Abstract
Background
Optimal use of disease‐modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis is vital if progression of disease is to be reduced. Methotrexate (MTX) and sulfasalazine (SASP) are widely used inexpensive DMARDs, recently often combined despite no firm evidence of benefit from previous studies.
Aim
To establish whether a combination of SASP and MTX is superior to either drug alone in patients with rheumatoid arthritis with a suboptimal response to 6 months of SASP.
Methods
A randomised controlled study of step‐up DMARD treatment in early rheumatoid arthritis. In phase I, 687 patients received SASP for 6 months. Those with a disease activity score (DAS) ⩾2.4 were offered additional treatment in phase II (SASP alone, MTX alone or a combination of the two). The primary outcome measure was change in DAS.
Results
At 6 months, 191 (28%) patients had a DAS <2.4, 123 (18%) were eligible but did not wish to enter phase II, 130 (19%) stopped SASP because of reversible adverse events and 165 (24%) entered phase II. DAS at 18 months was significantly lower in those who received combination treatment compared with those who received either SASP or MTX: monotherapy arms did not differ. Improvement in European League Against Rheumatism and American College of Rheumatology 20, 50 and 70 scores favoured combination therapy.
Conclusions
In this “true‐to‐life” study, an inexpensive combination of DMARDs proved more effective than monotherapy in patients with rheumatoid arthritis with a suboptimal response to SASP. There was no increase in toxicity. These results provide an evidence base for the use of this combination as a component of tight control strategies.
The immune‐mediated synovitis in rheumatoid arthritis results in cartilage loss and bone erosion and contributes to the associated morbidity and premature mortality.1 Optimal treatment in early disease may provide a window of opportunity leading to improved outcome.2 Current guidelines3 advise early and sustained use of disease‐modifying anti‐rheumatic drugs (DMARDs), of which methotrexate (MTX) and sulfasalazine (SASP) are the most commonly used.
Haagsma et al4 conducted two studies using the combination of drugs used in this study. In the first, a controlled open step‐up study in 40 patients “resistant” to SASP, the combination was significantly better than MTX alone. In the second,5 the individual drugs were compared with the combination in a parallel design from the outset. A modest trend favouring the combination of SASP and MTX was seen, with comparable results from the two individual drugs. Nausea was documented as an adverse event more often in the combination group. Dougados et al6 were unable to show a clinically relevant superiority of the combination SASP and MTX in 205 patients treated with combination or single drugs from the outset. Similarly, Dougados et al7 were unable to show significant benefit from a step‐up study of adding SASP in patients who had failed on leflunomide monotherapy.
Current practice aims for maximum achievable improvement in inflammatory disease in rheumatoid arthritis, demonstrated most effectively in the strategy study of Tight Control in Rheumatoid Arthritis (TICORA).8 Whereas combination therapies are widely used “as building blocks” in a tight control approach, it is essential to have a firm evidence base on which to recommend this treatment to patients. Three approaches to combination therapy have been used:
DMARDs used in parallel from the outset—for example, SASP, MTX and hydroxychloroquine (HCQ).9,10,11
Step‐down approach: initial combination, then as disease control is achieved, drugs are dropped—for example, the COBRA study.12
Step‐up approach: addition of drugs in those with an initial inadequate response—for example, as shown with the addition of ciclosporin A to MTX poor responders13 (this study did not include a ciclosporin alone arm).
Three systematic reviews have, however, reported that the overall evidence is not conclusive for the use of combination therapy.14,15,16 Nevertheless, since these were undertaken, several additional trials have been published, some in early and others in late disease.
The much improved response to parallel triple therapy reported in late disease by O'Dell et al10 with SASP, HCQ and MTX has been shown to be sustained to 2 years.14 The step‐down approach of Boers et al12 combining MTX, SASP and high‐dose steroid in early rheumatoid arthritis was associated with an improved clinical response initially, but the difference was not sustained beyond the first year (in contrast, radiographic progression was improved).17
Grigor et al8 used an intensive strategy in the TICORA study. Although this study showed excellent results, and the required intensive regimen of monthly clinic visits was cost neutral, the skilled rheumatological input may be difficult to resource in many areas, and protocol‐driven initial approaches with two drugs of proved value may be a useful initial strategy.
The BeSt study compared (1) sequential monotherapy, (2) step‐up combination therapy, (3) initial combination with tapered high‐dose prednisolone and (4) combination with infliximab, and showed that strategies 3 and 4 resulted in earlier functional improvement and less radiographic damage after 1 year than the other two strategies.18
Anti‐tumour necrosis factor (TNF) therapy is effective in combination with traditional DMARDs,19 but is restricted in the UK according to the National Institute for Health and Clinical Excellence guidelines,20 prohibitively expensive in many countries,21 and the long‐term risks are not yet clarified22,23,24; a recent report suggested increased risk of tumour and infection.25 Older established drugs such as SASP and MTX are inexpensive, and there is extensive information about their toxicity. It is thus possible to use them in a “true‐to‐life” setting, where the patients with rheumatoid arthritis with comorbidities are generalisable rather than the artificially selected patients entering anti‐TNF studies. Cost to healthcare providers is highly relevant. SASP monotherapy is approximately £12/month (€17), MTX £4/month (€6), and etanercept or adalimumab £775/month (€1087).26
This study was designed to determine whether the addition of MTX or a switch to MTX was superior to continuation with SASP in a cohort of patients with early rheumatoid arthritis who had shown a suboptimal response to 6 months of SASP.
Methods
Study design
Step‐up approach: a randomised controlled study in eight Scottish NHS sites—four in Glasgow, three in Lanarkshire and one in Inverness. Multi Centre Research Ethics Committee approval was granted and all patients gave written informed consent. Between May 1999 and June 2003, 687 patients with rheumatoid arthritis were recruited. Age was 18–80 years and disease duration <10 years. All patients had active disease defined by the disease activity score (DAS) of >2.4. The DAS is a validated composite of erythrocyte sedimentation rate (ESR), Ritchie articular index, joint swelling count and patient global assessment of disease activity.27,28 Patients were excluded if they had: prior exposure to either MTX or SASP, known sulphonamide allergy, significant renal (creatinine >150 mmol/dl) or liver (alanine aminotransferase aspartate aminotransferase >80 IU/l, alkaline phosphatase >700 IU/l, γ‐glulamyl transferase ×3) disease, abnormal white cell count (<4×109/l), pre‐existing pulmonary fibrosis, known or planned pregnancy or use of oral steroids >7.5 mg/day. Patients screened who did not meet the entry criteria or were not willing to participate in the study were documented.
Intervention
Drug therapy phase 1
All patients received enteric‐coated SASP 500 mg daily, increasing by 500 mg weekly until the target dose of 40 mg/kg/day (or the maximum tolerated dose), to a maximum dose of 4 g/day for the initial 6 months. Prochlorperazine or similar antiemetic was prescribed if required.
Assessment at the end of phase I
Disease activity was reassessed at 6 months, and patients with DAS ⩾2.4 were included in phase II of the study.
Randomisation
Patients were assigned to one of the three groups by an independent off‐site administrator using randomisation software.29 They were stratified according to rheumatoid factor (positive or negative), erosions (erosive or non‐erosive) and disease duration (<2, 2–5 and >5 years).
Drug therapy phase II
Double‐blind three‐treatment groups:
Continue SASP at the dose achieved by 6 months with the addition of MTX initially 7.5 mg/week (3×2.5 mg), increasing by 2.5 mg/month (1×2.5 mg) until the maximal permitted dose of 25 mg or toxicity occurred.
Continue SASP at the dose achieved by 6 months, with the addition of placebo MTX, initially 3 tablets/week, increasing by 1 tablet/month until the maximal permitted dose of 25 mg weekly or toxicity occurred.
Placebo SASP at the previously achieved number of tablets by 6 months, with the addition of MTX, initially 7.5 mg/week, increasing by 2.5 mg/month until the maximal dose of 25 mg/week or toxicity occurred.
Folic acid 5 mg/week given 3 days after MTX/MTX placebo.
Concomitant non‐steroidal anti‐inflammatory drugs and other drugs were continued. Intra‐articular or intramuscular corticosteroid was permitted, but not within 1 month of the 6, 12 or 18‐month assessments.
Assessments in phase II
Research nurses performed assessments at 6, 9, 12, 15 and 18 months. The patients whose DAS was considered “too good” (DAS <2.4) to receive combination therapy and who continued with SASP or an alternative drug were also assessed at 18 months.
Outcome measures
Primary: Reduction in DAS.
Secondary: Proportion of patients achieving a good response (European League Against Rheumatism (EULAR), ie, DAS <2.4, and a fall in score from baseline by >1.2) and American college of Rheumatology (ACR) 20, 50 and 70 responses (defined by at least 20%, 50% and 70% improvement in joint swelling and joint tenderness counts, and three of five other variables, ie, ESR, health assessment questionnaire, pain score, and assessors' and patients' global assessments30).
In addition, although the power of the study did not allow detailed analysis, individual side effects were recorded and x rays of hands and feet were performed at 6 and 18 months.
Radiology
Two experienced radiologists scored radiographs of hands and feet at 6 and 18 months using the van der Heijde modification of the Sharp score.31 Films were scored with the sequence known, but with the radiologists blinded to treatment groups.
Statistical analysis
On the basis of the difference between the 6‐month and final DAS, it was estimated that completers per group had 90% power to detect a difference of 1 unit in change from baseline DAS at the 2.5% significance level (adjusted for two comparisons), assuming a standard deviation (SD) of 1.2 in change from baseline DAS. In all, 50 completers per group would achieve >95% power based on the same assumptions. Allowing for withdrawals, it was calculated that 50–60 patients needed to be enrolled in each group in phase II. Experience from previous studies7,32 highlighted the need to continue recruiting into phase I until sufficient patients were available to enter phase II.
Analysis was undertaken as intention‐to‐treat last observation on treatment carried forward. Outcome measurements with a non‐Gaussian distribution were expressed as medians and interquartile ranges, and were analysed by the Kruskal–Wallis test, and pairwise comparison between groups was performed using Mann–Whitney U test. ACR responses were assessed using Fisher's exact test.
To assess interobserver variability in radiological scoring, we calculated the correlation between the two radiologists' scores.
The change in erosion score, joint space narrowing and total Sharp score was assessed by Wilcoxon matched pairs (within group) and by Kruskal–Wallis test (across the three groups).
Results
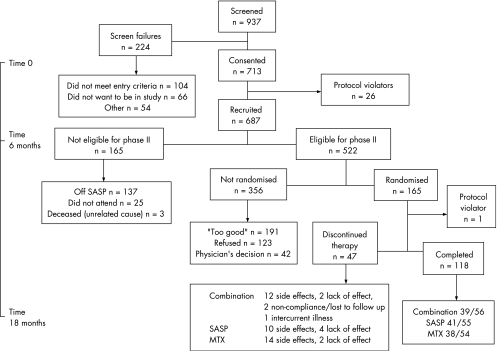
Table 1 shows the demographic features of the patients studied, and fig 1 shows the details of the screening, recruitment and randomisation.
Table 1 Demographic features.
| Phase 1, | Phase II | |||
|---|---|---|---|---|
| entire group (n = 687) | Combination group (n = 56) | SASP alone (n = 55) | MTX alone (n = 54) | |
| Age (years) | 55 (18–80) | 56 (30–78) | 55 (18–77) | 53 (34–79) |
| Disease duration (years) | 1.0 (1–10) | 1.0 (1–9) | 1.0 (1–7) | 1.0 (1–9) |
| Mean | 1.8 | 1.9 | 1.6 | 1.8 |
| Female (%) | 77 | 75 | 75 | 79 |
| Smokers (%) | 42 | 46 | 37 | 58 |
| RF positive (%) | 65 | 68 | 64 | 65 |
| BMI | 28 (16–58) | 28 (21–58) | 28 (18–40) | 29 (16–44) |
| DAS | 4.0 (2.4–7.8) | 3.63 (2.4–5.3) | 3.67 (2.5–5.4) | 3.5 (2.4–5.9) |
| Total Sharp score | — | 17.0 (0–149) | 14.0 (0–92) | 12.0 (0–195) |
BMI, body mass index; DAS, disease activity score; MTX, methotrexate; RF, rheumatoid factor; SASP, sulfasalazine.
The values are represented as median (range).
Overall 70% of patients had a disease duration of ⩽1 year, 22% >1–5 years, and 8% 6–10 years.
Figure 1 Consort diagram. MTX, methotrexate; SASP, sulfasalazine.
We screened 937 patients, and after exclusions 687 entered the study. At 6 months, 165 were not eligible to enter phase II because they had discontinued SASP (n = 137) (mainly because of side effects (n = 130 (19%)), did not attend (n = 25) or had died from unrelated causes (n = 3). Thus, 522 patients were eligible for phase II. Of the 356 who were not randomised, 191 had disease activity considered “too good” (DAS <2.4), 123 were satisfied with their progress and refused additional treatment, and in 42 entry to phase II was considered by the doctor to be inappropriate because of intercurrent disease.
Of the 165 patients randomised, 118 completed phase II (41/55 (75%) receiving SASP alone, 38/54 (70%) receiving MTX alone and 39/56 (70%) receiving the combination). Table 1 shows the baseline characteristics of the total group and of those who entered phase II.
Drug doses
Median (range) drug doses in phase II were 2.5 (0–4) g daily for SASP (and 15 mg weekly for MTX placebo in this group), 15 (0–20) mg weekly for MTX (and 2.5 g daily for SASP placebo in this group) and 2.5 g SASP daily and 12.5 mg MTX weekly in the combination group. Oral corticosteroids were not used.
Safety outcome
The reasons for discontinuation of treatment in phase I were as expected: nausea and vomiting 50 (7%), diarrhoea 10 (1%), abnormal liver function tests 12 (2%), rash 14 (2%), headache 9 (1%), reduced white cell count 16 (2%), reduced platelets 2, mouth ulcers 4, pregnancy 2, proteinuria, dizziness, peripheral neuropathy, pneumonitis, bleeding gums, lower limb cellulitis, generalised pruritus, haematuria, pyrexia and orange tears one patient each. Table 2 summarises the reasons for discontinuation of treatment in phase II.
Table 2 Summary of reasons for discontinuation of treatment in phase II (6–18 months).
| Combination group | SASP alone | MTX alone | |
|---|---|---|---|
| Intercurrent illness | 1 | — | — |
| Side effects | 12 | 10 | 14 |
| Lack of effect | 2 | 4 | 2 |
| Non‐compliance/lost to follow‐up | 2 | — | — |
| Total | 17 | 14 | 16 |
MTX, methotrexate; SASP, sulfasalazine.
Efficacy outcome
At 6 months, there was a significant improvement in the SASP group as expected (data available but not shown). Of the 137 patients off SASP, 1 patient had stopped treatment because of lack of effect, 1 because of inadequate effect/dose limited by toxicity, 130 had experienced side effects as noted above and 5 were non‐compliant.
Table 3 shows the changes in DAS between 6 and 18 months for intention‐to‐treat last observation on treatment carried forward.
Table 3 Analysis for last drug observation carried forward.
| Variable | Combination (n = 56) | SASP (n = 55) | MTX (n = 54) | Comb v SASP* p Value | Comb v MTX* p Value | SASP v MTX* p Value |
|---|---|---|---|---|---|---|
| DAS | –0.67 (–1.38 to –0.21) | –0.3 (–0.8 to 0) | –0.26 (–0.99 to 0) | 0.039 | 0.023 | 0.79 |
| HAQ | –0.5 (–10.25 to 0.06) | –0.25 (–9.13 to 0.13) | –0.19 (–10.25 to 0.13) | 0.51 | 0.57 | 0.99 |
| Ritchie articular index | –4 (–7.5 to –0.5) | –3 (–9 to 1) | 0 (–6 to 3) | 0.43 | 0.019 | 0.13 |
| Swollen joint count | –3 (–4 to –0.5) | –3 (–6 to 0) | –2 (–6 to 0) | 0.94 | 0.81 | 0.74 |
| Pain score | –8 (–27.5 to 2) | 0 (–13 to 7) | 0 (–23 to 11) | 0.071 | 0.25 | 0.58 |
| Patient global | –11.5 (–27.5 to 0.5) | 0 (–15 to 5) | –7 (–26 to 2) | 0.06 | 0.72 | 0.14 |
| Physician global | –12.5 (–25 to 0) | –4 (–15 to 5) | –5 (–22 to 0) | 0.044 | 0.62 | 0.13 |
| ESR | 0 (–8.5 to 1) | 0 (–4 to 9) | 1 (–3 to 6) | 0.087 | 0.033 | 0.86 |
| CRP | 0 (–5.5 to 1) | 0 (–1 to 2) | 0 (–3 to 2) | 0.18 | 0.24 | 0.90 |
CRP, C reactive protein; DAS, disease activity score; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire; MTX, methotrexate; SASP, sulfasalazine.
A positive value indicates an increase in the variable over the trial period. Data are median (IQR) increase in score. Changes are 18‐month values minus 6‐month values.
*Mann–Whitney U test used.
Thus, DAS in the combination arm was significantly better than either SASP or MTX alone, but the two monotherapy arms were not significantly different (combination v SASP p = 0.039; combination v MTX p = 0.023; SASP v MTX p = 0.79; table 3).
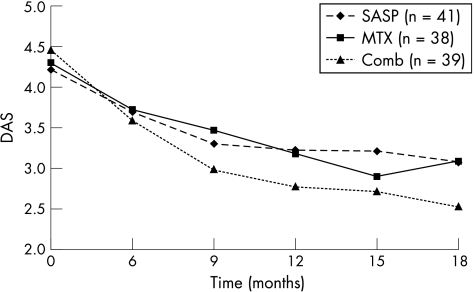
Figure 2 shows median DAS at 0, 6, 9, 12, 15 and 18 months for those who entered and completed phase II. Table 4 shows ACR response at 0–6 months for those considered “too good” or those who refused to enter phase II (n = 123), and for those who did enter phase II (and who clearly had a suboptimal response).
Figure 2 Changes in mean disease activity score (DAS) for those who entered and completed phase II.
Table 4 ACR 20, 50 and 70 responses in phase I (0–6 months)30.
| “Too good” (n = 192) | Refused (n = 123) | Entered phase II (n = 133) | |
|---|---|---|---|
| ACR 20 response | 77 | 42 | 20 |
| ACR 50 response | 47 | 5 | 2 |
| ACR 70 response | 18 | – | 1 |
ACR, American College of Rheumatology.
Table 5 shows change in EULAR DAS in the three groups. Phase II showed a trend for more patients to be in the remission or good DAS category in the combination group and fewer in the high DAS group at 18 months.
Table 5 European League Against Rheumatism Disease Activity Score response at 18 months33.
| Combination | SASP | MTX | |
|---|---|---|---|
| Good response, n (%) | 7 (18) | 3 (7) | 2 (5) |
| Remission, n (%) | 4 (10) | 2 (5) | 1 (3) |
| DAS | |||
| Low | 20 | 11 | 9 |
| Moderate | 45 | 55 | 48 |
| High | 14 | 20 | 31 |
DAS, disease activity score; MTX, methotrexate; SASP, sulfasalazine.
Table 6 shows ACR 20, 50 and 70 responses in phase II (intention‐to‐treat), and indicate that the combination group achieved a higher proportion in the 20%, 50% and 70% responding groups compared with the single drugs alone, although this was not statistically significant. Table 7 shows the ACR responses during 0–18 months for phase II.
Table 6 ACR responses at 6–18 months in those who entered phase II (intention‐to‐treat).
| Combination (n = 56) | SASP (n = 55) | MTX (n = 54) | SASP OR (95% CI) | p Value* | MTX OR (95% CI) | p Value* | |
|---|---|---|---|---|---|---|---|
| ACR 20 response | 16 (29%) | 10 (18%) | 8 (15%) | 1.25 (0.56 to 2.79) | 0.68 | 2.01 (0.85 to 4.76) | 0.14 |
| ACR 50 response | 6 (11%) | 3 (6%) | 4 (7%) | 1.43 (0.43 to 4.81) | 0.76 | 1.79 (0.49 to 6.49) | 0.53 |
| ACR 70 response | 2 (4%) | 1 (2%) | 1 (2%) | 1.50 (0.24 to 9.34) | 1.00 | 3.00 (0.30 to 29.78) | 0.62 |
ACR, American College of Rheumatology; MTX, methotrexate; SASP, sulfasalazine.
The ORs are found with the combination group as the reference level.
*Fisher's exact text used.
Table 7 ACR 20, 50 and 70 response at 0–18 months for those who were randomised in phase II.
| Combination (n = 56) | SASP alone (n = 55) | MTX alone (n = 54) | |
|---|---|---|---|
| ACR 20 response | 48 | 32 | 33 |
| ACR 50 response | 25 | 10 | 7 |
| ACR 70 response | 13 | 7 | 4 |
ACR, American College of Rheumatology; MTX, methotrexate; SASP, sulfasalazine.
Radiographic examination showed no significant difference in total Sharp score and in total erosions (hands and feet), and joint space narrowing between 6 and 18 months in the SASP, MTX and combination groups was similar, with no significant difference between the three groups (data available but not shown).
Discussion
Studies such as this provide evidence of effective “building blocks”, which can be used to achieve tight control of rheumatoid arthritis. Although a fixed combination of DMARDs within a rigid protocol will inevitably have limitations, this true‐to‐life study nevertheless yields useful results. Thus, 28% of the patients with rheumatoid arthritis (most of whom had early inflammatory arthritis) showed “too good” a response to SASP alone to warrant consideration for combination therapy. A further 19% were withdrawn because of reversible side effects in the first 6 months, and were ineligible for combination therapy.
In those with an inadequate response, 165 agreed to enter phase II—after 12 months, the combination of SASP and MTX was superior to monotherapy with either drug, and no additional toxicity was observed. The MTX alone arm was at a slight disadvantage because of the lag phase before the drug became effective.
As DMARD therapy in rheumatoid arthritis needs to be sustained, if benefit is to be shown, tolerability is of importance. The “intention‐to‐treat” approach showed that no harm had resulted from combination therapy. Although 25% of the patients in SASP, 30% in MTX and 30% in combination groups did not complete 18 months of therapy, efficacy was greater with combination compared with SASP or MTX alone.
An in vitro study from Jansen et al34 concluded that the potent SASP inhibition of reduced folate carrier may lead to lack of additivity when SASP and MTX are used together, suggesting that the in vitro effect is not relevant to use in vivo.
Unlike studies undertaken for regulatory approval or those including anti‐TNFα treatments, this study had a “true‐to‐life” recruitment protocol with very few exclusion criteria, making these results applicable to routine clinical practice. Most patients had early disease (70% <1 year, 22% 1–5 years) and 8% had a disease duration of 6–10 years; minimisation ensured distribution of disease duration between the three groups.
This inexpensive intervention of a “step‐up” combination of SASP and MTX therapy proved effective and affordable in an unselected population of patients with rheumatoid arthritis. Although the extent of benefit observed was relatively modest, a small beneficial shift across large numbers of patients is relevant to the rheumatoid arthritis population in general, and other drugs—eg, hydroxychloroquine—could then be added in some patients. This strategy costs only 2% of that using anti‐TNF therapy, and is not associated with increased toxicity. These findings should have a direct benefit to patients with rheumatoid arthritis and allow clear protocols to emerge. Further studies to determine which patients would benefit from a third additional treatment, and to what extent this would prevent radiological progression of disease are still required. This information is needed to work towards tight control strategies in all patients with rheumatoid arthritis.
This study was not powered to show slowing of radiological progression, and predictably this was not observed. In part, the very small number of erosions over a relatively short period (18 months) limits the usefulness of radiological assessment, and other studies have not attempted x ray scoring in this setting. Similarly, function as determined by the health assessment questionnaire is a multifactorial measure, of which synovitis is one component.
The “step‐up” approach was used rather than “sustained continuous” or “step‐down” regimens, because this approach is often adopted in clinical practice. In a double‐blind controlled randomised study, O'Dell et al compared parallel sustained treatment with MTX, SASP and HCQ against MTX alone, and SASP and HCQ in patients with established rheumatoid arthritis.10,11 Low‐dose oral prednisolone was permitted in their studies. Although the patients had more severe disease than in the Management of Atrial Fibrillation (AF) Suppression in the MASCOT study, the results are comparable, as both showed improvement in measures of clinical synovitis, but no difference in ESR as a single measure. O'Dell et al did not report on radiological outcome.
Other studies reporting the benefit of sustained initial combination therapy with either MTX and SASP, or MTX, SASP and HCQ have been open or single‐blind studies, and not all were randomised. Most have shown that the three drugs combined is better than a combination of two drugs or single agents. Only one study has reported that radiological outcome is better in those receiving a combination of MTX, SASP and HCQ.9
One open step‐up study of this combination showed benefit,4 but two double‐blind placebo‐controlled studies have shown that the combination of MTX and SASP from the outset showed only a trend to superiority over monotherapy with either agent.5,6 The differences between our results and these studies may simply be that a step‐up approach selects a subgroup of patients more likely to derive benefit.
The use of targeted biological treatments is an alternative to combinations of small‐molecule conventional DMARDs. When used with MTX, all the licensed anti‐TNFα treatments in early disease show benefit over MTX, but at a greater financial cost, and with, to date, unknown long‐term toxicity.22,23,24,25 A direct comparison of anti‐TNFα treatment with standard approaches in the BeSt study (single‐blind design18) confirmed that a step‐up approach was superior to sustained monotherapy, with no difference in the number of patients who achieved a sustained fall in DAS score ⩾2.4 over 52 weeks in the step‐up or step‐down approach (as in the COBRA study) or with an anti‐TNFα treatment in combination with MTX. This study did, however, report earlier benefit with high‐dose steroid or infliximab, and radiological advantage at 1 year.
We followed a robust study design, but there are limitations which merit emphasis. A larger than expected number of patients eligible for phase II preferred not to enter the double‐blind phase (24%). This was also true in a previous study from this centre.32 The longer‐term outcome of this group compared with those who were randomised is being recorded. DAS tended to improve between 6 and 18 months, but did not reach significance. The effect of patient attitude on future designs of combination DMARDs and the economic evaluation of biological agents in early treatment of rheumatoid arthritis needs to be noted.
The results in terms of ACR and EULAR response are intermediate between those noted by Dougados, who added SASP to leflunomide,7 and those achieved in the TICORA8 and anti‐TNF studies. The ACR and EULAR responses have been shown to have comparable validity.35 The flexible treatment strategy used in TICORA was tailored to the needs of the individual patient, and all were seen monthly by the same rheumatologist. Although this strategy was effective, staff required for this approach are not always available because there are too few skilled healthcare workers in many areas. The addition of an anti‐TNF drug is expensive, in some instances prohibitively so. There are also uncertainties about toxicity in a true‐to‐life setting and in the long term. It is anticipated that this information will emerge from ongoing prospective data registries in several countries.
For health resource planning, this study provides precise information about how often unselected patients show sufficient response to their initial DMARD (SASP), and hence do not require additional therapy, and about the proportion who refuse a combination even when disease activity suggests that this would be valuable.
This combination study shows that step‐up treatment for patients with an inadequate response to SASP is of significant clinical benefit, carries no additional toxicity and is achievable at minimal extra cost, an important consideration in the responsible use of healthcare resources.
Acknowledgements
We thank Sisters Fiona McGhie, Liz McIvor, E Anne Thomson for metrology help, and Dr M Field, Dr S Fraser, Dr A McEntegart, Dr P McGill, Dr E Murphy and Prof R Sturrock for allowing their patients to participate.
Abbreviations
DAS - disease activity score
DMARD - disease‐modifying antirheumatic drug
ESR - erythrocyte sedimentation rate
EULAR - European League Against Rheumatism
HCQ - hydroxychloroquine
MTX - methotrexate
SASP - sulfasalazine
TICORA - tight control in rheumatoid arthritis
TNF - tumour necrosis factor
Footnotes
Funding: This study was funded by the Arthritis Research Campaign. Wyeth Pharmaceuticals supplied methotrexate and matching placebo. Pharmacia supplied sulpfasalazine and matching placebo.
Competing interests: None declared.
The study was funded by the Arthritis Research Campaign, which had no role in data collection, data analysis, data interpretation, writing of the report, or in the decision to submit the paper for publication. Wyeth (Maidenhead, UK) and Pharmacia (Milton Keynes, UK) provided drugs and placebo, but had no role in the conduct or analysis of the study.
References
- 1.Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts. Arthritis Rheum 2001441234–1236. [DOI] [PubMed] [Google Scholar]
- 2.O'Dell J R. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum 200246283–285. [DOI] [PubMed] [Google Scholar]
- 3. SIGN Guidelines number 48. Management of early RA, December 2000
- 4.Haagsma C J, van Reil P L C M, de Rooij D J R A, Vree T B, Russel F J M, van't Hof M A.et al Combination of methotrexate and sulphasalazine vs methotrexate alone: a randomized open clinical trial in rheumatoid arthritis patients resistant to sulphasalazine therapy. Br J Rheumatol 1994331049–1055. [DOI] [PubMed] [Google Scholar]
- 5.Haagsma C J, van Riel P L C M, de Jong A J L, van de Putte L B A. Combination of sulphasalazine and methotrexate versus the single components in early rheumatoid arthritis: a randomised, controlled, double‐blind, 52 week clinical trial. Br J Rheumatol 1997361982–1988. [DOI] [PubMed] [Google Scholar]
- 6.Dougados M, Combe B, Cantagrel A, Goupille P, Olive P, Schattenkirchner M.et al Combination therapy in early rheumatoid arthritis: a randomised, controlled, double blind 52 week clinical trial of sulphasalazine and methotrexate compared with the single components. Ann Rheum Dis 199958220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougados M, Emery P, Lemmel E M, Zerbini C A F, Brin S, van Riel P. When a DMARD fails, should patients switch to sulfasalazine or add sulfasalazine to continuing leflunomide? Ann Rheum Dis 20056444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grigor C, Capell H, Stirling A, McMahon A D, Lock P, Vallance R.et al Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single‐blind randomised controlled trial. Lancet 2004364263–269. [DOI] [PubMed] [Google Scholar]
- 9.Möttönen T, Hannonen P, Leriisalo‐Repo M, Nissila M, Kautiainen H, Lorpela M.et al Comparison of combination therapy with single‐dose drug therapy in early rheumatoid arthritis: a randomised trial. Lancet 19993531568–1573. [DOI] [PubMed] [Google Scholar]
- 10.O'Dell J R, Haire C E, Erikson N, Drymalski W, Palmer W, Eckhoff P J.et al Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med 19963341287–1291. [DOI] [PubMed] [Google Scholar]
- 11.O'Dell J R, Leff R, Paulsen G, Haire C, Mallek J, Eckhoff P J.et al Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine or a combination of the three medications: results of a two year, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum 2002461164–1170. [DOI] [PubMed] [Google Scholar]
- 12.Boers M, Verhoeven A C, Markusse H M, van de Laar M A F J, Westhovens R, van Denderen J C.et al Randomised comparison of combined step‐down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997350309–318. [DOI] [PubMed] [Google Scholar]
- 13.Tugwell P, Pincus T, Yocum D, Stein M, Gluck O, Kraag G.et al Combination therapy with cyclosporine and methotrexate in severe rheumatoid arthritis. The Methotrexate‐Cyclosporine Combination Study Group. N Engl J Med 1995333137–141. [DOI] [PubMed] [Google Scholar]
- 14.Felsen D T, Anderson J J, Meenan R F. The efficacy and toxicity of combination therapy in rheumatoid arthritis. Arthritis Rheum 1994371487–1491. [DOI] [PubMed] [Google Scholar]
- 15.Verhoeven A, Boers M, Tugwell P. Combination therapy in rheumatoid arthritis: updated systematic review. Br J Rheum 199837612–619. [DOI] [PubMed] [Google Scholar]
- 16.Smolen J, Aletarn D, Keystone E. Superior efficacy of combination therapy in rheumatoid arthritis: fact or fiction? Arthritis Rheum 2005522975–2983. [DOI] [PubMed] [Google Scholar]
- 17.Landewe R B M, Boers M, Verhoeven A C, Westhovens R, van de Laar M A F J, Markusse H M.et al COBRA combination therapy in patients with early rheumatoid arthritis. Arthritis Rheum 200246347–356. [DOI] [PubMed] [Google Scholar]
- 18.Goekoop Ruiterman Y, de Vries‐Bouwstan J, Allant C, van Zeber D, Kersters P, Hazes J M.et al Clinical and radiographic outcomes of four different treatment strategies people with early RA (the BeSt study). A randomised controlled trial. Arthritis Rheum 2005523381–3390. [DOI] [PubMed] [Google Scholar]
- 19.Furst D E, Breedveld F C, Kalden J R, Smolen J S, Burmester G R, Bijlsma J W J.et al Updated consensus statement on biological agents, specifically tumour necrosis factor α (TNFα) blocking agents and interleukin‐1 receptor antagonist (IL‐1ra), for the treatment of rheumatic diseases. Ann Rheum Dis 200564iv2–NaN14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Clinical Excellence Guidance on the use of etanercept and infliximab for the treatment of rheumatoid arthritis: technology appraisal guidance no. 36. London: National Clinical Institute, 20027
- 21.Symmons D P M. Anti‐tumour necrosis factor α therapy: can we afford it? Ann Rheum Dis 200564969–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Askling J, Fored C M, Baecklund E, Brandt L, Backlin C, Ekbon A.et al Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis 2005641414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askling J, Fored C M, Brandt L, Baecklund E, Bertilsson L, Feltelius N.et al Risks of solid cancers in patients with rheumatoid arthritis and after treatment with tumour necrosis factor antagonists. Ann Rheum Dis 2005641421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winthrop K L. Update on tuberculosis and other opportunistic infections associated with drugs blocking tumour necrosis factor α. Ann Rheum Dis 200564iv29–iv30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bongartz T, Sutton A J, Sweeting M J, Buchan I, Matteson E L, Montori V. Anti‐TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta‐analysis of rare harmful effects in randomized controlled trials. JAMA 20062952275–2285. [DOI] [PubMed] [Google Scholar]
- 26.British National Formulary 4 Mar 2006
- 27.van der Heijde D M F M, van't Hof M A, van Riel P L C M, Theunisse L A M, Lubberts E W, van Leeuwen M A.et al Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis 199049916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Gestel A M, Prevoo M L, van't Hof M A, van Rijswijk M D, van de Putte L B, van Riel P L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Arth Rheum 19963934–40. [DOI] [PubMed] [Google Scholar]
- 29.Treasure T, MacRae K D. Minimisation: the platinum standard for trials? Randomisation doesn't guarantee similarity of groups: minimisation does [editorial], Br Med J 1998317362–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felsen D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C.et al American College of Rheumatology preliminary definitions of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. [DOI] [PubMed] [Google Scholar]
- 31.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 199926743–745. [PubMed] [Google Scholar]
- 32.Porter D R, Capell H A, Hunter J. Combination therapy in rheumatoid arthritis—no benefit of addition of hydroxychloroquine to patients with a suboptimal response to intramuscular gold therapy. J Rheumatol 199320645–649. [PubMed] [Google Scholar]
- 33.Fransen J, van Riel P L C M. The disease activity score and the EULAR response criteria. Clin Exp Rheumatol 200523(Suppl 39)S93–S99. [PubMed] [Google Scholar]
- 34.Jansen G, van der Heijden J, Oerlemans R, Lems W F, Ifergan I, Scheper R J.et al Sulfasalazine is a potent inhibitor of the reduced folate carrier. Arthritis Rheum 2004502130–2139. [DOI] [PubMed] [Google Scholar]
- 35.van Gestel A M, Anderson J J, van Riel P L, Boers M, Haagsma C J, Rich B.et al ACR and EULAR improvement criteria have comparable validity in rheumatoid arthritis trials. American College of Rheumatology European League of Association for Rheumatology. J Rheumatol 199926705–711. [PubMed] [Google Scholar]