The magnitude of elevation of urinary Helix‐II (41% v controls) in this population with hip osteoarthritis was similar to that reported previously for urinary CTX‐II (45%) in the same patients, indicating that these two biochemical markers have a similar sensitivity to detect increased cartilage degradation in hip osteoarthritis.
One of the main potential uses of biochemical markers is to identify patients at high risk for rapid progression of joint destruction. When patients with hip osteoarthritis were considered as a single group, we found that increased levels of urinary Helix‐II were associated with decreased minimal JSW measured at the time of the second radiographs—that is, when cartilage loss had already occurred, this marker explained up to 33% of the inter‐individual variance of JSW at the hip. This suggests that a sustained increased rate of type II collagen degradation as detected by this specific urinary marker leads to more rapid destruction of cartilage. These findings are also supported by the 71% higher levels of urinary Helix‐II in patients with a rapidly destructive disease than those with a slowly progressive disease.
Recent test tube experiments have shown that Helix‐II and CTX‐II are released from human cartilage by different types of cathepsins and matrix‐metalloproteases, which are proteolytic enzymes believed to play a part in cartilage degradation in osteoarthritis.23 Immunohistochemistry experiments using full‐depth human osteoarthritis cartilage also showed that the spatial distribution within the tissue is different between Helix‐II and CTX‐II, further suggesting that these two markers reflect different biological processes of cartilage degradation.24 Thus, it is possible that urinary Helix‐II and CTX‐II may reflect different and potentially complementary mechanisms of cartilage damage associated with osteoarthritis.
In this study, we found that although both urinary CTX‐II and Helix‐II were increased in patients with hip osteoarthritis, the correlation between these two biological markers of cartilage degradation was rather modest (r = 0.32, p = 0.04), in agreement with our previous finding in patients with early rheumatoid arthritis.10 When both urinary Helix‐II and CTX‐II levels in the highest tertile were included together in a multiple variable model, we found that the two markers were significantly associated with an increased OR of rapidly progressive disease even after adjustment for age and BMI. Thus these data suggest that urinary Helix‐II and CTX‐II may provide independent information on disease progression in hip osteoarthritis. Interestingly, in patients with early rheumatoid arthritis, we previously reported that urinary Helix‐II and CTX‐II were also associated with each other independently of radiological disease progression.10
Our study has some limitations. The number of patients, especially those with rapidly progressive disease, is limited resulting in wide CI for OR and clearly these data will need to be confirmed in larger longitudinal studies. The status of other potential sources of CTX‐II and Helix‐II in the body, such as the knees, hands and spine, was not evaluated in this study and could have confounded the results.25 We did not carry out radiographic evaluation of the joints of the controls. Consequently, we cannot exclude the possibility of some controls having asymptomatic osteoarthritis. However, this would actually have resulted in an underestimation of the difference in urinary Helix‐II and CTX‐II levels between patients with hip osteoarthritis and controls.
In conclusion, increased urinary Helix‐II levels were found in patients with a rapidly destructive disease of the hip. Urinary Helix‐II and CTX‐II were, independently of each other, associated with a rapidly destructive disease, suggesting that the combination of these two biochemical markers reflecting different aspects of cartilage degradation may be useful for the clinical investigation of patients with hip osteoarthritis.
Abbreviations
BMI - body mass index
CTX‐II - type II collagen C telopeptide
Helix‐II - type II collagen helical peptide
JSW - joint space width
Footnotes
Competing interests: None declared.
References
- 1.Gray M L, Eckstein F, Peterfy C, Kim Y J, Sorensen A G. Toward imaging biomarkers for osteoarthritis. Clin Orthop Relat Res 2004427(Suppl)S175–S181. [DOI] [PubMed] [Google Scholar]
- 2.Lohmander L S. Markers of altered metabolism in osteoarthritis. J Rheumatol 200431(Suppl 70)28–353. [PubMed] [Google Scholar]
- 3.Kraus V B. Biomarkers in osteoarthritis. Curr Opin Rheumatol 200517641–646. [DOI] [PubMed] [Google Scholar]
- 4.Garnero P, Delmas P D. Biomarkers in osteoarthritis. Curr Opin Rheumatol 200315641–646. [DOI] [PubMed] [Google Scholar]
- 5.Burrage P S, Mix K S, Brinckerhoff E. Matrix metalloproteases: role in arthritis. Front Biosci 200611529–543. [DOI] [PubMed] [Google Scholar]
- 6.Hollander A P, Heathfield T F, Webber C, Iwata Y, Bourne R, Rorabeck C.et al Increased damage of type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest 1994931722–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Downs J T, Lane C L, Nestor N B, McLellan T J, Kelly M A, Karam G A.et al Analyis of collagenase‐cleavage of type II collagen using a neoepitope ELISA. J Immunol Methods 200124725–34. [DOI] [PubMed] [Google Scholar]
- 8.Lohmander L S, Atley L M, Pietka T A, Eyre D R. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum 2003483130–3139. [DOI] [PubMed] [Google Scholar]
- 9.Christgau S, Garnero P, Fledelius C, Moniz C, Ensig M, Gineyts E.et al Collagen type II C‐telopeptide fragments as an index of cartilage degradation. Bone 200129209–215. [DOI] [PubMed] [Google Scholar]
- 10.Charni N, Juillet F, Garnero P. Urinary type II collagen helical peptide (Helix II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 2005521081–1090. [DOI] [PubMed] [Google Scholar]
- 11.Deberg M, Labasse A, Christgau S, Cloos P, Bang‐Henriksen D, Chapelle J ‐ P.et al New serum biochemical markers (Coll 2‐1 and Coll 2‐1 NO2) for studying oxidative‐related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cart 200513258–265. [DOI] [PubMed] [Google Scholar]
- 12.Garnero P, Ayral X, Rousseau J C, Christgau S, Sandell L J, Dougados M.et al Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum 2002462613–2624. [DOI] [PubMed] [Google Scholar]
- 13.Garnero P, Landewe R, Maarten B, Verhoeven A, Van Der Linden S, Christgau S.et al Association of baseline levels of markers of bone and cartilage degradation are associated with long term progression of joint damage in patients with early rheumatoid arthritis: the Cobra Study. Arthritis Rheum 2002462847–2856. [DOI] [PubMed] [Google Scholar]
- 14.Garnero P, Gineyts E, Christgau S, Finck B, Delmas P D. Association of baseline levels of urinary glucosyl‐galactosyl‐pyridinoline and type II Collagen C‐telopeptide are associated with progression of joint destruction in patients with early rheumatoid arthritis. Arthritis Rheum 20024621–30. [DOI] [PubMed] [Google Scholar]
- 15.Reijman M, Hazes J M, Bierna‐Zeinstra S M, Koes B W, Christgau S, Christiansen C.et al A new marker for osteoarthritis: cross‐sectional and longitudinal approach. Arthritis Rheum 2004502471–2476. [DOI] [PubMed] [Google Scholar]
- 16.Mazières B, Garnero P, Guéguen A, Abbal M, Berdah L, Lequesne M.et al Molecular markers of cartilage breakdown and synovitis are strong independent predictors of structural progression of hip osteoarthritis. The ECHODIAH Cohort. Ann Rheum Dis 200665354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K.et al Development of criteria for the classification and reporting of osteoarthritis. Diagnostic and therapeutic criteria committee of the American Rheumatism Association. Arthritis Rheum 1986291039–1049. [DOI] [PubMed] [Google Scholar]
- 18.Garnero P, Conrozier T, Christgau S, Mathieu P, Delmas P D, Vignon E. Urinary type II collagen C‐telopeptide levels are increased in patients with a rapidly destructive hip osteoarthritis. Ann Rheum Dis 200362939–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lequesne M, Ray G. La coxopathie destructrice rapide idiopathique. Rev Rhum 198956115–119. [PubMed] [Google Scholar]
- 20.Conrozier T, Tron A M, Balblanc J C, Mathieu P, Piperno M, Fitousi F.et al Measurement of the hip joint space using computerized image analysis. Rev Rhum 199360105–111. [PubMed] [Google Scholar]
- 21.Conrozier T, Lequesne M, Favret H, Taccoen A, Vignon M, Vignon E. Measurement of the radiological hip joint space width. An evaluation of various methods of measurement. Osteoarthritis Cart 20019281–286. [DOI] [PubMed] [Google Scholar]
- 22.Mouritzen U, Christgau S, Lehmann H ‐ J, Tanko L B, Christiansen C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann Rheum Dis 200362332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garnero P, Desmarais S, Charni N, Percival M D. The type II collagen fragments HELIX‐II and CTX‐II reveal distinct enzymatic pathways of cartilage collagen degradation: diagnostic and therapeutic implications in rheumatoid arthritis and osteoarthritis. Arthritis Rheum 200544(Suppl)S56 [Google Scholar]
- 24.Bay Jensen A ‐ C, Charni N, Andersen T L, Juillet F, Kjaersgaard‐Andersen K, Wagner P.et al The type II collagen degradation markers, Helix‐II and CTX‐II, have distinct distributions in tissue sections of human articular cartilage, and are affected differently by menopausal status. OARSI (2005)
- 25.Garnero P, Sornay‐Rendu E, Arlot M, Christiansen C, Delmas P D. Association between spine disc degeneration and type II Collagen degradation in postmenopausal women: the OFELY study. Arthritis Rheum 2004503137–3144. [DOI] [PubMed] [Google Scholar]