Abstract
Objectives
To examine the validity of power Doppler ultrasound imaging to identify synovitis, using histopathology as gold standard, and to assess the performance of ultrasound equipments.
Methods
44 synovial sites in small and large joints, bursae and tendon sheaths were depicted with ultrasound. A synovial biopsy was performed on the site depicted and a synovial sample was taken for histopathological evaluation. The performance of three ultrasound devices was tested using flow phantoms.
Results
A positive Doppler signal was detected in 29 of 35 (83%) of the patients with active histological inflammation. In eight additional samples, histological examination showed other pathological synovial findings and a Doppler signal was detected in five of them. No significant correlation was found between the amount of Doppler signal and histological synovitis score (r = 0.239, p = NS). The amount of subsynovial infiltration of polymorphonuclear leucocytes and surface fibrin correlated significantly with the amount of power Doppler signal: r = 0.397 (p<0.01) and 0.328 (p<0.05), respectively. The ultrasound devices differed in showing the smallest detectable flow.
Conclusions
A negative Doppler signal does not exclude the possibility of synovitis. A positive Doppler signal in the synovium is an indicator of an active synovial inflammation in patients. A Doppler signal does not correlate with the extent of the inflammation and it can also be seen in other synovial reactions. It is important that the quality measurements of ultrasound devices are reported, because the results should be evaluated against the quality of the device used.
Grey‐scale ultrasound imaging (B mode) is a useful method to find soft tissue lesions in synovial structures such as joints, tendons and bursae, as well as bone erosion.1,2,3,4,5,6,7,8,9,10,11 Circulation is a crucial part of the synovial inflammation process.12,13 Colour Doppler and power Doppler ultrasound (PDU) imaging can detect synovial blood cell movements—that is, perfusion and vessels. PDU is theoretically more sensitive than colour Doppler ultrasound in small‐vessel imaging, because PDU provides increased sensitivity to low‐volume, low‐velocity blood flow at the microvascular level.14 It has been shown that the Doppler signal increases in active synovitis and decreases after a successful intra‐articular or systemic treatment showing therapeutic response.15,16,17,18,19,20,21,22,23,24,25,26 Thus, the activity of a synovial inflammatory state is hoped to be assessed with ultrasound.
The concurrent validity of Doppler ultrasound imaging to show synovitis is not known. There are only a few studies on the correlation between Doppler imaging and the histology of the synovium in patients with rheumatic diseases.27,28,29 The relatively few joints studied in these papers have been end‐stage rheumatic disease of knees and hips. And in two of these three studies only vascularity was reported, not the inflammatory state of the synovial tissue.
Quality‐assurance measurements of ultrasound devices have not been reported in scientific clinical articles. Practical work among ultrasound equipments has raised a doubt that some devices might have a more sensitive Doppler mode than others. Thus, the performance of Doppler imaging equipment and probes may play an essential part in evaluating the significance of the results.
Our paper aimed to firstly use PDU to depict small and large joints, bursae and tendon sheaths in patients with rheumatic diseases, and to compare the results with histological samples, and, secondly, to examine the performance of ultrasound devices, especially to show very slow flows.
Materials and methods
Ultrasound equipment and probes
The ultrasound equipment used by JMK was the Esaote Technos, 2000(Esaote Biomedica, Genova, Italy) equipped with two linear probes: LA424 (frequency range 8–14 MHz) and LA523 (frequency range 5–10 MHz). The first probe was used in hand and foot joints, and the second in elbow, shoulder and knee joints. The device was used in the clinical part of the study. Two other scanners from the x ray department were coincidentally chosen for quality‐assurance measurements. JMK, UH and HH carried out the quality‐assurance tests on a Toshiba Aplio 50 with a 12‐MHz linear probe PLT‐1240AT (Toshiba Medical Systems, Tokyo, Japan), an Aloka SSD 5000 with a 10‐MHz linear transducer UST 5545 and a 7.5‐MHz linear transducer UST 5710 (Aloka, Tokyo, Japan) and an Esaote.
Quality‐assurance measurements of the power Doppler mode
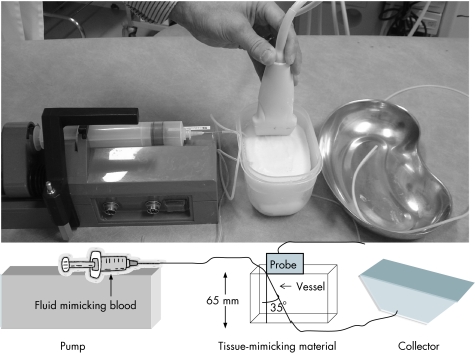
Quality‐assurance measurements were conducted on fast flows (10–200 cm/s) in the Esaote and Toshiba and very slow flows (0.032–3.4 cm/s) in all three devices. The fast flows were determined using a Model 43 Doppler String Phantom (CIRS, Norfolk, Virginia, USA). As there is no commercial phantom for slow flow measurements, a phantom was constructed by UH. We were especially interested in determining the slowest detectable flow. The ultrasound devices were measured using “laboratory settings”, which make it possible to use the most sensitive Doppler mode to detect slow flows (eg, minimal pulse repetition frequency (PRF) value); in the Esaote, we also used machine settings used in clinical work (clinical settings). The flow phantom consisted of a block of tissue‐mimicking material made of polyvinyl alcohol cryogel. The block was made at the Department of Applied Physics, Kuopio University, Finland, and the material was investigated to ensure that it corresponded with soft tissue (speed of sound 1540 m/s). A silicone vessel (inner diameter 1 mm) was put through the 65‐mm‐thick block at an angle of 35° to the ultrasound beam (fig 1). The vessel corresponded to a small blood vessel. A fluid mimicking blood (BMF, Sidac Engineering, North York, Ontario, Canada) was pumped through the vessel. The pump used, Perfusor Secura FT (B Braun, Bethlehem, Pennsylvania, USA), can produce a regular gradual flow of 1–99 ml/h, which corresponded to a flow of 0.32–34 mm/s in the vessel used. The real mean flow was checked by following the air‐bubble movement in the vessel for a distance of 40 cm. The real velocity of the flow in the vessel and the flows measured by the devices were checked three times at every pump speed. The flow measurements inside the block were conducted to the depth of 25–35 mm.
Figure 1 Phantom set‐up for very slow flows. A fluid mimicking blood was pumped through a silicone vessel (inner diameter 1 mm), which was put through a tissue mimicking material at an angle of 35° to the ultrasound beam.
Patients, ultrasound imaging, biopsy specimens and histopathological examination
An ultrasound scan and a percutaneous synovial biopsy of the site scanned were performed by JMK on 44 outpatients with monarthritis or polyarthritis in 44 synovial sites: 25 knee, 7 wrist, 3 tibiotalar, 2 metatarsophalangeal joints, 1 glenohumeral, metacarpophalangeal and elbow joint, as well as 2 subdeltoid bursae, 1 tibialis posterior and 1 peroneus tendon sheath. Table 1 shows the clinical characteristics, laboratory findings, drugs and x ray status according to Larsen's method30 of the patients. There were no changes in the drugs or intra‐articular injections during the 3 months preceding the intervention. The diagnoses established were based on the criteria of the American College of Rheumatology31 and the European Spondylarthropathy Study Group.32 Consecutively, all the patients of the clinic scheduled for a synovial biopsy volunteered for the study. The indication for a synovial biopsy was clinical in each case.33
Table 1 Clinical characteristics (mean (SD)), laboratory findings (mean (SD)), drugs and x ray status of the patients.
| Age (years) | 55 (15) |
| Females:males | 28:16 |
| Disease duration (years) | 8.4 (11) |
| Seropositive:seronegative | 16:28 |
| Disease | |
| Rheumatoid arthritis | 14 |
| Monarthritis | 11 |
| Oligoarthritis | 9 |
| Psoriatic arthritis | 3 |
| Juvenile arthritis | 3 |
| Undifferentiated spondyloarthropathy | 2 |
| Polymyalgia rheumatica | 2 |
| VAS of the site, pain (0–100 mm) | 40 (20) |
| Duration of pain at the site (years) | 2.6 (3.6) |
| Swollen joint count | 2 (2.2) |
| ESR (mm/h) | 18 (20) |
| CRP (mg/l) | 13 (36) |
| B haemoglobin (g/l) | 138 (13) |
| Drugs | |
| No | 24 |
| 1 DMARD | 9 |
| 2 DMARDs (combination) | 4 |
| 3–4 DMARDs (combination) | 7 |
| x Ray status of the sites, Larsen scores | |
| L 0 | 19 |
| L 1 | 17 |
| L 2 | 2 |
| L 3 | 5 |
| L 4 | 1 |
| L 5 | 0 |
CRP, C reactive protein; DMARD, disease‐modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; VAS, Visual Analogue Scale.
The ultrasound examination was performed at room temperature (22°C) and the sonographer used minimal probe pressure after applying gel on the skin. The scanning of the anatomical sites was carried out with standard scans2 to locate effusion and synovial hypertrophy, and to estimate the power Doppler signal. The effusion was defined as hypoechoic or anechoic fully compressible material, the synovial hypertrophy as echogenic or hypoechoic slightly compressible or non‐compressible material, fixed intra‐articular tissue and the Doppler signal as a stagnant (or tube‐like) pulsating colour spot found inside the synovial structure. The colour box was adjusted to cover the region of interest. The settings of the Esaote were PRF 500 MHz, colour emission frequency 8.3 MHz (LA424), 7.1 MHz (LA523), low wall filter and movement artefact suppression parameter 3 (range 0 (absent) to 4 (high)). The colour priority, dynamic range and persistence were set high. The colour gain was increased until background noise appeared and then reduced until the noise was suppressed. Flow was also shown on two planes. The image with maximum colour activity was chosen for analysis. The grey scale as well as PDU findings were reported subjectively and graded on a semiquantitative scale from 0 to 3: 0 for no effusion, synovial proliferation or colour signal; 1 for mild effusion, proliferation or colour signal; 2 for moderate effusion, proliferation or colour signal; and 3 for a substantial increase of effusion, proliferation or colour signal.
A percutaneous synovial biopsy of the scanned synovial structure was performed under ultrasound guidance immediately after the ultrasound imaging. In 10 patients, there was a maximum of a 24‐h delay between the two procedures. The patients' drugs were kept constant during the delay. An exact description of the biopsy method and the handling of the samples have been published earlier.33
The synovial samples were all examined once under blinded conditions by the histopathologist MH. Only sections with an intact synovial lining layer were included. Seven histological parameters of the specimen were determined separately: multiplication of synovial lining, villous hypertrophy of the synovial surface, surface fibrin deposition, subsynovial infiltration of polymorphonuclear leucocytes, subsynovial infiltration of mononuclear leucocytes, proliferation of blood vessels and fibrosis. Each parameter was graded on a semiquantitative scale from 0 to 3 according to the amount of the character: 0 points for no existence; 1 point for mild evidence; 2 points for moderate evidence; and 3 points for a substantial increase in the particular character. The points were counted for an overall histopathological score (points 0–21) in each sample. A score of ⩾2 points in an individual sample was regarded as pathological. The histological sample was regarded as actively inflammatory (active synovitis) if leucocytes were present and as a pathological synovial reaction if any of the other characters were present without leucocytes.
The study was approved by the local ethical committee and each patient gave informed consent.
Statistical analysis
Statistical analyses were carried out using SPSS V.13 software. Spearman's r correlation analyses between variables were tested for two‐tailed probability values. Values of p<0.05 were considered significant.
Results
Quality assurance measurement of the devices and transducers
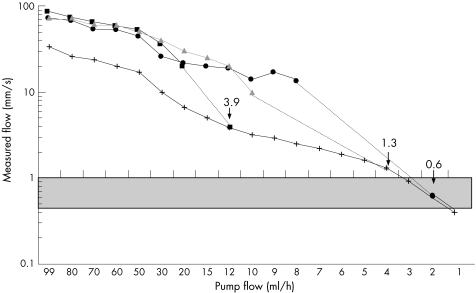
The Esaote and Toshiba performed well when used to measure fast flows. The devices measured the same results as the phantom. When very slow flows were tested, all three pieces of equipment measured peak flow values as approximately 2–3 times higher than the real mean flow values. When the flow was reduced, there was a point where the monitor no longer showed a flow curve, but a Doppler signal could still be seen. The slowest detectable flow as seen in colours using laboratory settings was 0.6, 1.3 and 3.9 mm/s in the Toshiba, Esaote (LA424) and Aloka (UST 5545), respectively (fig 2). In the LA523 (Esaote), the value was 1.6 mm/s and that in the UST 5710 (Aloka) was 6.7 mm/s. The slowest detectable flows on clinical settings of the Esaote were 2.2 and 2.5 mm/s for the probes LA424 and LA523, respectively. The most sensitive Doppler mode could be achieved in probe LA424, with a colour emission frequency of 8.3 MHz, PRF of 250 Hz, low wall filter, scan correlation parameter 1 and movement artefact suppression parameter 1. The corresponding settings for the LA523 were colour emission frequency 7.1 MHz, PRF of 250 Hz, low wall filter, scan correlation parameter 1 and movement artefact suppression parameter 1.
Figure 2 Measurements of very slow peak flows in the phantom system by the three ultrasound devices and transducers (Aloka UST 5545 ▪_▪, Esaote LA424 ▴‐▴ and Toshiba PLT‐1204AT•‐•). The real mean flows measured are shown as x–x. The dotted line denotes values where flow could be detected only visually as colours but could not be measured as a flow curve by devices ending at the point (arrows) of slowest detectable flow (values are mm/s). One device could show flow at capillary flow level (grey area).
Histopathological findings versus ultrasound imaging
Histological examination detected active synovitis in 35 of 44 (79%) samples and other pathological synovial reactions in 8 additional samples—that is, abnormal findings were found in 43 of 44 (98%) of the samples. Grey‐scale ultrasound showed effusion in 35 of 44 (80%), proliferation in 39 of 44 (89%), effusion or proliferation or both in 42 of 44 (95%) of the patients. When a positive power Doppler was added, the figure was 43 of 44 (98%). No significant correlation was found between the amount of fluid or synovial proliferation detected with ultrasound, and the overall histopathological score (r = 0.134, p = NS and r = 0.222, p = NS, respectively). In one case of ultrasound‐detected effusion, there were no histopathological changes and in one case, there was a severe chronic histological inflammation without any grey scale or PDU findings. A positive Doppler signal could be detected in 34 of 44 (77%) of the patients, in 29 of 35 (83%) patients with an active histological inflammation and in 34 of 43 (79%) of the patients with any pathological synovial event. Histological examination showed other pathological synovial reactions in 8 of 43 (18.6%) of the samples, such as multiplication of synovial lining, villous hypertrophy of the synovial surface, excess of fibrin surface deposition, proliferation of blood vessels and fibrosis. A Doppler signal was found in five of them (table 2). There were two cases where only fibrosis could be found histologically, but PDU showed a strong intra‐articular signal (ie, proliferative stage of the fibrosis).
Table 2 Ultrasound findings of the material in 44 patients.
| Grey‐scale US | |
| Effusion | 35/44 (80%) |
| Synovial proliferation | 39/44 (89%) |
| Effusion or synovial proliferation or both | 42/44 (95%) |
| Grey‐scale pathology or PDU+ or both | 43/44 (98%) |
| PDU+ | 34/44 (77%) |
| PDU+ in histological inflammation | 29/35 (83%) |
| PDU+ in any histological reaction | 34/43 (79%) |
| PDU+ in other pathological synovial reactions than inflammation | 5/8 (62.5%) |
PDU, power Doppler ultrasound; PDU+, positive power Doppler signal found in the synovium; US, ultrasound.
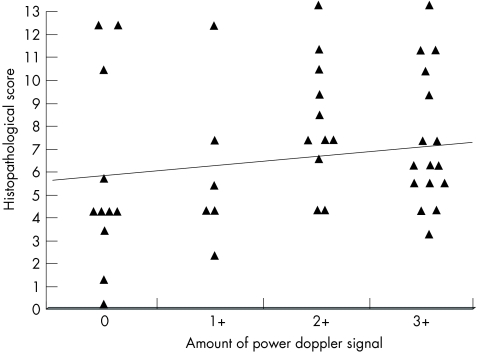
No significant correlation was found between the amount of the power Doppler signal in the synovium and the overall histopathological score (r = 0.239, p = NS; fig 3). The amounts of subsynovial infiltration of polymorphonuclear leucocytes and surface fibrin were the only histological parameters that correlated significantly with the amount of power Doppler signal: r = 0.397 (p<0.01) and 0.328 (p<0.05), respectively (table 3). No divergence was observed between the various synovial sites examined.
Figure 3 Correlation between the amount of power Doppler signal and histopathological score in 44 synovial sites (r = 0.239, p = NS).
Table 3 Correlations of power Doppler signal amount to histopathological parameters.
| r | p Value | |
|---|---|---|
| Histopathological score | 0.239 | NS |
| Villous hypertrophy of the synovialsurface | −0.172 | NS |
| Surface fibrin | 0.328 | <0.05 |
| Multiplication of synovial lining | 0.071 | NS |
| Subsynovial polymorphonuclear leucocytes | 0.397 | <0.01 |
| Subsynovial mononuclear leucocytes | 0.134 | NS |
| Proliferation of blood vessels | −0.03 | NS |
| Fibrosis | 0.131 | NS |
NS, not significant.
Discussion
Performance measurements of the devices
This study showed that only one device could show a Doppler signal at the capillary flow level. The quality and performance of the Esaote seemed acceptable, but the lack of vascular signals on the PDU in patients with evident histological synovitis shows that there is possibly flow in the synovium under the detection threshold of the device. The case becomes even clearer if we take into account that the clinical settings do not allow the most sensitive performance of the device. Browne et al34 have shown that ultrasound devices and transducers differ in showing slow flows and that the instrument setting has a marked effect on the sensitivity of the scanner. The sensitivity of depicting microvascular perfusion in rheumatological Doppler imaging is particularly important because the flow in a capillary vessel is about 0.45–1 mm/s, depending on the physiological situation.35
Perfusion in the synovium can be evaluated by using a subjective semiquantitative scale of colour signals, a quantitative measurement of colour pixels or by analysing the Doppler curves. We chose to assess the power Doppler signal subjectively on a scale of 0–3. This method is easy to use in daily work and it has been shown to correlate with digital image analysis.28 The phantom part of the paper showed that a visual observation of the flow (ie, assessing the colours) seemed to be more sensitive than measuring the flow from the Doppler curve (pulsed‐wave Doppler).
Histopathology and correlation with ultrasound scanning
We did not find any significant correlation between the amount of Doppler signal and the histopathological score of synovitis. This is at odds with the prevailing conception but this is also the only study of its kind. The histological analysis by Schmidt et al27 was different. The authors found a good agreement between the presence of colour signals and histologically verified pannus tissue.
The finding that the amount of Doppler signal and the histopathological state do not correlate significantly makes the use of the semiquantitative scale of Doppler signal questionable in the grading of synovitis in clinical work as well as in scientific papers. Instead, a dichotomous scale (yes/no) could be better, and here only a positive Doppler signal seems to be significant in terms of reliability in evaluating synovitis. The finding also casts a shadow on using PDU in treatment monitoring. A diminishing of colour in PDU found in the follow‐up of the joint treated is inevitably an indicator of a good response, but, according to this study, a negative Doppler signal does not exclude inflammation.
The heterogeneity of the diseases investigated was a problem for this study. Owing to the small number of patients in the subgroups, a detailed histological analysis versus diagnoses or markers for disease activity was not carried out.
It is not possible to calculate the concurrent validity of PDU in this material because of a lack of healthy specimens. However, the sensitivity of PDU in showing synovial inflammation or other pathological synovial reactions in this material seemed to be quite good. It is also important to realise that it is difficult to give generalised validity figures about Doppler imaging because the results are inevitably device dependent. Using a device with a more sensitive Doppler mode could give different results, something that remains to be proved.
Conflicting reports of the Doppler signal in a normal joint are available. Most researchers report normal joints to be negative, but colour Doppler activity in normal hand joints has been reported in 11–18%36,37 of cases. The distinguishing feature between normal and pathological joints is that pathological grey‐scale changes are missing in a normal joint.
We did not find a positive correlation between the amount of Doppler signal and vessels detected histologically. This was in accordance with Schmidt's study.27 However, Walther et al28,29 found a close correlation between the power Doppler flow and the amount of vessels in two studies. Biopsy methods (percutaneous v open) do not explain the difference, as Schmidt and Walther both used open biopsies. The staining method in Walther's paper was different: haematoxylin and eosin and factor VIIII staining for the detection of vessels. The preparation and staining of the sample was not described in Schmidt's paper.
Using histological examination as a gold standard is problematic. Firstly, there is no accepted uniform scoring system of inflammation in the evaluation of synovitis. We used a system of seven parameters, which included typical phenomena in the inflammation process found using haematoxylin and eosin staining. Secondly, what is actually an active (significant) synovitis assessed in histopathology using haemotoxylin and eosin staining? We defined active synovitis as leucocytes found in the synovium. However, there were no leucocytes in 8 of 43 (18.6%) samples with pathological findings (in five of them the Doppler signal was positive), but other mixed reactions could be found. Fibrosis inevitably represents the reparative state of inflammation, but other synovial reactions may signal an early or a late phase of the inflammation process. Thus, the PDU could not distinguish between active synovitis and other types of pathological synovial reactions. Thirdly, only relatively little information is available on the histology of the synovium in a normal living person. One study38 found several phenomena usually related to the inflammatory state in a normal synovium. Therefore, the line between normal and pathological synovia remains obscure. A histological assessment is nevertheless widely regarded as the gold standard to which other methods of assessment should be related.
In conclusion, this paper shows that a negative power Doppler signal in the synovium does not exclude the possibility of synovitis, but that a positive Doppler signal is a significant marker for synovitis in patients. Even a minor colour signal detected in the synovium is important in terms of histopathology, but the amount of colour in PDU does not correlate significantly with the severity of histopathological synovitis. A PDU signal can be seen in various synovial reactions. In Doppler imaging, the significance of the results should be evaluated against the performance of the equipment used.
Abbreviations
PDU - power Doppler ultrasound
PRF - pulse repetition frequency
Footnotes
Funding: This study was supported by an EVO grant.
Competing interests: None.
References
- 1.Manger B, Kalden J R. Joint and connective tissue ultrasonography—a rheumatologic bedside procedure? A German experience. Arthritis Rheum 199538736–742. [DOI] [PubMed] [Google Scholar]
- 2.Backhaus M, Burmester G R, Gerber T, Grassi W, Machold K P, Swen W A.et al Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 200160641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt W A, Schmidt H, Schicke B, Gromnica‐Ihle E. Standard reference values for musculoskeletal ultrasonography. Ann Rheum Dis 200463988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grassi W, Tittarelli E, Pirani O, Ovaltroni D, Cervini C. Ultrasound examination of metacarpophalangeal joints in rheumatoid arthritis. Scand J Rheumatol 1993993243–247. [DOI] [PubMed] [Google Scholar]
- 5.Naredo E, Iagnocco A, Valesini G, Uson J, Beneyto P, Crespo M. Ultrasonographic study of painful shoulder. Ann Rheum Dis 2003621026–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koski J M, Anttila P, Hämäläinen M, Isomäki H. Hip joint ultrasonography: correlation with intra‐articular effusion and synovitis. Br J Rheumatol 199029189–192. [DOI] [PubMed] [Google Scholar]
- 7.Kane D, Balint P V, Sturrock R D. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol 200330966–971. [PubMed] [Google Scholar]
- 8.Karim Z, Wakefield R J, Quinn M, Conaghan P G, Brown A K, Veale D J.et al Validation and reproducibility of ultrasonography in the detection of synovitis in the knee. Arthritis Rheum 200450387–394. [DOI] [PubMed] [Google Scholar]
- 9.Wakefield R J, Gibbon W W, Conaghan P G, O'Connor P, McGonagle D, Pease C.et al The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum 2000432762–2770. [DOI] [PubMed] [Google Scholar]
- 10.Grassi W, Filippucci E, Farina A, Cervini C. Sonographic imaging of tendons. Arthritis Rheum 200043969–976. [DOI] [PubMed] [Google Scholar]
- 11.Iagnocco A, Coari G, Palombi G, Valesini G. Sonography in the study of metatarsalgia. J Rheumatol 2001281338–1340. [PubMed] [Google Scholar]
- 12.Paleolog E M. Angiogenesis in rheumatoid arthritis. Arthritis Res 20024(Suppl)S81–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor P C. VEGF and imaging of vessels in rheumatoid arthritis. Arthritis Res 20024(Suppl)S99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinoli C, Derchi L E, Rizzatto G, Solbiati L. Power Doppler sonography: general principles, clinical applications, and future prospects. Eur Radiol 199881224–1235. [DOI] [PubMed] [Google Scholar]
- 15.Newman J S, Adler R S, Bude R O, Rubin J M. Detection of soft‐tissue hyperemia: value of power Doppler sonography. Am J Roentgenol 1994163385–389. [DOI] [PubMed] [Google Scholar]
- 16.Newman J S, Laing T J, McCarthy C J, Adler R S. Power Doppler sonography of synovitis: assessment of therapeutic response‐preliminary observations. Radiology 1996198582–584. [DOI] [PubMed] [Google Scholar]
- 17.Wakefield R J, Brown A K, O'Connor P J, Emery P. Power Doppler sonography: improving disease activity assessment in inflammatory musculoskeletal disease. Arthritis Rheum 200348285–288. [DOI] [PubMed] [Google Scholar]
- 18.Hau M, Schultz H, Tony H P, Keberle M, Jahns R, Haerten R.et al Evaluation of pannus and vascularization of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis by high‐resolution ultrasound (multidimensional linear array). Arthritis Rheum 1999422303–2308. [DOI] [PubMed] [Google Scholar]
- 19.Weidekamm C, Koller M, Weber M, Kainberger F. Diagnostic value of high‐resolution B‐mode and doppler sonography for imaging of hand and finger joints in rheumatoid arthritis. Arthritis Rheum 200348325–333. [DOI] [PubMed] [Google Scholar]
- 20.Strunk J, Lange U, Kurten B, Schmidt K L, Neeck G. Doppler sonographic findings in the long bicipital tendon sheath in patients with rheumatoid arthritis as compared with patients with degenerative diseases of the shoulder. Arthritis Rheum 2003481828–1832. [DOI] [PubMed] [Google Scholar]
- 21.Qvistgaard E, Rogind H, Torp‐Pedersen S, Terslev L, Danneskiold‐Samsoe B, Bliddal H. Quantitative ultrasonography in rheumatoid arthritis: evaluation of inflammation by Doppler technique. Ann Rheum Dis 200160690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carotti M, Salaffi F, Manganelli P, Salera D, Simonetti B, Grassi W. Power Doppler sonography in the assessment of synovial tissue of the knee joint in rheumatoid arthritis: a preliminary experience. Ann Rheum Dis 200261877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hau M, Kneitz C, Tony H P, Keberle M, Jahns R, Jenett M. High‐resolution ultrasound detects a decrease in pannus vascularisation of small finger joints in patients with rheumatoid arthritis receiving treatment with soluble tumour necrosis factor alpha receptor (etanercept). Ann Rheum Dis 20026155–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terslev L, Torp‐Pedersen S, Qvistgaard E, Danneskiold‐Samsoe B, Bliddal H. Estimation of inflammation by Doppler ultrasound: quantitative changes after intra‐articular treatment in rheumatoid arthritis. Ann Rheum Dis 2003621049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribbens C, Andre B, Marcelis S, Kaye O, Mathy L, Bonnet V.et al Rheumatoid hand joint synovitis: gray‐scale and power Doppler US quantifications following anti‐tumor necrosis factor‐alpha treatment: pilot study. Radiology 2003229562–569. [DOI] [PubMed] [Google Scholar]
- 26.Terslev L, Torp‐Pedersen S, Qvistgaard E, Kristoffersen H, Rogind H, Danneskiold‐Samsoe B.et al Effects of treatment with etanercept on rheumatoid arthritis evaluated by Doppler ultrasonography. Ann Rheum Dis 200362178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt W A, Volker L, Zacher J, Schlafke M, Ruhnke M, Gromnica‐Ihle E. Colour Doppler ultrasonography to detect pannus in knee joint synovitis. Clin Exp Rheumatol 200018439–444. [PubMed] [Google Scholar]
- 28.Walther M, Harms H, Krenn V, Radke S, Faehndrich T ‐ P, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 200144331–338. [DOI] [PubMed] [Google Scholar]
- 29.Walther M, Harms H, Krenn V, Radke S, Kirschner S, Gohlke F. Synovial tissue of the hip at power Doppler US: correlation between vascularity and power Doppler US signal. Radiology 2002225225–231. [DOI] [PubMed] [Google Scholar]
- 30.Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol (Diagn) 197681079–1087. [DOI] [PubMed] [Google Scholar]
- 31.Arnett F C, Edworthy S M, Bloch D A, Mc Shane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987: revised criteria of the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 32.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A.et al The European Spondylarthropathy Study Group: preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991341218–1227. [DOI] [PubMed] [Google Scholar]
- 33.Koski J M, Helle M. Ultrasound guided synovial biopsy using portal and forceps. Ann Rheum Dis 200564926–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Browne J E, Watson A J, Hoskins P R, Elliott A T. Validation of a sensitivity performance index test protocol and evaluation of colour Doppler sensitivity for a range of ultrasound scanners. Ultrasound Med Biol 2004301475–1483. [DOI] [PubMed] [Google Scholar]
- 35.Stuecker M, Baier V, Reuther T, Hoffmann K, Kellam K, Altmayer P. Capillary blood cell velocity in human skin capillaries located perpendiculary to the skin surface: measured by a new laser Doppler anemometer. Microvasc Res 199652188–192. [DOI] [PubMed] [Google Scholar]
- 36.Terslev L, Torp‐Pedersen S, Qvistgaard E, von der Recke P, Bliddal H. Doppler ultrasound findings in healthy wrists and finger joints. Ann Rheum Dis 200463644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terslev L, Torp‐Pedersen S, Bang N, Koenig M J, Nielsen M B, Bliddal H. Doppler ultrasound findings in healthy wrists and finger joints before and after use of two different contrast agents. Ann Rheum Dis 200564824–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith M D, Barg E, Weedon H, Papengelis V, Smeets T, Tak P P.et al Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints. Ann Rheum Dis 200362303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]