Abstract
Objective
To evaluate the efficacy of anti‐tumour necrosis factor (TNF) treatments (given for rheumatological manifestations) in reducing uveitis flares in patients with spondylarthropathy in daily practice.
Methods
A retrospective observational study of all patients with spondylarthropathy with at least one uveitis flare treated with anti‐TNF in one centre (December 1997–December 2004). The number of uveitis flares per 100 patient‐years was compared before and during anti‐TNF treatment; each patient was his or her own control. The relative risk (RR) and the number needed to treat (NNT) were calculated.
Results
46 patients with spondylarthropathy treated with anti‐TNF drugs had at least one uveitis flare (33 treated with anti‐TNF antibodies, infliximab or adalimumab, and 13 with soluble TNF receptor, etanercept). The mean age at first symptoms was 26 years, 71% were men. Patients were followed for 15.2 years (mean) before anti‐TNF versus 1.2 years during anti‐TNF treatment. The number of uveitis flares per 100 patient‐years before and during anti‐TNF were, respectively: for all anti‐TNF treatments,—51.8 v 21.4 (p = 0.03), RR = 2.4, NNT = 3 (95% confidence interval (CI) 2 to 5); for soluble TNF receptor—54.6 v 58.5 (p = 0.92), RR = 0.9; and for anti‐TNF antibodies—50.6 v 6.8 (p = 0.001), RR = 7.4, NNT = 2 (95% CI 2 to 5).
Conclusion
Anti‐TNF treatments were efficacious in decreasing the number of uveitis flares in patients with spondylarthropathy. Anti‐TNF antibodies decreased the rate of uveitis flares, whereas soluble TNF receptor did not seem to decrease this rate. These results could have consequences for the choice of anti‐TNF treatment in certain patients.
Uveitis is a well‐known extra‐rheumatological manifestation of spondylarthropathies, which may lead to severe functional impairment.1 One study has shown considerably higher levels of tumour necrosis factor (TNF) in the aqueous humor2 and inflamed joints3 of patients with spondylarthropathy. Anti‐TNF drugs have shown their efficacy in preventing relapses of rheumatological manifestations of spondylarthropathies.4 Thus, from a physiopathology point of view, anti‐TNF treatment seems to be efficacious for spondylarthropathy‐related uveitis flare. However, it is established that etanercept (soluble TNF receptor) is not efficacious in inflammatory bowel disease, whereas infliximab and adalimumab (anti‐TNF antibodies) do well.5,6 All anti‐TNF drugs seem efficacious in treating psoriasis.7 The question remains for uveitis. Small studies are available concerning the efficacy of anti‐TNF drugs in treating uveitis8,9 and tend to show at least some efficacy. One larger study published recently10 compared the efficacies of infliximab and etanercept in decreasing the number of uveitis flares in 717 patients with ankylosing spondylitis in seven placebo‐controlled studies. During the treatment with anti‐TNF, the incidence of anterior uveitis flares was 3.4/100 patient‐years with infliximab and 6.4/100 patient‐years with etanercept. With placebo, flares occurred at a rate of 16.2/100 patient‐years. This was significantly different from the rates for treatment with infliximab than for etanercept (p = 0.001), with a larger reduction for infliximab than for etanercept. There was no significant difference between the two compounds (p = 0.27).
The objective of this study was to compare the efficacies of etanercept, infliximab and adalimumab in reducing uveitis flares in patients with spondylarthropathy in daily practice conditions.
Patients and methods
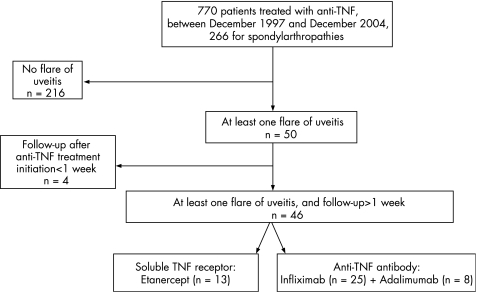
A systematic retrospective observational study was conducted in a tertiary referral centre (Cochin Hospital, Paris, France). Inpatients and outpatients were selected through a computer survey of patient files for visit or hospitalisation between December 1997 and December 2004 using the keywords anti‐TNF, etanercept, infliximab and adalimumab. Data were collected between December 2004 and March 2005, face‐to‐face, with complete clinical and radiological records, or from the computer database. Figure 1 shows the patient selection process.
Figure 1 Flow chart of patient selection process.
All patients who had received at least one anti‐TNF for rheumatological manifestations were initially selected, including patients with a spondylarthropathy according to Amor criteria,11 whatever the clinical form (axial such as in ankylosing spondylitis, peripheral or other). Patients with at least one uveitis flare noted in the charts at any time point were selected. When the precise number of uveitis flares occurring in a patient was not explicit, the patient was asked by telephone. As is common in retrospective studies, some information was not available, such as the clinical characteristics of the uveitis and its specific treatment. The only exclusion criterion was a follow‐up in our centre for <1 week after the initiation of anti‐TNF. Only the first treatment course was considered. Two groups were retrospectively determined: soluble TNF receptor (etanercept) and anti‐TNF antibody (infliximab and adalimumab). Patient characteristics collected were sex, age at first symptoms, age at the initiation of the anti‐TNF, clinical presentation (entheseal, peripheral, axial, extra‐articular, undetermined), presence of human leucocyte antigen B27 antigen, type of anti‐TNF drug, concomitant disease‐modifying anti‐rheumatic drugs (DMARDs) at anti‐TNF initiation (methotrexate, leflunomide, gold salt, hydroxychloroquine, sulfasalazine) and corticosteroids, prescribed for rheumatological manifestations. The period before anti‐TNF treatment was calculated from the date of first symptoms of spondylarthropathy to the date of initiation of the anti‐TNF. The study period was the period from the date of the initiation of the anti‐TNF to the date of interruption of treatment, or to the end of the study (December 2004). One uveitis flare was considered as one event. Each patient was his or her own control.
Statistical analysis
Descriptive data were analysed with SAS V.8.0 statistical software. Double data entry was performed. The characteristics of patients treated with anti‐TNF antibodies and soluble TNF receptor were compared using χ2 and t tests. The rate of uveitis flares before anti‐TNF treatment was calculated as the number of events (uveitis) that occurred before anti‐TNF initiation divided by the disease duration before anti‐TNF treatment (rate/100 patient‐years). The rate of uveitis flares during anti‐TNF treatment was defined and calculated as the number of events (uveitis) which occurred during anti‐TNF treatment divided by the duration of the anti‐TNF treatment (rate/100 patient‐years). Matched data analysis was used to compare the rate of uveitis before and during anti‐TNF. The relative risk (RR) ratio of uveitis flares was defined as the rate of uveitis flares before anti‐TNF treatment divided by the rate of uveitis flares during anti‐TNF treatment (for all anti‐TNF drugs, whatever the type, soluble TNF receptor, anti‐TNF antibodies, adalimumab and infliximab). The number of patients needed to treat (NNT) was defined as the number of patients needed to treat to avoid one uveitis flare in one patient over 1 year. The NNT was calculated as the inverse ratio of the absolute risk reduction (defined as number of uveitis flares/100 patient‐years during treatment−number of uveitis flares/100 patient‐years before treatment). The advantage of the NNT is that it reflects an absolute risk reduction, and, because it is related to the control event rate, it reflects the true baseline or underlying risk of the study population. For rational decision making in daily clinical practice, absolute measures such as NNT are more meaningful than relative measures.12
Results
Between 1997 and 2004, 770 patients received at least one course of anti‐TNF, of whom 266 had a spondylarthropathy; 50 patients had at least one uveitis flare at any time point (fig 1), among whom 46 were followed for >1 week after anti‐TNF initiation for their rheumatological manifestations: 13 patients received a soluble TNF receptor and 33 patients an anti‐TNF antibody. Eight patients received adalimumab, 25 patients infliximab. Table 1 shows the patient characteristics.
Table 1 Characteristics of 46 patients with spondylarthropathy with at least one uveitis flare, and treated with anti‐tumour necrosis factor (TNF) antibody and soluble TNF receptor between December 1997 and December 2004.
| Characteristics | All patients (n = 46) | Anti‐TNF antibodies infliximab andadalimumab (n = 33) | Soluble TNF receptor etanercept (n = 13) | p Value* |
|---|---|---|---|---|
| Age at first symptoms, mean (SD), years | 25.9 (9.2) | 26.0 (9.6) | 25.9 (8.5) | 0.7 |
| Age at initiation of anti‐TNF treatment, mean (SD), years | 40.0 (10.3) | 41.1 (9.6) | 37.3 (11.8) | 0.3 |
| Male, n (%) | 33 (71.7) | 24 (72.7) | 9 (69.2) | 1 |
| Predominant clinical form of spondylarthropathy for which anti‐TNF was prescribed | 1 | |||
| Axial, n (%) | 33 (71.8) | 23 (69.9) | 10 (76.9) | |
| Peripheral, n (%) | 9 (19.5) | 6 (18.1) | 3 (23.1) | |
| Enthesitic, n (%) | 1 (2.2) | 1 (3.0) | 0 | |
| Undetermined, n (%) | 3 (6.5) | 3 (9.0) | 0 | |
| HLA B27: ± known: n (%)† | 36/40 (90.0) | 26/28 (92.9) | 10/12 (83.3) | 0.6 |
| One concomitant DMARD: yes, n (%) | 15 (32.6) | 12 (36.4) | 3 (23.1) | 0.5 |
| Concomitant corticosteroids: yes, n (%) | 12 (26.1) | 11 (33.3) | 1 (7.7) | 0.1 |
*p Value of the comparison between the patients treated with antibody and those treated with soluble receptor.
†Positivity is given on known data.
SD, standard deviation; DMARD, disease‐modifying anti‐rheumatic drug; HLA, human leucocyte antigen; TNF: tumor necrosis factor.
Mean age at first symptoms was 26 years, mean age at initiation of anti‐TNF treatment was 40 years, 33 patients (71.7%) were male. Human leucocyte antigen B27 was positive in 36 patients (90% of available data). Although patients treated with soluble receptor received less DMARDs and corticosteroids, these results did not differ significantly between the treatment groups. No patient received sulfasalazine, the only DMARD for which a reduction in the number of uveitis flares was shown. The mean disease duration before anti‐TNF initiation (table 2) was 15.2 v 1.2 years during anti‐TNF treatment. The mean number of uveitis flares per patient before and during anti‐TNF treatment was 6.3 v 0.2 with all anti‐TNF drugs. The number of uveitis flares/100 patient‐years before and during anti‐TNF treatment was 51.8 v 21.4 with all anti‐TNF drugs (p = 0.03), 54.6 v 58.5 with soluble TNF receptor (p = 0.92), 50.6 v 6.8 with anti‐TNF antibody (p = 0.001), 47.4 v 9.0 with infliximab (p = 0.008) and 60.5 v 0 with adalimumab (p = 0.04).
Table 2 Uveitis flares before and during each kind of anti‐tumour necrosis factor (TNF) treatment (n = 46 patients), in patients with spondylarthropathy: comparison between uveitis flares during the treatment with those during treatment with anti‐TNF antibody and soluble TNF receptor.
| Anti‐TNF (n = patients) | Period before anti‐TNF treatment | Period during anti‐TNF treatment | p Value* | ||||
|---|---|---|---|---|---|---|---|
| Duration of period (years) Mean (SD) | Number of uveitis flares/patient Mean (SD) | Number of uveitis flares/100 patient‐years Mean (SD) | Treatment period (years) Mean (SD) | Number of uveitis flares/patient Mean (SD) | Number of uveitis flares/100 patient‐years Mean (SD) | ||
| All anti‐TNF n = 46 | 15.2 (10.2) | 6.3 (9.7) | 51.8 (65.0) | 1.2 (1.1) | 0.2 (1.0) | 21.4 (74.9) | 0.03 |
| Soluble TNF receptor (etanercept) n = 13 | 11.5 (10.4) | 3.6 (4.1) | 54.6 (78.2) | 1.2 (1.1) | 0.5 (0.8) | 58.5 (121.9) | 0.92 |
| Anti‐TNF antibodies (adalimumab and infliximab) n = 33 | 16.7 (9.8) | 7.3 (11.1) | 50.6 (61.0) | 1.2 (1.1) | 0.1 (1.0) | 6.8 (39.3) | 0.001 |
| Infliximab n = 25 | 16.8 (10.4) | 7.3 (12.1) | 47.4 (58.9) | 1.4 (1.3) | 0.2 (1.2) | 9.0 (45.2) | 0.008 |
| Adalimumab n = 8 | 16.2 (8.7) | 7.2 (7.8) | 60.5 (70.4) | 0.6 (0.2) | 0 | 0 | 0.04 |
TNF, tumour necrosis factor.
*p Value comparing the number of uveitis flares/100 patient‐years before and during the treatment. Each patient is his or her own control.
Owing to the absence of uveitis flare during treatment with adalimumab, it was impossible to calculate the RR and the number of patients needed to treat to avoid one uveitis flare for this molecule. The RR was 2.4 for all anti‐TNF, 0.9 with soluble TNF receptor, 7.4 with anti‐TNF antibodies and 5.2 with infliximab. The NNT was, respectively, 3 (95% confidence interval (CI) 2 to 5) for all anti‐TNF; 125 (95% CI 12 to 10) for TNF soluble receptor; 2 (95% CI 2 to 5) for anti‐TNF antibodies; and 3 (95% CI 2 to 4) for infliximab. In this study, two patients who never had uveitis before anti‐TNF developed uveitis while taking etanercept.
Discussion
This retrospective study, evaluating the efficacy of anti‐TNF in reducing the occurrence of uveitis flares in patients with spondylarthropathy treated with anti‐TNF drugs for their rheumatological manifestations, suggests a difference in the efficacies of soluble TNF receptor and anti‐TNF antibody treatments. In patients with a spondylarthropathy, uveitis flares decreased with anti‐TNF treatment, with an RR of 2.4. Soluble TNF receptor treatment did not reduce flares(RR 0.9), whereas anti‐TNF antibodies greatly reduced flares (RR 7.4). The NNT was 3 for all anti‐TNF treatments pooled together, 2 for anti‐TNF antibodies, 3 for infliximab and non‐significant for etanercept. This means that treating three patients with anti‐TNF avoids one uveitis flare in one patient over 1 year. Treating two patients with anti‐TNF antibodies avoids one uveitis flare in one patient over 1 year, whereas etanercept is not efficacious. As a remark, the treatment duration with adalimumab was shorter; the absence of flare with this drug in this relatively short period of treatment increases the difference between soluble TNF‐blockers and anti‐TNF antibodies.
A recent comparison of uveitis flares during anti‐TNF and placebo treatment10 proved the efficacy of anti‐TNF treatment in reducing uveitis flares. However, the results of this study are at variance with those of Braun et al,10 as etanercept was found to be inefficacious. Further, in this study, patients had a higher rate of uveitis flares without treatment than in Braun's meta‐analysis (15.6/100 patient‐years in the placebo group in Braun et al's meta‐analysis versus 51.8/100 patient‐years before treatment in our study). This difference observed in the number of uveitis flares/100 patient‐years might be explained by different inclusion criteria (any patient participating in an anti‐TNF clinical trial in Braun's meta‐analysis versus only patients who had at least one episode of uveitis flare in our study). Another important difference between the present study and the only other sizeable study10 is that the present study is a daily practice study, whereas Braun's was based on phase III clinical trials. It is recognised that phase III trials, although necessary to show efficacy, only imperfectly reflect what one should look for in daily practice.
The limitations of our study are that it is retrospective, uveitis flares were based solely on patient report, and it contains a small number of patients. The discrepancy in the period of study before and after treatment (15.2 v 1.2 years) could also be a limitation given the sporadic natural history of uveitis in spondylarthropathies and the fact that the number of uveitis flares seems to decrease with time. However, the number of uveitis flares observed in patients treated with etanercept was remarkably stable over time (before and during treatment), which strengthens the results and in particular the differences observed between the different drugs.
This study was retrospective, and as such, the populations treated by etanercept and anti‐TNF antibodies were not strictly similar, as etanercept‐treated patients were less often treated with a concomitant DMARD and corticosteroids. This could partly explain the difference in the number of uveitis flares between the anti‐TNF groups, as methotrexate and corticosteroids have some efficacy on uveitis flares. Nevertheless, the differences in DMARDs and corticosteroids did not reach statistical significance. The other difference between the two groups of patients is that patients receiving etanercept had on average fewer flares before treatment, although this was also not significant.
This study also has its strengths. It was performed in clinical practice conditions, with a long period of follow‐up, and was exhaustive using all patients of the centre, thus avoiding selection bias. Furthermore, the great stability noted in the rate of uveitis flares for patients treated with etanercept argues against memory bias from patients, or reporting bias, and strengthens our results.
The difference in efficacies of soluble TNF receptor and anti‐TNF antibodies has been observed in inflammatory bowel disease, another extra‐rheumatological manifestation of spondylarthropathy. As infliximab binds the transmembrane‐bound TNF of T cells, it induces apoptosis of activated lymphocytes, thereby alleviating a fundamental defect in Crohn's disease in the regulation of T cells, which is not an effect of soluble TNF receptor.13,14 This suggests that in Crohn's disease an additional mechanism such as cell lysis seems to be necessary. The same explanation could be put forward for the difference in the efficacies of these two types of treatment for uveitis. Moreover, there remains the question of a paradoxical effect of etanercept on ophthalmological or digestive manifestations of spondylarthropathies: case reports described Crohn colitis flares during treatment with etanercept, while the rheumatological manifestations of spondylarthropathy were perfectly controlled.15 In the present study, two patients who never had uveitis before anti‐TNF treatment developed uveitis after treatment with etanercept (which did not occur with anti‐TNF antibody treatment).
The difference in the efficacies of the different anti‐TNF treatments for uveitis flares may have implications in clinical practice. Indeed, our results suggest that using an anti‐TNF antibody should perhaps be considered first rather than using a soluble TNF receptor, in patients with spondylarthropathy having uveitis flares. These findings should be confirmed by prospective controlled studies.
Abbreviations
DMARD - disease‐modifying anti‐rheumatic drug
NNT - number needed to treat
TNF - tumour necrosis factor
References
- 1.Durrani O M, Tehrani N N, Marr J E, Moradi P, Stavrou P, Murray P I. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol 2004881159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos Lacomba M, Marcos Martin C, Gallardo Galera J M, Gomez Vidal M A, Collantes Estevez E, Ramirez Chamond R.et al Aqueous humor and serum tumor necrosis factor‐alpha in clinical uveitis. Ophthalmic Res 200133251–255. [DOI] [PubMed] [Google Scholar]
- 3.Braun J, de Keyser F, Brandt J, Mielants H, Sieper J, Veys E. New treatment options in spondyloarthropathies: increasing evidence for significant efficacy of anti‐tumor necrosis factor therapy. Curr Opin Rheumatol 200113245–249. [DOI] [PubMed] [Google Scholar]
- 4.Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W.et al Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 200261187–1193. [DOI] [PubMed] [Google Scholar]
- 5.Doubremelle M, Bourreille A, Zerbib F, Heresbach D, Metman E H, Beau P.et al Treatment of Crohn's disease with anti‐TNF alpha antibodies (infliximab): results of a multicentric and retrospective study. Gastroenterol Clin Biol 200226973–979. [PubMed] [Google Scholar]
- 6.Papadakis K A, Shaye O A, Vasiliauskas E A, Ippoliti A, Dubinsky M C, Birt J.et al Safety and efficacy of adalimumab (D2E7) in Crohn's disease patients with an attenuated response to infliximab. Am J Gastroenterol 200510075–79. [DOI] [PubMed] [Google Scholar]
- 7.Tobin A M, Kirby B. TNF alpha inhibitors in the treatment of psoriasis and psoriatic arthritis. BioDrugs 20051947–57. [DOI] [PubMed] [Google Scholar]
- 8.Munoz‐Fernandez S, Hidalgo V, Fernandez‐Melon J, Schlincker A, Martin‐Mola E. Effect of infliximab on threatening panuveitis in Behcet's disease. Lancet 20013581644. [DOI] [PubMed] [Google Scholar]
- 9.Falappone P C, Iannone F, Scioscia C, Grattagliano V, Covelli M, Lapadula G. The treatment of recurrent uveitis with TNF‐alpha inhibitors. Reumatismo 200456185–189. [PubMed] [Google Scholar]
- 10.Braun J, Baraliakos X, Listing J, Sieper J. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti‐tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum 2005522447–2451. [DOI] [PubMed] [Google Scholar]
- 11.Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic 19905785–89. [PubMed] [Google Scholar]
- 12.Laupacis A, Sackett D L, Roberts R S. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 19883181728–1733. [DOI] [PubMed] [Google Scholar]
- 13.Van den Brande J M, Braat H, van den Brink G R, Versteeg H H, Bauer C A, Hoedemaeker I.et al Infliximab but not etanercept induces apoptosis in lamina propria T‐lymphocytes from patients with Crohn's disease. Gastroenterology 20031241774–1785. [DOI] [PubMed] [Google Scholar]
- 14.Sands B E. Why do anti‐tumor necrosis factor antibodies work in Crohn's disease? Rev Gastroenterol Disord 20044(Suppl 3)S10–S17. [PubMed] [Google Scholar]
- 15.Oh J, Arkfeld D G, Horwitz D A. Development of Crohn's disease in a patient taking etanercept. J Rheumatol 200532752–753. [PubMed] [Google Scholar]