Abstract
Background
Modulation of Jak‐STAT signalling may provide an effective therapeutic strategy in inflammatory arthritis (IA).
Objective
To examine the effect of successful disease‐modifying antirheumatic drug (DMARD) treatment on the expression of Jak‐STAT in a cohort of patients with active rheumatoid arthritis.
Methods
Synovial tissue biopsy specimens from 16 patients with active rheumatoid arthritis, taken before and after initiation of DMARD treatment, were examined for the presence of janus kinase (Jak)3, signal transducer and activator of transcription (STAT)1, STAT4 and STAT6 expression using immunohistochemistry.
Results
Successful treatment with DMARDs results in reduction in STAT1 expression in the lining, and STAT1 and STAT6 in the sublining of rheumatoid arthritis synovial tissue. Although the overall expression of STAT4 and Jak3 was not significantly altered by DMARD treatment, there was a significant reduction in the expression of the STAT4 and Jak3 bright cells, thought to be an activated dendritic cell subpopulation.
Conclusion
Results show that Jak3, STAT1, STAT4 expression and STAT6 sublining expression decrease in response to successful treatment of rheumatoid arthritis with standard DMARDs. Therefore, altering the expression of these pathways may represent an alternative treatment option, either through promoting up‐regulation of inhibitory pathways, or suppressing inflammatory paths.
Rheumatoid arthritis is a chronic inflammatory disease, with synovial inflammation which is perpetuated by cytokines,1 particularly those produced by macrophages, such as tumour necrosis factor α (TNFα) and interleukin 1 (IL1). The clinical application of treatments targeting TNFα and IL1 have been successful in treating the synovial inflammation in rheumatoid arthritis, but only 60% of patients will obtain a partial response and a minority will experience no benefit.2 Transcription factors bridge the gap between cytokine–receptor interaction at the cell surface and the transcriptional effects of this interaction in the cell nucleus. A limited number of inducible transcription factors seem to play a pivotal part in the regulation of inflammatory genes (eg, activator protein‐1, CCAT/enhancer‐binding proteins (C/EBPs), signal transducer and activator of transcription (STAT), nuclear factor of activated T cells (NF‐AT) and nuclear factor‐kappa B (NF‐κB).3 The janus kinase and signal transducer and activator of transcription (Jak‐STAT) pathway is the signalling target of a multitude of cytokines, including IFNγ, IL2, IL4, IL6, IL7, IL10, IL12 and IL15, all of which are thought to have biologically important roles in rheumatoid synovial inflammation.4,5,6
We have previously demonstrated the expression of Jak3 and STAT1, STAT4 and STAT6 in the synovial tissue from patients with rheumatoid arthritis, seronegative spondyloarthropathies, osteoarthritis and normal synovial tissue.7 The up‐regulation of Jak‐STAT expression in inflammatory arthritis suggests that these intracellular second messengers may be appropriate therapeutic targets. This hypothesis would be supported by the down‐regulation of STAT or Jak expression in the synovial membranes of patients with rheumatoid arthritis, when the disease activity has been down‐modulated in response to disease‐modifying anti‐rheumatic drug (DMARD) treatment. This paper documents the change in expression of Jak3, STAT1, STAT4 and STAT6 in a group of patients with rheumatoid arthritis, before and after successful treatment with DMARDs.
Methods
All patients with rheumatoid arthritis fulfilled the American College of Rheumatology (ACR) criteria for rheumatoid arthritis.8 All patients gave informed consent, and the study protocol was approved by the research and ethics committee of the Repatriation General Hospital, Adelaide, South Australia. All patients were followed up at 3–6‐month intervals, with a range of clinical (tender and swollen joint counts, visual analogue scales for pain, patient and physician global assessments and a modified Health Assessment Questionnaire (HAQ)), and laboratory (C reactive protein (CRP, normal <6 mg/l) and rheumatoid factor (RF, normal <20 IU/ml) (by nephelometry) as well as erythrocyte sedimentation rate) investigations and x ray examinations of hands and feet performed annually. Response to DMARD treatment was assessed by calculating a Disease Activity Score (DAS)9 and an ACR response.10
Synovial membrane samples were obtained from clinically involved knee joints of 16 patients with active rheumatoid arthritis under direct vision using a 2.7‐mm mini‐arthroscope (Dyonics, Andover, Massachusetts, USA) and standard approaches as previously described.11 Table 1 presents the demographic details of the patients included in this study. Patients A to K had a significant clinical response to DMARD treatment, whereas patients L to P had no response to DMARD treatment. Synovial biopsy specimens were obtained from the same knee joint before and at 6‐month intervals after initiation of DMARD treatment. This study used synovial biopsy samples taken at baseline and at the time of maximal clinical response (or no response) after starting treatment with a DMARD (table 1).
Table 1 Clinical characteristics of patients with rheumatoid arthritis at the time of biopsy and ACR response at the time of the second biopsy.
| Patient biopsy | Disease duration (months) | Erosions | DMARD | RF | CRP | DAS28 | HAQ | ACR% | EULAR | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | Initial | 3 | No | SAS | 43 | 130 | 5.5 | 2.6 | High activity | |
| 4 months | 7 | No | 35 | 1 | 2.6 | 1.2 | 50 | Low activity | ||
| B | Initial | 3 | No | IM Gold | 141 | 15 | 5.2 | 1.9 | High | |
| 11 months | 14 | No | 20 | 5 | 0.7 | 0.4 | 80 | Remission | ||
| C | Initial | 9 | Yes | IM Gold | 263 | 84 | 6.4 | 2.9 | High | |
| 17 months | 24 | Yes | 320 | 4 | 1.5 | 0.5 | 80 | Remission | ||
| D | Initial | 36 | Yes | IM Gold + | 486 | 162 | 6.9 | 2.8 | High | |
| 10 months | 48 | Yes | Mtx | 194 | 19 | 4.4 | 1.5 | 40 | Moderate | |
| E | Initial | 3 | No | Mtx | 20 | 20 | 5.6 | 2.4 | High | |
| 41 months | 36 | No | 20 | 1 | 0.6 | 0 | 90 | Remission | ||
| F | Initial | 180 | Yes | Mtx | 504 | 16 | 5.8 | 1.8 | High | |
| 8 months | 192 | Yes | 145 | 9 | 1.8 | 0.25 | 70 | Remission | ||
| G | Initial | 3 | No | IM Gold | 351 | 62 | 5.2 | 2.7 | High | |
| 6 months | 9 | No | 26 | 2 | 0.7 | 0.25 | 90 | Remission | ||
| H | Initial | 6 | No | IM Gold | 143 | 26 | 6.3 | 2.6 | High | |
| 14 months | 20 | Yes | 20 | 1 | 0.7 | 0 | Remission | Remission | ||
| I | Initial | 12 | No | IM Gold | 404 | 116 | 5.8 | 1.9 | High | |
| 30 months | 42 | Yes | 25 | 1 | 0.9 | 0 | 90 | Remission | ||
| J | Initial | 168 | Yes | IM Gold + | 400 | 167 | 6.9 | 3 | High | |
| 11 months | 180 | Yes | Mtx | 36 | 12 | 2.6 | 0 | 90 | Low | |
| K | Initial | 180 | No | Mtx | 20 | 83 | 6 | 2.7 | High | |
| 24 months | 204 | Yes | 20 | 1 | 0.8 | 1.3 | 90 | Remission | ||
| L | Initial | 108 | Yes | CyA | 213 | 51 | 5.9 | 2.3 | High | |
| 8 months | 116 | Yes | 240 | 45 | 6.2 | 2.7 | 0 | High | ||
| M | Initial | 36 | Yes | IM Gold | 486 | 102 | 6.9 | 2.8 | High | |
| 6 months | 42 | Yes | 345 | 86 | 5.4 | 2.3 | 0 | High | ||
| N | Initial | 3 | Yes | IM Gold | 504 | 16 | 5.8 | 1.8 | High | |
| 9 months | 12 | Yes | 520 | 26 | 5.5 | 2.3 | 0 | High | ||
| O | Initial | 6 | No | IM Gold + Mtx | 451 | 24 | 4.8 | 2.7 | High | |
| 9 months | 15 | No | 540 | 53 | 4.7 | 2.3 | 0 | High | ||
| P | Initial | 6 | No | SAS | 43 | 130 | 5.5 | 2.6 | High | |
| 8 months | 14 | No | 68 | 89 | 5.2 | 2.3 | 0 | High | ||
ACR, American College of Rheumatology; CRP, C reactive protein (mg/l); CyA, iclosporin A; DMARD, disease‐modifying antirheumatic drug; EULAR, EULAR response criteria9; IM Gold, intramuscular sodium aurothiomalate; mtx, methotrexate; SAS, sulphasalazine.
Erosions, radiological evidence of joint erosions on hand and feet x‐rays; RF, rheumatoid factor titre (IU/l); HAQ, Health Assessment Questionnaire.
Percentage response based on American College of Rheumatology response criteria.10
Disease Activity Score based on a 28 joint count.9
Immunohistochemistry
Cryosections of thickness of 4 µm were prepared on 3‐aminopropyltriethoxysilane (APTS); Sigma, St Louis, Missouri, USA)‐treated glass slides and fixed in ice‐cold acetone for 4 min. Sections were brought to room temperature, washed in phosphate‐buffered saline, and immunohistochemical labelling for Jak3, STAT1, STAT4 and STAT6 (Santa Cruz Biotechnology, California, USA) as well as cell lineage markers (CD68 Macrophage, DAKO, Botany, NSW, Australia), CD55‐positive synovial lining fibroblast (Serotec, Oxford, UK), CD3‐positive T lymphocytes (Becton Dickinson, New Jersey, USA), CD45Ro‐positive memory T lymphocytes (DAKO, Botavy, Australia), CD22‐positive B lymphocytes (Serotec) was carried out on all tissues using a double enhancement method as previously published. 7 To exclude bias from run‐to‐run variability, sections from the same patient before and after treatment were stained on the same day.
For double immunohistochemistry, sections were incubated with STAT4 followed by a secondary and tertiary antibody. Subsequently, tissue was blocked with 0.1 M TRIS 0.02 M glycine for 60 min at room temperature. A 20% normal donkey serum block was applied for 60 min and the second primary antibody for the cell lineage markers (CD68, CD55, CD3, CD22) was added overnight at 4°C in a humidified chamber. Biotinylated donkey antimouse (Jackson ImmunoResearch, Pennsylvania, USA) was added for 40 min followed by alkaline phosphatase‐antialkaline phosphatase (APAAP) (DAKO) 1:50 for 60 min at room temperature. Signals were detected with fast blue substrate. Counterstaining was not performed on double‐stained sections.
Semiquantitative analysis
Synovial tissue in the lining and sublining region was evaluated by two independent observers, blinded to the order of biopsies and response to DMARD treatment, using a semiquantitative scoring system as previously described,12 where 0, ⩽5% staining; 1, 5–25% staining; 2, 26–50% staining; 3, 51–75% staining; and 4, ⩾75% staining.7 Scores were compared and where differences occurred, a consensus opinion was obtained. As previously described,7 bright cells were defined as intensely staining individual cells, in contrast with the lower intensity of staining of the inflammatory cell infiltrate in the rest of the synovial membrane.
Statistical analysis
Non‐parametric statistics were used to analyse the mean ranks of the semiquantitative scores; p<0.05 was considered significant. Spearman's rank correlations were performed for changes in Jak3 and STAT 1, STAT 4 and STAT 6 with changes in synovial inflammatory cell infiltrates (CD68, CD55, CD3, CD45Ro, CD22 and CD38), with p values corrected for multiple comparisons.
Results
Eleven patients included in this study had a significant response to DMARD treatment, with ACR responses achieved with treatment being at least 40%, on the basis of the published ACR response criteria10 and EULAR response being moderate or greater in all 11 patients.9 An additional group of five patients with rheumatoid arthritis who also had sequential synovial biopsies from a single knee joint before and after DMARD treatment and had no response to treatment were studied similarly.
Effect of DMARD treatment on Jak/STATexpression in rheumatoid arthritis synovial tissue
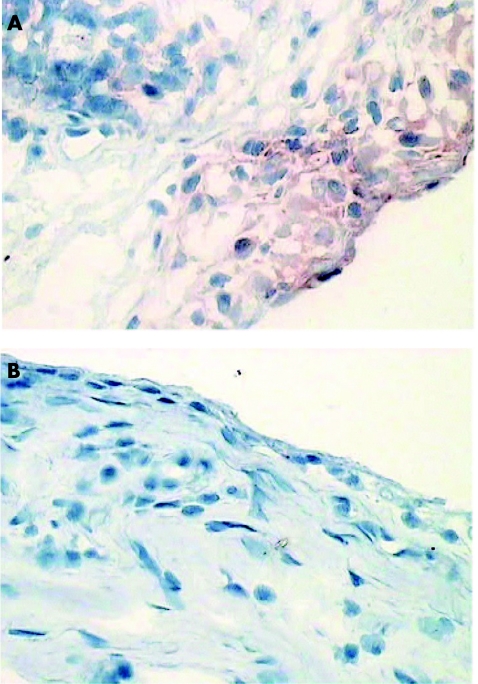
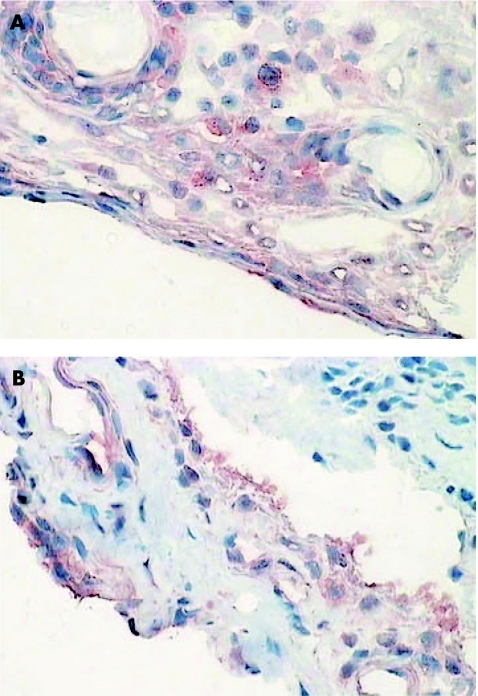
Expression of STAT1 in both the lining (p<0.046) and sublining (p = 0.018) of rheumatoid arthritis synovial tissue was found to be significantly down‐regulated after effective DMARD treatment (table 2; fig 1). By contrast, there was no significant change in overall expression of STAT4 or Jak3 in rheumatoid arthritis synovial tissue in response to DMARD treatment. There was no major difference in STAT6 lining expression, but sublining expression was significantly reduced after DMARD treatment. However, these results must be interpreted with caution, as the overall level of inflammatory cells in the synovial sublining was also dramatically reduced and the semiquantitative score cannot reliably be corrected for this change in cellular infiltrate (fig 2).
Table 2 Results of semiquantitative scoring for STAT1, STAT4, STAT6 and Jak3 expression in synovial tissue of rheumatoid arthritis patients before and after disease‐modifying antirheumatic drug treatment.
| Median SQA STAT1 (range) | Median SQA STAT4 (range) | Median SQA STAT6 (range) | Median SQA Jak3 (range) | ||
|---|---|---|---|---|---|
| Responders (n = 11) Before DMARD treatment | Lining | 4 (1–4) | 0 (0–1) | 3 (0–4) | 0 (0–2) |
| Sublining | 2 (0–4) | 0 (0–2) | 2 (0–4) | 0 (0–3) | |
| Responders (n = 11) After DMARD treatment | Lining | 1 (0–3)* | 0 (0) | 2 (0–4) | 0 (0) |
| Sublining | 0 (0–3)* | 0 (0) | 0 (0–3)* | 0 (0) | |
| Non‐responders (n = 5) Before DMARD treatment | Lining | 3 (1–4) | 1 (1–2) | 2 (1–3) | 0 (0–2) |
| Sublining | 2 (1–3) | 1 (1–2) | 1 (1–2) | 0 (0–2) | |
| Non‐responders (n = 5) After DMARD treatment | Lining | 2 (1–2) | 1 (1–2) | 1 (1–2) | 0 (0–2) |
| Sublining | 2 (1–3) | 2 (1–2) | 2 (1–2) | 0 (0–1) |
DMARD, disease‐modifying antirheumatic drug; SQA, semi‐quantitative analysis; STAT, signal transducer and activator of transcription.
Responders, patients who have had a significant clinical response to DMARD treatment; Non‐responders, patients who have not had a significant clinical response to DMARD treatment.
*p<0.05 compared with baseline biopsy.
Figure 1 Synovial biopsy specimens taken before (A) and after (B) disease‐modifying antirheumatic drug treatment, stained for signal transducer and activator of transcription 1.
Figure 2 Synovial biopsy specimens taken before (A) and after (B) disease‐modifying antirheumatic drug treatment, stained for signal transducer and activator of transcription 6.
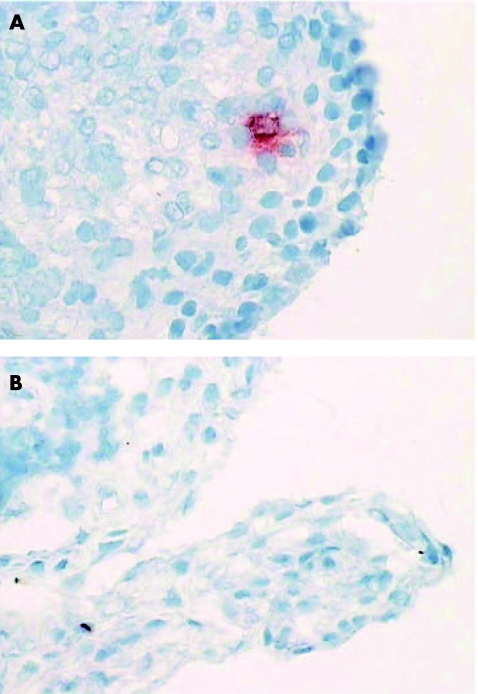
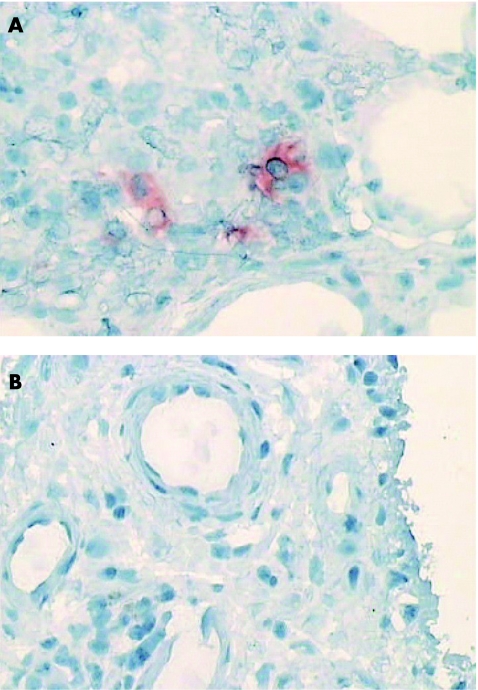
Jak3 and STAT4 bright cell expression is reduced after successful DMARD treatment
Jak3 (fig 3) and STAT4 (fig 4) bright cell expression (presumptive dendritic cells7) was down‐regulated after treatment with DMARDs in our cohort of treatment responders (Jak3, p = 0.025; STAT4, p = 0.014). Similarly, STAT6 bright cell expression was also significantly downregulated in rheumatoid arthritis patients responding to DMARD treatment (p = 0.011). We also noted that 3 patients had evidence of a few intensely staining cells for STAT1. These intensely staining cells were of different morphology to the Jak3, STAT4 and STAT6 bright cells, which have previously been characterised as presumptive dendritic cells and were more consistent with a macrophage morphology. The expression of these occasional STAT1 bright cells did not change significantly in response to treatment (p>0.05), in contrast with the Jak3, STAT4 and STAT6 positive bright cells.
Figure 3 Synovial biopsy specimens taken before (A) and after (B) disease‐modifying antirheumatic drug treatment, stained for janus kinase 3, showing a reduction in bright cells (presumptive dendritic cells).
Figure 4 Synovial biopsy specimens taken before (A) and after (B) disease‐modifying antirheumatic drug treatment, stained for signal transducer and activator of transcription 4, showing a reduction in bright cells (presumptive dendritic cells).
Effect of successful DMARD treatment on inflammatory cell expression of Jak3 and STAT1, STAT4 and STAT6
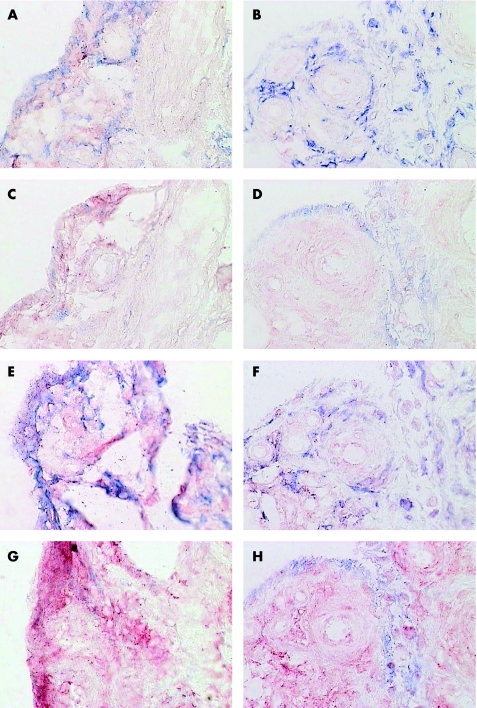
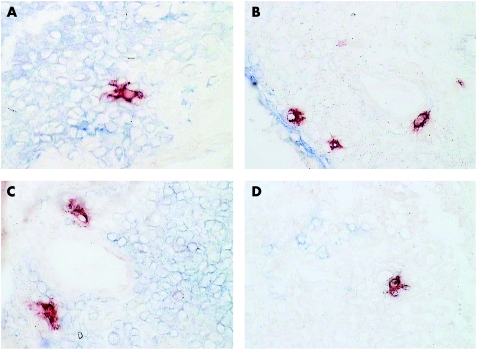
Single and double immunohistochemical labelling was performed to establish which inflammatory cells in the synovial membrane expressed Jak3 and STAT1, STAT4 and STAT6 and whether the changes in expression of Jak3 and STAT1, STAT4 and STAT6 with DMARD treatment correlated with changes in particular inflammatory cell subsets. STAT1 is expressed mainly by CD55‐positive synovial fibroblasts in the lining but also by CD68‐positive macrophages in the lining and sublining and T cells in the sublining. There was a pronounced reduction in STAT1 expression by all cells in the synovial membrane with DMARD treatment. STAT6 is expressed by all cellular components of the synovial membrane, and the expression of STAT6 is reduced in all cells with the exception of CD55‐positive synovial lining fibroblasts as a result of DMARD treatment (fig 5). There was a negative correlation between STAT6 staining and CD55 staining in the lining of the synovial tissue, but there were no other significant correlations between changes in Jak3, STAT1, STAT4 and STAT6 staining and changes in cellular infiltrate of the synovial membrane, when p values were corrected for multiple comparisons. STAT4 and Jak3 are expressed mainly by putative dendritic cells7 in the synovial sublining (fig 6), with some expression by CD68‐positive macrophages and T cells in the synovial sublining. The main effect of successful DMARD treatment is the disappearance of the putative dendritic cells, which stain brightly for Jak3 and STAT4 (figs 3 and 4).
Figure 5 Double labelling of pretreatment (A,C,E,G) and post‐treatment (B,D,F,H) synovial biopsy specimens with anti‐signal transducer and activator of transcription (STAT)1 or anti‐STAT6 (A,E,C; red) and cell lineage markers (alkaline phosphatase, blue). (A,B) Anti‐STAT1 and anti‐CD68 (macrophages); (C,D) Anti‐STAT1 and anti‐CD55 (synovial lining fibroblasts); (E,F) Anti‐STAT6 and anti‐CD68; (G,H) Anti‐STAT6 and anti‐CD55.
Figure 6 Double labelling of a pretreatment synovial biopsy specimen with anti‐signal transducer and activator of transcription (STAT)4 (A, E, C; red) and cell lineage markers (alkaline phosphatase, blue). (A) Anti‐STAT4 and anti‐CD68 (macrophages); (B): anti‐STAT4 and anti‐CD55 (synovial lining fibroblasts); (C) anti‐STAT4 and anti‐CD3 (T cells); (D) anti‐STAT4 and anti‐CD22 (B cells).
Lack of change in synovial expression of Jak3, STAT1, STAT4 and STAT6 after unsuccessful DMARD TREATMENT
An additional group of five patients with rheumatoid arthritis who did not achieve a significant clinical response to DMARD treatment were included in this study. The duration of treatment was short in this group, and it was not ethical to continue with ineffective treatment for any significant period of time. However, all five patients did not show any significant change in Jak3 or STAT1, STAT4 or STAT6 expression when the biopsies taken before and after DMARD treatment were compared. In addition, there was no decrease in the expression of brightly stained Jak3 and STAT4 cells (putative dendritic cells) in the synovial biopsies taken before and after DMARD treatment, in contrast with the decrease in these cells in clinical responders to DMARD treatment (table 2).
Discussion
This study has shown that Jak3, STAT1 and STAT4 expression, and STAT6 sublining expression decrease in response to successful treatment of rheumatoid arthritis with standard DMARDs.
We have recently reviewed the role of the Jak/STAT pathway in the context of a possible therapeutic target for the treatment of rheumatoid arthritis.6 STAT1 seems to have an anti‐inflammatory and pro‐apoptotic role in the rheumatoid synovium, possibly through up‐regulation of suppressor of cytokine signalling‐1,14,15,16 producing feedback inhibition of cytokine‐induced Jak/STAT activation. Down‐regulation of STAT1 expression in response to successful DMARD treatment is consistent with a potential role in modulating the inflammatory response of active rheumatoid arthritis. Although we were unsuccessful in showing activated STAT1 (pSTAT1) staining using immunohistochemistry methods (extremely weak staining, results not shown), others using different antibody preparations have shown that pSTAT1 is increased in rheumatoid arthritis tissues as compared with controls. Moreover, expression of pSTAT1 was found to be proportional to overall STAT1 expression and therefore reflects increased pSTAT1 activity.14 Earlier work by the same group15 had shown increased expression of STAT1 mRNA on microarray analysis in those patients with more active rheumatoid arthritis.
IL4, known to have an anti‐inflammatory role in the rheumatoid synovium, signals through STAT6 and inhibits NFκB and jun kinase pathways.17 It has been proposed that modulating the Th1/Th2 balance by altering the expression of STAT6 may be an effective means of reducing inflammation.18 Our initial research showed that STAT6 was widely expressed in all arthritis synovial tissues tested and was even easily detectable in normal synovium.7 Therefore, we have some concerns about targeting STAT6 as a disease modulator, because its wide level of expression suggests that it may play important homoeostatic functions in the synovium. Our findings show that although STAT6 expression is maintained in the synovial lining, expression in the sublining is reduced after DMARD treatment. This result must be interpreted with caution as its reduction is largely due to the dramatic decline in sublining inflammatory cell infiltrate in rheumatoid arthritis synovial tissue after DMARD treatment.
Jak3, STAT4 and STAT6 bright cell expression was reduced significantly in response to successful DMARD treatment. We have previously hypothesised that these may be dendritic cells undergoing activation,7 and as such, targeting these signal transduction pathways may represent a novel means of modulating dendritic cell function in rheumatoid arthritis. The expression of Jak3 is largely limited to haematopoietic cell lines and this makes it an attractive target for treatment‐induced disease modulation, in view of the major role that these cells play in chronic inflammation in rheumatoid arthritis. We have previously shown increased Jak3 expression in the lining and sublining of patients with rheumatoid arthritis compared with those with osteoarthritis and normal tissues,7 and hence a Jak3 inhibitor may be a useful addition to therapeutics in rheumatoid arthritis. Specific inhibitors to Jak3 already exist and are being tested in transplant models.18 Although our study did not show any difference in Jak3 expression after DMARD treatment, the baseline synovial expression of Jak3 was lower in this study than we have previously shown,7 possibly related to earlier disease and lower disease activity in this patient group. Modulating the activity of these presumptive dendritic cells in rheumatoid arthritis, possibly through the use of a Jak3 inhibitor, may provide a novel means of altering the natural history of rheumatoid arthritis.
Marked changes in expression of signal transduction components in patients with rheumatoid arthritis responding to DMARD treatment support the development and testing of Jak and STAT inhibitors as novel alternative therapeutic agents. Therefore, modulation of these pathways may represent an alternative treatment option, either through promoting up‐regulation of inhibitory pathways or suppressing inflammatory pathways.
Footnotes
This study was supported by the Daw Park research Foundation, National Health and Medical Research Council of Australia and the Arthritis Foundation of Australia.
Competing interests: None declared.
References
- 1.Weyand C M, Goronzy J J, Takemura S, Kurtin P J. Cell‐cell interaction in synovitis. Interactions between T cells and B cells in rheumatoid arthritis. Arthritis Res 20002457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Firestein G. Evolving concepts of rheumatoid arthritis. Nature 2003423356–361. [DOI] [PubMed] [Google Scholar]
- 3.Makarov S S, NF‐κΒ in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia and tissue destruction Arthritis Res. 2001;3:200–206. doi: 10.1186/ar300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello C A, Moldawer L L.Proinflammatory and anti‐inflammatory cytokines in rheumatoid arthritis. A primer for clinicians. Thousand Oaks, CA: Amgen, 2002
- 5.Gadina M, Hilton D, Johnston J A, Morinobu A, Lighvani A, Zhou Y.et al Signaling by type I and II cytokine receptors: ten years after. Curr Opinion Immunol 200113363–373. [DOI] [PubMed] [Google Scholar]
- 6.Walker J G, Smith M D. The Jak‐STAT pathway in rheumatoid arthritis. J Rheum 2005321650–1653. [PubMed] [Google Scholar]
- 7.Walker J G, Ahern M J, Coleman M, Weedon H, Papangelis V, Beroukas D.et al Expression of Jak3, STAT1, STAT4 and STAT6 in inflammatory arthritis: unique Jak3 and STAT4 expression in dendritic cells in seropositive rheumatoid arthritis. Ann Rheum Dis 200665149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnett F C, Edworthy S M, Bloch D A, Mc Shane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 9.Prevoo M L, van't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–46. [DOI] [PubMed] [Google Scholar]
- 10.Felson D T, Anderson J J, Boers M, Bombardier C, Chernoff M, Fried B.et al The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum 199336729–740. [DOI] [PubMed] [Google Scholar]
- 11.Smith M, Chandran G, Youssef P, Darby T, Ahern M. Day case knee arthroscopy under regional anaesthesia performed by rheumatologists. Aust NZ J Med 199626108–109. [DOI] [PubMed] [Google Scholar]
- 12.Tak P P, Lubbe PAvd, Cauli A, Daha M R, Smeets T J, Kluin P M.et al Reduction of synovial inflammation after anti‐CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum 1995381457–1465. [DOI] [PubMed] [Google Scholar]
- 13.Ivashkiv L B, Hu X. Signaling by STATs. Arthritis Res 20046159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasperkovitz P, Verbeet N, Smeets T, Rietschoten J V, Kraan M, Kraan TvdP.et al Activation of the STAT1 pathway in rheumatoid arthritis. Ann Rheum Dis 200463233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraan T V d P, Gaalen F v, Kasperkovitz P, Verbeet N, Smeets T, Kraan M.et al Rheumatoid arthritis is a heterogeneous disease. Evidence for differences in the activation of the STAT‐1 pathway between tissues. Arthritis Rheum 2003482132–2145. [DOI] [PubMed] [Google Scholar]
- 16.Hooge A, Loo F, Koenders M I, Bennink M B, Arntz O J, Kolbe T.et al Local Activation of STAT‐1 and STAT‐3 in the inflamed synovium during zymosan‐induced arthritis. Arthritis Rheum 2004502014–2023. [DOI] [PubMed] [Google Scholar]
- 17.Hirayama T, Dai S, Abbas S, Yamanaka Y, Abu‐Amer Y.et al Inhibition of inflammatory bone erosion by constitutively active STAT‐6 through blockade of JNK and NF‐kappaB activation. Arthritis Rheum 2005522719–2729. [DOI] [PubMed] [Google Scholar]
- 18.O'Shea J J. Targeting the Jak/STAT pathway for immunosuppression. Ann Rheum Dis 200463(Suppl II)ii67–ii71. [DOI] [PMC free article] [PubMed] [Google Scholar]